Hepatocyte-Specific β-Catenin Deletion During Severe Liver Injury Provokes Cholangiocytes to Differentiate Into Hepatocytes
Abstract
Liver regeneration after injury is normally mediated by proliferation of hepatocytes, although recent studies have suggested biliary epithelial cells (BECs) can differentiate into hepatocytes during severe liver injury when hepatocyte proliferation is impaired. We investigated the effect of hepatocyte-specific β-catenin deletion in recovery from severe liver injury and BEC-to-hepatocyte differentiation. To induce liver injury, we administered choline-deficient, ethionine-supplemented (CDE) diet to three different mouse models, the first being mice with deletion of β-catenin in both BECs and hepatocytes (Albumin-Cre; Ctnnb1flox/flox mice). In our second model, we performed hepatocyte lineage tracing by injecting Ctnnb1flox/flox; Rosa-stopflox/flox-EYFP mice with the adeno-associated virus serotype 8 encoding Cre recombinase under the control of the thyroid binding globulin promoter, a virus that infects only hepatocytes. Finally, we performed BEC lineage tracing via Krt19-CreERT; Rosa-stopflox/flox-tdTomato mice. To observe BEC-to-hepatocyte differentiation, mice were allowed to recover on normal diet following CDE diet–induced liver injury. Livers were collected from all mice and analyzed by quantitative real-time polymerase chain reaction, western blotting, immunohistochemistry, and immunofluorescence. We show that mice with lack of β-catenin in hepatocytes placed on the CDE diet develop severe liver injury with impaired hepatocyte proliferation, creating a stimulus for BECs to differentiate into hepatocytes. In particular, we use both hepatocyte and BEC lineage tracing to show that BECs differentiate into hepatocytes, which go on to repopulate the liver during long-term recovery. Conclusion: β-catenin is important for liver regeneration after CDE diet–induced liver injury, and BEC-derived hepatocytes can permanently incorporate into the liver parenchyma to mediate liver regeneration.
Abbreviations
-
- AAV8-TBG-Cre
-
- adeno-associated virus serotype 8 encoding Cre recombinase under the hepatocyte-specific thyroid binding globulin promoter
-
- ALP
-
- alkaline phosphatase
-
- ALT
-
- alanine aminotransferase
-
- ANOVA
-
- analysis of variance
-
- AST
-
- aspartate aminotransferase
-
- BECs
-
- biliary epithelial cells
-
- CDE
-
- choline-deficient, ethionine-supplemented
-
- EpCAM
-
- epithelial cell adhesion molecule
-
- EYFP
-
- enhanced yellow fluorescence protein
-
- GS
-
- glutamine synthetase
-
- IHC
-
- immunohistochemistry
-
- KO
-
- knockout
-
- LR
-
- liver regeneration
-
- PBS
-
- phosphate-buffered saline
-
- WT
-
- wild-type
Despite the liver's capacity for regeneration, chronic liver disease and cirrhosis is the twelfth leading cause of death in the United States.1 This significant morbidity is attributable to lack of treatments for advanced liver disease besides liver transplantation, for which there is a severe shortage of donor organs.2 Often, hepatocytes and biliary epithelial cells (BECs) can replicate to replenish their respective cell types and eventually restore hepatic mass following injury. However, livers of patients with chronic liver disease exhibit ductular reaction,3-5 with the degree of BEC expansion correlating with disease severity.6, 7 The role of ductular reaction in liver regeneration (LR) remains controversial, although studies are beginning to show BECs may be giving rise to hepatocytes. Indeed, experimentally, when endogenous hepatocyte proliferation is impaired, reactive BECs8, 9 can differentiate into hepatocytes to mediate repair.10, 11 Alternatively, hepatocytes can differentiate into BECs, which can then be incorporated into biliary ductules12, 13 and may revert back to hepatocytes when injury is withdrawn.14 There is evidence of BEC-to-hepatocyte differentiation in both humans15, 16 and animal models of liver injury where hepatocyte proliferation is blocked17-19 or after near total loss of hepatocytes.20
The choline-deficient, ethionine-supplemented (CDE) diet is a well-known liver injury diet that induces proliferation of reactive BECs.21, 22 Recent lineage tracing studies have demonstrated limited contribution of BECs to hepatocytes in CDE diet–fed mice,23-25 leading to the conclusion that BECs do not contribute significantly to the restoration of hepatocyte mass after chronic liver injury. However, the CDE diet does not block hepatocyte proliferation25 and thus is unable to provide the correct milieu for BEC-to-hepatocyte transdifferentiation. Because the Wnt/β-catenin signaling pathway is a major driver of hepatocyte proliferation during LR,26, 27 we investigated if CDE diet–induced injury to conditional β-catenin knockout mice will necessitate BEC-mediated liver repair. We used multiple mouse models to perform hepatocyte and BEC lineage tracing in mice that underwent modulation of β-catenin expression and were administered CDE diet. Our results demonstrate that loss of β-catenin in hepatocytes during CDE diet–induced liver injury indeed impairs hepatocyte proliferation, triggering the expansion of BECs, which subsequently differentiate into hepatocytes. We have successfully established a model that will lend itself well to the study of the mechanisms of BEC expansion and differentiation.
