Old Remedies to Heal the Liver: Novel Effects of Digoxin in Hepatic Sterile Inflammation
Abbreviations
-
- ASH
-
- alcoholic steatohepatitis
-
- HIF
-
- hypoxia inducible factor
-
- HIF-1α
-
- hypoxia inducible factor 1 alpha
-
- IL
-
- interleukin
-
- NASH
-
- nonalcoholic steatohepatitis
-
- PKM2
-
- pyruvate kinase M2
The hepatic inflammatory response is one of the main driving forces of disease progression in chronic liver diseases—primarily by promoting a sustained hepatic fibrogenesis, which can lead to cirrhosis.1 Thus, reducing persistent inflammatory activity over time is an important goal of therapy, as it may abrogate disease progression. Important advances have been made over the past decade in the understanding of the molecular pathways and cellular players involved in liver inflammation. In particular, a more detailed knowledge of the underlying mechanisms of initiation and perpetuation of sterile inflammation have shed light on the pathophysiology of both alcoholic steatohepatitis (ASH) and nonalcoholic steatohepatitis (NASH),2 which are by far the most common causes of liver disease in industrialized countries, and are major causes of morbidity and mortality worldwide.3