Materials and Methods
MOUSE STRAINS, VIRAL INFECTIONS, TAMOXIFEN ADMINISTRATION, IN VIVO RNAi, AND DIET
All animals are housed in temperature- and light-controlled facilities and are maintained in accordance with the Guide for Care and Use of Laboratory Animals and the Animal Welfare Act. Albumin-Cre;Ctnnb1flox/flox mice or knockout (KO) 1 and wild-type (WT) 1 littermate controls28 were maintained on a C57BL/6 background. Ctnnb1flox/flox; Rosa-stopflox/flox-EYFP reporter mice were generated through breeding Ctnnb1flox/flox mice with Rosa-stopflox/flox-EYFP mice (Jackson Laboratories). Krt19-CreERT; Rosa-stopflox/flox-tdTomato reporter mice were described previously.17 To label hepatocytes and generate KO2 mice, 23-25-day-old Ctnnb1flox/flox; Rosa-stopflox/flox-EYFP mice were injected intraperitoneally with 1×1012 genome copies of adeno-associated virus serotype 8 encoding Cre recombinase under the hepatocyte-specific thyroid binding globulin promoter (AAV8-TBG-Cre) (Penn Vector Core) followed by a 12-day washout period. To generate WT2 mice, the same AAV8-TBG-Cre was injected into or Ctnnb1+/+; Rosa-stopflox/flox-EYFP mice. To label BECs, Krt19-CreERT; Rosa-stopflox/flox-tdTomato mice were given 3 doses of 12.5 mg/kg tamoxifen during postnatal week 1, followed by 2 weeks of washout. For the liver injury time point, 4-5-week-old mice were given choline-deficient diet (Envigo Teklad Diets) supplemented with 0.15% ethionine drinking water (Acros Organics, 146170100) for 2-3 weeks (Krt19-CreERT; Rosa-stopflox/flox-tdTomato mice). For recovery time points, animals were switched back to normal chow diet for 3 days up to 6 months after 2 weeks of CDE diet. For in vivo knockdown of β-catenin expression, both C57BL6/NJ (Charles River) and Krt19-CreERT; Rosa-stopflox/flox-tdTomato mice were given biweekly or weekly subcutaneous injections of 5 mg/kg Ctnnb1-siRNA dissolved in phosphate-buffered saline (PBS) or PBS as a control starting 1 week prior to CDE diet administration and continuing throughout CDE diet and recovery periods. The small interfering RNA (siRNA) (Dicerna Pharmaceuticals) was conjugated to N-acetylgalactosamine, which allows hepatocyte-specific knockdown of target gene expression.29 Serum biochemistry analysis was performed by automated methods at the University of Pittsburgh Medical Center clinical chemistry laboratory. All studies were performed according to the guidelines of the National Institutes of Health and the University of Pittsburgh Institutional Animal Use and Care Committee.
IMMUNOHISTOCHEMISTRY
See Supporting Methods.
IMMUNOFLUORESCENCE
See Supporting Methods.
CELL QUANTIFICATION
In the negative lineage tracing model, samples were triple stained for β-catenin, enhanced yellow fluorescence protein (EYFP), and Hnf4α as described above. For each sample, eight images at ×200 magnification of periportal regions were taken and in each image the number of EYFP+/Hnf4α+ and EYFP-/Hnf4α+ cells were counted. A minimum of 700 cells were counted for every sample. In the positive lineage tracing model, images were obtained on a Zeiss Axiovert 200 microscope using a Zeiss Axiocam MR camera. Cell counts were performed manually on blinded slides and more than 20 consecutive fields at ×200 magnification. Hepatocytes and cholangiocytes were defined as Hnf4α and CK19 positive cells, respectively. Cells were counted with ImageJ cell counter program.
WESTERN BLOTTING
See Supporting Methods.
RT-PCR
See Supporting Methods.
STATISTICS
For analysis of serum biochemistry between two groups, a two-tailed t test was performed. For analysis of gene expression data between more than two groups, a one-way analysis of variance (ANOVA) was performed. For analysis of cell counts, such as proliferating hepatocytes, a Mann-Whitney U test was performed. For analysis of change in body weight over time, a two-way ANOVA was performed. A P < 0.05 was considered significant, and plots are mean ± SD. All statistical analysis and graph generation was performed using GraphPad Prism 7 software.
Results
β-CATENIN IS IMPORTANT FOR LR AFTER CDE DIET–INDUCED LIVER INJURY
To test whether lack of β-catenin in hepatocytes impairs hepatocyte proliferation in a chronic liver injury setting, we placed KO1 mice28 lacking β-catenin in hepatocytes and BECs, and WT1 mice on CDE diet for 2 weeks (Fig. 1A). KO1 showed severe histological abnormalities including steatohepatitis (Fig. 1F), with gross liver morphology displaying pale and smller livers (Fig. 1B). Serum liver injury markers, alanine aminotransferase (ALT) (Fig. 1C), serum aspartate aminotransferase (AST) (Supporting Fig. S1D), and bilirubin (Fig. 1D) were significantly elevated in KO1 compared with WT1 on CDE diet. There was no difference in serum alkaline phosphatase (ALP) levels in WT1 and KO1 mice (Supporting Fig. S1E). Both WT1 and KO1 displayed characteristic expansion of reactive BECs, which are positive for BEC-marker CK19 (Supporting Fig. S1A), although KO1 displayed a more robust BEC expansion as determined by increased expression of BEC markers Krt19 and Sox9 (Supporting Fig. S1F,G). KO1 also displayed increased fibrosis by Sirius Red staining (Supporting Fig. S1A) and increased expression of profibrotic genes Col1a1 (Supporting Fig. S1B) and Acta2 (Supporting Fig. S1C,H). We detected increased cell death as evidenced by terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick-end labeling staining (Supporting Fig. S1A) and increased hepatocyte senescence in KO1 as evidenced by an increase in p21-positive hepatocytes (Supporting Fig. S1A) and increased levels of p21 protein (Supporting Fig. S1H) compared with WT1. Collectively, we detect severe liver injury in KO1 mice on CDE diet.
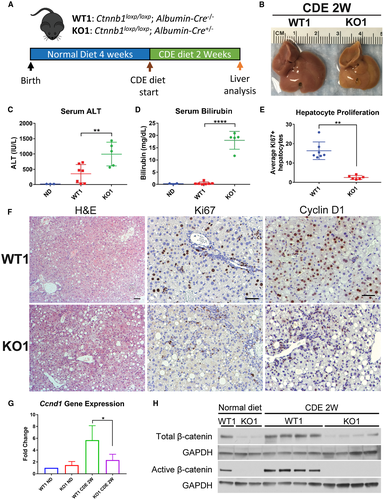
To determine if there was a defect in LR in KO1, we assessed hepatocyte proliferation via Ki67 and Cyclin D1 immunohistochemistry (IHC) (Fig. 1F). Whereas WT1 displayed robust hepatocyte proliferation and Cyclin D1 expression especially in periportal hepatocytes, KO1 displayed significantly impaired hepatocyte proliferation (Fig. 1E) and reduced Ccnd1 expression (Fig. 1G) and protein (Supporting Fig. S1H). As expected, low levels of β-catenin protein were observed in KO1 livers, likely due to nonparenchymal cells (Fig. 1H). However, there was no expression of active-β-catenin (hypophosphorylated) in KO1 mice even after CDE diet (Fig. 1H). Alternatively, there was increased total and active-β-catenin levels in WT1 mice on CDE diet compared with normal diet (Fig. 1H). This suggests activation of β-catenin signaling is contributing to hepatocyte proliferation, and deletion of β-catenin leads to impaired hepatocyte proliferation and defective LR after CDE diet.
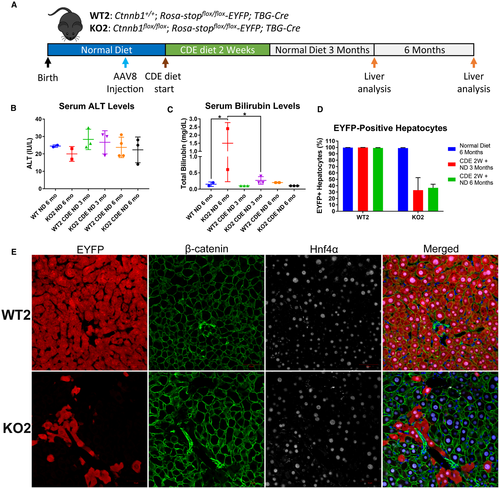
LACK OF β-CATENIN IN HEPATOCYTES IMPAIRS HEPATOCYTE PROLIFERATION AND PROMOTES INJURY FROM THE CDE DIET
As KO1 mice on CDE diet displayed severe liver injury, impaired hepatocyte proliferation, and robust expansion of BECs, we hypothesized BEC differentiation to hepatocytes would be activated to mediate restoration of hepatocyte mass. To test this hypothesis, we generated KO2 mice.23 Genes in the Rosa locus are ubiquitously expressed, but prior to Cre recombination, a floxed stop codon inactivates expression of the Rosa-driven EYFP reporter gene.30 AAV8 infects only hepatocytes, and greater than 99% of hepatocytes can be permanently labeled with EYFP after AAV8-TBG-Cre injection.25, 23, 31 We administered a single injection of AAV8-TBG-Cre and allowed 12-day washout before administering CDE diet (Fig. 2A) to allow for clearance of residual viral genome.32 In KO2, hepatocytes lack β-catenin expression and are permanently labeled with EYFP (Fig. 2B). Importantly, BECs are not infected with AAV8 and thus retain β-catenin and do not express EYFP. Therefore, hepatocytes originating from BECs will be negative for EYFP and express β-catenin (Fig. 2F). As controls, WT2 mice showed EYFP-labeled hepatocytes that retain β-catenin expression. IHC confirmed β-catenin expression in hepatocytes in WT2 mice but not in KO2 mice (Supporting Fig. S2A).
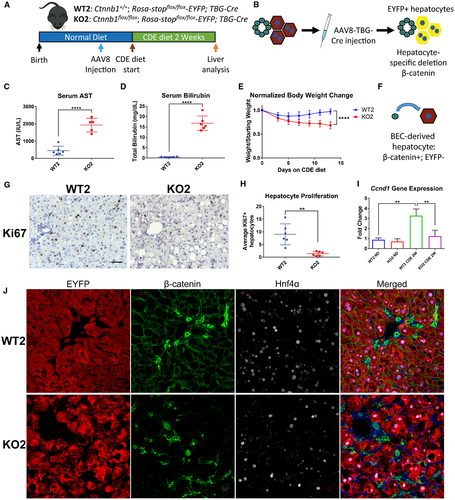
KO2 on CDE diet, like KO1, showed severe liver injury reflected by significantly increased serum AST (Fig. 2C), serum ALT (Supporting Fig. S2D), and bilirubin levels (Fig. 2D) as compared with WT2. WT2 mice on CDE diet initially lost weight but began to recover after 5-7 days. In contrast, KO2 mice continued to lose weight over the course of CDE diet administration (Fig. 2E), suggesting failure to recover from injury. Both WT2 and KO2 mice displayed expansion of BECs (Fig. S2A), confirmed by increased Krt19 (Supporting Fig. S2F) and Sox9 (Supporting Fig. S2G) expression. KO2 and WT2 mice developed fibrosis after 2 weeks of CDE diet, evident by increased Sirius Red staining (Supporting Fig. S2A) and increased expression of Col1a1 (Supporting Fig. S2B) and Acta2 (Supporting Fig. S2C). KO2 mice on CDE diet also displayed increased p21-positive hepatocytes (Supporting Fig. S2A) and overall p21 protein levels (Supporting Fig. S2H).
When hepatocyte proliferation was assessed by Ki67 staining, WT2 mice also displayed robust periportal hepatocyte proliferation, which was nearly absent in KO2 mice (Fig. 2G,H). WT2 mice, like WT1, displayed increased Ccnd1 gene (Fig. 2I) and protein expression (Supporting Fig. S2H) after CDE diet, which was significantly abrogated in KO2. This suggests severely impaired hepatocyte proliferation in KO2 mice after 2 weeks of CDE diet. We did not detect any β-catenin-positive, EYFP-negative hepatocytes in KO2 (Fig. 2J), suggesting no BEC-derived hepatocytes (Fig. 2F) were present at this time. Corroborating this observation, we did not detect an increase in total β-catenin levels in KO2 mice after 2 weeks of CDE diet in comparison with KO2 mice left on normal diet. However, we did detect active β-catenin in KO2 mice on CDE diet, which was absent in KO2 mice on normal diet (Supporting Fig. S2H). This suggests active-β-catenin is present in β-catenin-positive BEC compartment, and that BEC differentiation into hepatocytes had yet to occur.
DEFECTIVE HEPATOCYTE PROLIFERATION IN KO2 MICE DRIVES BEC-TO-HEPATOCYTE DIFFERENTIATION AFTER CDE DIET-INDUCED HEPATIC INJURY
To facilitate BEC-driven repair, we next administered CDE diet to WT2 and KO2 mice for 2 weeks, followed by recovery on normal diet for 2 weeks17, 19, 33 (Fig. 3A). Both WT2 and KO2 displayed normal serum ALT (Fig. 3C), although KO2 displayed slightly elevated serum bilirubin (Fig. 3D) and ALP (Fig. 3E) compared with WT2. Excitingly, in KO2 mice we detected clusters of β-catenin-positive, EYFP-negative cells, which stained positively for hepatocyte marker Hnf4α (Fig. 3G), indicating BEC-derived hepatocytes (Fig. 3B). We did not detect expansion of EYFP-negative hepatocytes in WT2 mice, suggesting that BECs do not give rise to hepatocytes in animals when hepatocyte proliferation is not impaired, consistent with previous reports.14, 23, 25 An alternative explanation for these cells is that they were hepatocytes that escaped initial Cre recombination, which could result in EYFP-negative, β-catenin-positive hepatocytes. However, we would predict the efficiency of AAV8-TBG-Cre to be the same in both WT2 and KO2 mice. Therefore, we quantified the number of EYFP-positive/negative hepatocytes in our WT2 and KO2 mice over the course of CDE diet injury and recovery (Fig. 3F). First, we quantified the number of EYFP-positive hepatocytes in WT2 and KO2 mice 12 days after injection with AAV8-TBG-Cre, corresponding to the initial labeling efficiency before the onset of liver injury. We found that greater than 99% of hepatocytes were EYFP positive, consistent with previous reports.23, 25 After 2 weeks of CDE diet, the percentage of EYFP-negative hepatocytes was not significantly increased in either WT2 or KO2. However, after 2 weeks of recovery after CDE diet–induced liver injury, in KO2 mice approximately 20% of periportal hepatocytes were EYFP negative, whereas the percentage of EYFP-negative hepatocytes in WT2 mice did not increase (Fig. 3F).
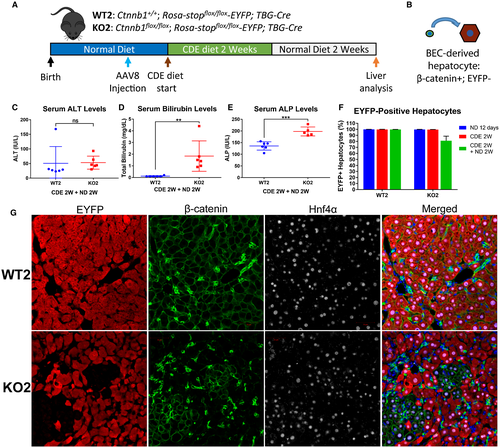
Correspondingly, tiled images (and serial higher-magnification images from a representative area) from KO2 mice of representative lobes stained for β-catenin revealed no clusters of β-catenin-positive hepatocytes after 2 weeks of CDE diet (Supporting Fig. S3A,B), whereas after 2 weeks of CDE diet followed by 2 weeks of recovery on normal diet, many clusters of β-catenin-positive hepatocytes were evident across the entire lobe (and serial higher-magnification images from a representative area) (Supporting Fig. S3C,D). Likewise, hepatic Ctnnb1 expression tended to increase in KO2 mice after CDE diet and recovery, although it was still significantly lower than WT2 mice. In age-matched control mice left on normal diet for 6 weeks after AAV8-TBG-Cre injection, hepatic gene expression of Ctnnb1 was greatly reduced in KO2 mice compared with WT2 mice (Supporting Fig. S3E), indicating lack of repopulation of β-catenin-positive hepatocytes in the absence of liver injury. These results, in combination with lack of expansion of EYFP-negative hepatocytes in WT2 after CDE diet injury and recovery, support BEC-to-hepatocyte conversion in KO2 over expansion of Cre-recombinase escaped cells.
BEC-DERIVED β-CATENIN-POSITIVE HEPATOCYTES ARE MORE PROLIFERATIVE THAN ENDOGENOUS HEPATOCYTES
Analysis of hepatic sections from KO2 mice after 2 weeks of recovery on normal chow following 2 weeks of CDE diet showed that nearly all β-catenin-positive hepatocytes were located in clusters adjacent to BECs in the periportal region (Supporting Fig. S3B). This may also explain the lack of reappearance of pericentral β-catenin target glutamine synthetase (GS)26, 34 in KO2 mice at this time (Fig. 4C). The reactive BEC response was extensive after 2 weeks of CDE diet and recovery, as evidenced by large numbers of epithelial cell adhesion molecule (EpCAM)–positive BECs (Supporting Fig. S4A). The extent of BEC expansion was similar in WT2 and KO2, as indicated by comparable hepatic expression of BEC markers Krt19 (Supporting Fig. S4B) and Sox9 (Supporting Fig. S4C). There was ongoing fibrosis in both WT2 and KO2 as evidenced by Sirius Red staining even after 2 weeks of recovery on normal diet (Supporting Fig. S4A), which is likely due to ongoing reactive BEC response, which is known to secrete profibrogenic cytokines.35 This level of fibrosis also indicated that repair of CDE diet–induced liver injury was not complete after 2 weeks of recovery on normal diet.
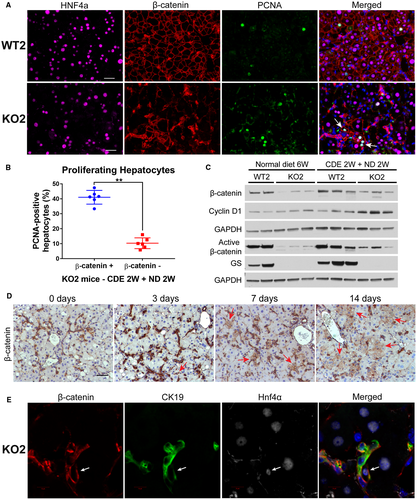
To determine if BEC-derived, β-catenin-positive hepatocytes were driving restoration of hepatocyte mass in KO2 mice after CDE diet–induced liver injury, we performed triple immunofluorescence for Hnf4α, β-catenin, and proliferating cell nuclear antigen (PCNA) (Fig. 4A). Within a given cluster of β-catenin-positive hepatocytes in KO2, the majority of β-catenin-positive hepatocytes stained positively for PCNA. Indeed, periportal β-catenin-positive hepatocytes were significantly more proliferative than surrounding β-catenin-negative hepatocytes in KO2 (Fig. 4B). Likewise, clusters of periportal hepatocytes in KO2 were strongly Cyclin D1 positive in comparison with surrounding hepatocytes (Supporting Fig. S4A, red arrows). KO2 also displayed increased total Cyclin D1 protein compared with WT2 after 2 weeks of CDE diet and recovery (Fig. 4C). There was also a noticeable increase in total and active-β-catenin in KO2 after recovery in comparison with age-matched KO2 left on normal diet for 6 weeks (Fig. 4C), indicating repopulation by β-catenin-positive cells in KO2 during repair. Collectively, these results demonstrate BEC-derived β-catenin-positive hepatocytes are preferentially proliferating to replenish lost hepatic mass in KO2 mice.
BEC DIFFERENTIATION TO HEPATOCYTES OCCURS EARLY IN RECOVERY ON NORMAL DIET
To determine if the BEC-derived hepatocytes after 2 weeks of recovery on normal diet express BEC markers, we performed triple immunofluorescence with a wide-spectrum cytokeratin antibody (PanCK) in addition to EYFP and hepatocyte marker Hnf4α (Supporting Fig. S4D). EYFP-negative hepatocytes were PanCK negative (Supporting Fig. S4D, white arrowhead), and as expected the majority of cytokeratin-positive BECs were negative for EYFP. Interestingly, rare EYFP-positive BECs were observed in both WT2 and KO2 mice (Supporting Fig. S4D, white arrows), potentially indicating hepatocyte-to-BEC transdifferentiation after CDE diet–induced liver injury. When we performed staining for a second BEC marker, EpCAM, we observed rare cells with hepatocyte-like morphology, which were EpCAM positive in KO2 mice (Supporting Fig. S4A, black arrows). This finding correlates with the observations of EpCAM-positive hepatocytes in human patients with BEC expansion after severe liver injury.16 These results demonstrate that after 2 weeks of recovery on normal diet, the majority of BEC-derived hepatocytes in KO2 mice do not express BEC markers.
To further explore the timing of BEC-to-hepatocyte differentiation during recovery from CDE diet, we harvested KO2 mice after 3 days or 7 days of recovery on normal diet post-CDE diet (Supporting Fig. S5A). Excitingly, very few small β-catenin-positive hepatocyte-like cells are evident after 3 days of recovery, which grow into small clusters of β-catenin-positive cells with hepatocyte morphology after 7 days of recovery (Fig. 4D, red arrows). These clusters are even larger following 2 weeks of recovery on normal diet, suggesting potential clonal expansion of BEC-derived, β-catenin-positive hepatocytes. Interestingly, serum ALT levels are dramatically reduced following 3 days of recovery on normal diet (Supporting Fig. S5B), whereas both serum bilirubin and ALP levels remain elevated after 3 days of recovery and only begin to normalize after 7 or 14 days of recovery, respectively (Supporting Fig. S5C,D), potentially suggesting abatement of acute liver injury may be required for expansion of these cells. Similar serum liver injury profiles and appearance of small β-catenin-positive hepatocytes were also observed in female KO2 mice after CDE diet and 3 days of recovery (data not shown), suggesting BEC-to-hepatocyte differentiation also occurs in female mice following recovery from CDE diet–induced liver injury.
We next investigated the expression of Hnf4α in these putative transdifferentiating BECs over time. After 2 weeks of CDE diet, virtually all β-catenin-positive cells were positive for CK19 and negative for Hnf4α (Supporting Fig. S5E). However, as early as after 3 days of recovery, we detected β-catenin-positive cells that were CK19 positive and weakly positive for Hnf4α (Supporting Fig. S5F). After 7 days of recovery on normal diet, rare β-catenin-positive cells were observed, which were strongly positive for both CK19 and Hnf4α (Fig. 4E). At this time point, clusters containing β-catenin+/Hnf4α+/CK19− hepatocytes were evident (Supporting Fig. S5G). The rarity of the β-catenin+/Hnf4α+/CK19+ cells in combination with the increased proliferation of β-catenin-positive hepatocytes suggests that few BECs differentiate into hepatocytes, which subsequently proliferate to restore lost hepatocyte mass in KO2 mice.
BEC-DERIVED HEPATOCYTES REPOPULATE THE LIVER DURING LONG-TERM RECOVERY
Previous studies have demonstrated that transdifferentiated cells, such as hepatocyte-derived BECs, may revert back to their original cell type when liver injury has abated.14 Alternatively, it has been shown that with persistent need for transdifferentiated cells, such as in mice that lack the intrahepatic biliary system, hepatocyte-to-BEC conversion is permanent and stable for life.13 To determine if BEC-derived hepatocytes would persist and further repopulate the liver, KO2 mice were allowed to recover on normal diet for either 3 or 6 months after CDE diet (Fig. 5A). As a control, we traced WT2 and KO2 mice for 6 months on normal diet to determine the persistence of EYFP labeling in the absence of injury. Serum ALT levels were normal in both WT2 and KO2 (Fig. 5B,C). Interestingly, KO2 mice left on normal diet for 6 months showed minor elevation of bilirubin compared with WT2 (Fig. 5C). KO2 mice after long-term recovery from CDE diet showed extensive expansion of EYFP-negative, β-catenin-positive hepatocytes (Fig. 5E), as evidenced by central veins that were partially or completely GS positive (Supporting Fig. S6A). When we quantified the number of EYFP-positive hepatocytes, we found that in control WT2 and KO2 left on normal diet for 6 months, there was no significant expansion of EYFP-negative hepatocytes, similar to WT2 after long-term recovery from CDE diet (Fig. 5D). However, in KO2 after 3 months of recovery from CDE diet, up to 70% of hepatocytes were EYFP negative, and this number remained stable after 6 months of recovery. In control KO2 mice left on normal diet for 6 months, virtually all EYFP-positive hepatocytes were β-catenin negative (Supporting Fig. S6B), as confirmed by β-catenin IHC (Supporting Fig. S6C). Hepatic expression of Ctnnb1 was significantly reduced in KO2 mice left on normal diet for 6 months compared with control WT2 mice, whereas expression of Ctnnb1 in KO2 after recovery from CDE diet began to approach WT2 levels (Supporting Fig. S6D). The lack of expansion of EYFP-negative hepatocytes in WT2 mice even after 6 months of recovery post-CDE diet, combined with the lack of expansion of EYFP-negative hepatocytes in KO2 left on normal diet, further supports our hypothesis that BEC-derived hepatocytes repopulate the liver in KO2 after CDE diet–induced liver injury.
IN VIVO Ctnnb1 RNAi IMPAIRS HEPATOCYTE PROLIFERATION AFTER CDE DIET-INDUCED LIVER INJURY
To prove BECs were indeed giving rise to hepatocytes in our model, we sought to perform direct lineage tracing of BECs using tamoxifen-inducible Krt19-Cre to label BECs with the reporter tdTomato. However, the use of Cre recombinase to label BECs precluded the use of Cre recombinase to delete Ctnnb1 specifically in hepatocytes. To achieve labeling of BECs with a reporter and simultaneous knockdown of Ctnnb1 expression in hepatocytes, we used Ctnnb1-siRNA conjugated to hepatocyte-targeting ligand N-acetylgalactosamine.36 To validate that Ctnnb1-siRNA injection would recapitulate the phenotype of CDE diet–fed mice with genetic hepatocyte-specific Ctnnb1 deletion, we performed weekly subcutaneous injections of Ctnnb1-siRNA in mice fed CDE diet. The first injection was performed 1 week before CDE diet exposure to ensure reduced β-catenin levels in hepatocytes at the time of CDE diet administration (Fig. 6A).
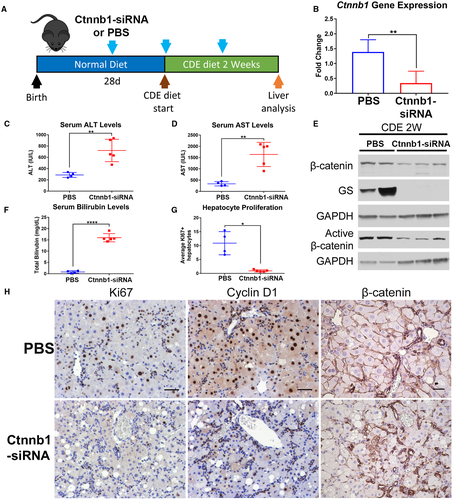
Real-time PCR analysis demonstrated significant reduction of hepatic Ctnnb1 gene expression in Ctnnb1-siRNA-injected mice compared with PBS-injected control mice on CDE diet (Fig. 6B). Ctnnb1-siRNA-injected mice fed CDE diet for 2 weeks displayed significantly elevated serum ALT (Fig. 6C), AST (Fig. 6D), and bilirubin levels (Fig. 6F) compared with control PBS-injected mice. We additionally confirmed reduction in both total and active β-catenin protein levels, as well as loss of expression of β-catenin downstream target GS, in Ctnnb1-siRNA-injected mice on CDE diet (Fig. 6E). Staining for Ki67 displayed robust hepatocyte proliferation in PBS-injected controls but not Ctnnb1-siRNA-injected mice on CDE diet (Fig. 6H), which was confirmed by quantification (Fig. 6G). There was also a notable decrease in Cyclin D1 staining in the livers of Ctnnb1-siRNA-injected mice on CDE diet (Fig. 6H). Finally, IHC for β-catenin confirmed loss of β-catenin expression specifically in hepatocytes of Ctnnb1-siRNA-injected mice (Fig. 6H). Collectively, these results demonstrate that Ctnnb1-siRNA-injected mice display increased liver injury and impairment of hepatocyte proliferation.
Becs Give Rise To Hepatocytes In Ctnnb1 Rnai-Treated Mice After CDE Diet-Induced Liver Injury
Having proved that injection of Ctnnb1-siRNA was sufficient to block hepatocyte proliferation in mice on CDE diet, we developed a mouse model of Krt19-CreERT+/--driven BEC lineage tracing17 in combination with hepatocyte-specific Ctnnb1 knockdown. To label BECs, tamoxifen was injected in Krt19-CreERT+/- mice during postnatal week 1, followed by a 2-week washout period to ensure elimination of any residual tamoxifen (Fig. 7A). Next, these mice were given weekly injections of Ctnnb1-siRNA throughout the course of CDE diet and recovery (Fig. 7A). In this model, BEC-derived hepatocytes can be directly traced because they will be tdTomato-positive (Fig. 7B). Prior to CDE diet administration, we verified that early postnatal tamoxifen administration labeled only BECs and not surrounding hepatocytes (Supporting Fig. S7A), implying that any tdTomato-positive hepatocytes would have to arise from a pre-existing tdTomato-positive BEC. We additionally determined the Krt19-CreERT+/- recombination efficiency in BECs in this model to be approximately 50% (Supporting Fig. S7B). After CDE diet and recovery, serum liver injury markers were approaching normal levels in both PBS and Ctnnb1-siRNA-injected mice (Supporting Fig. S7B-D). Excitingly, clusters of tdTomato-positive hepatocytes that were also Hnf4α positive (Fig. 7C) and CYP2D6 positive (Fig. 8A) were evident in the Ctnnb1-siRNA-injected mice but not in the mice injected with PBS as a control. Quantification revealed that approximately 6% of hepatocytes were tdTomato positive in Ctnnb1-siRNA-injected mice after CDE diet and recovery (Fig. 7D).
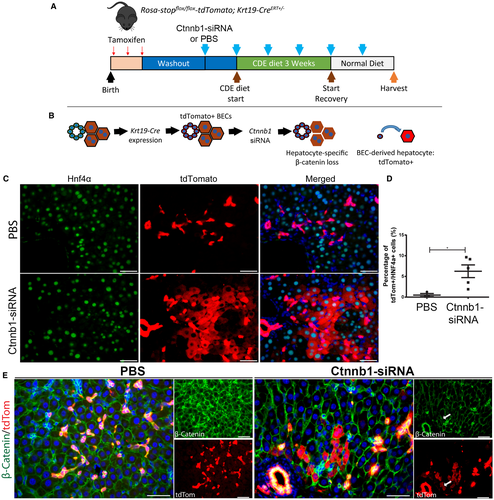
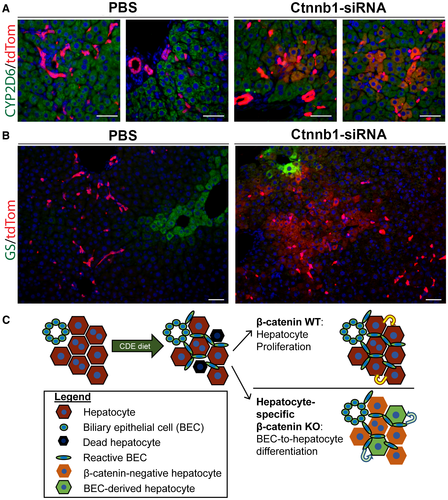
Immunofluorescence of β-catenin was strongly detected in the hepatocytes of PBS-injected mice but was significantly reduced in Ctnnb1-siRNA-injected mice (Fig. 7E). Importantly, tdTomato-positive hepatocytes were located adjacent to clusters of β-catenin-positive BECs in Ctnnb1-siRNA-injected mice. Finally, clusters of tdTomato-positive hepatocytes in Ctnnb1-siRNA-injected mice would occasionally extend all the way to the central vein, resulting in localized re-expression of β-catenin target GS (Fig. 8B). All together, these results demonstrate that BECs give rise to hepatocytes when hepatocyte proliferation is impaired, which was achieved in our model through the loss of β-catenin expression in hepatocytes in mice exposed to CDE diet–induced liver injury (Fig. 8C).
Discussion
The role of BECs in mediating LR has remained controversial, as several studies have reported a limited role for BEC differentiation to hepatocytes.23-25 Recent studies have demonstrated that widespread hepatocyte senescence,17 hepatocyte ablation,20 impaired hepatocyte proliferation,19 or prolonged exposure to severe liver injury37 can trigger differentiation of BECs to hepatocytes. Interestingly, a previous study reported ductular reaction and BEC-to-hepatocyte differentiation in aged Albumin-Cre β-catenin KO livers,38 suggesting expression of β-catenin in hepatocytes is important for long-term liver health. Here, we demonstrate that hepatocyte-specific loss of β-catenin expression in combination with CDE diet–induced liver injury triggers severe impairment of hepatocyte proliferation and leads to unequivocal repopulation of liver with BEC-derived hepatocytes.
Genetic loss of β-catenin in hepatocytes led to extensive injury in the CDE diet model with robust expansion of BECs in both male and female mice (data not shown). Further, knockdown of Ctnnb1 expression in hepatocytes via siRNA also impaired hepatocyte proliferation after CDE diet. Intriguingly, BEC expansion occurred normally despite β-catenin loss in these cells in Albumin-Cre Ctnnb1 KO mice on CDE diet. However, the appearance of active-β-catenin in KO2 mice, which retained β-catenin expression in BECs, suggests activation of β-catenin signaling in this compartment during CDE diet. This observation is in accordance with previous findings that activation of β-catenin in reactive BECs promotes differentiation toward the hepatocyte lineage.39 In our model, appearance of BEC-derived hepatocytes was evident as early as 3 days of recovery on basal diet after 2 weeks of CDE diet, when cells positive for both BEC and hepatocyte markers could be observed. Excitingly, Hnf4α+/CK19+ cells have also been observed in a recent study demonstrating BEC-derived hepatocytes during long-term severe liver injury.37 Our results suggest that differentiation of BECs to hepatocytes is limited or impaired during ongoing injury and that a period of recovery is necessary to allow BEC differentiation to hepatocytes, as also shown elsewhere.17, 19, 33
Greater than 99% of hepatocytes were labeled with EYFP after AAV8-TBG-Cre injection, and after 6 months of normal diet, the numbers of EYFP-negative hepatocytes in both WT2 and KO2 mice were not significantly increased. Additionally, over 99% of hepatocytes were still EYFP positive in both WT2 and KO2 mice after 2 weeks of CDE diet, suggesting no significant population of hepatocytes escaping initial Cre-recombination, as these cells would express β-catenin and would be expected to have a proliferative advantage during CDE diet–induced liver injury. In this negative tracing model, we found that after recovery, approximately 20% of periportal hepatocytes were BEC derived. Interestingly, in our positive lineage tracing model, we found only 6% of hepatocytes were BEC derived. This is attributable to the fact that initially only around 50% of BECs were labeled with tdTomato in this model, suggesting potentially 12% of hepatocytes were BEC derived in our model after factoring in the limited recombination efficiency. Furthermore, newly BEC-derived hepatocytes may be susceptible to siRNA-mediated Ctnnb1 knockdown, potentially reducing their proliferation during recovery from CDE diet, further reducing tdTomato-positive hepatocyte numbers.
Although our data convincingly prove the differentiation and long-term survival of BEC-derived hepatocytes, much remains to be elucidated about both the long-term effects of BEC-derived hepatocytes on liver health and the mechanisms underlying BEC-to-hepatocyte differentiation. For instance, it will be interesting to determine if BEC-derived hepatocytes are equally as adept at promoting resolution of liver injury, such as resolution of fibrosis. Additionally, although factors that promote ductular reaction are better known,40 our knowledge of the factors promoting BEC differentiation to hepatocytes is much more limited. Because our model establishes a clear timeline for BEC differentiation to hepatocytes, it will be invaluable in determining the factors that underlie this process. For example, as we have demonstrated the importance of β-catenin signaling in LR, this would potentially allow promotion of BEC-to-hepatocyte differentiation while circumventing activation of oncogenic β-catenin signaling in patients. Additionally, it will be important to determine the threshold of hepatic impairment that triggers BEC-to-hepatocyte differentiation, as this may lead to the development of biomarkers for patients with severe liver disease that are amenable to such treatments. These would be important steps in developing regenerative medicine therapies for the treatment of chronic liver disease.
Potential conflict of interest
Dr. Abrams owns stock in Dicerna Pharmaceuticals.




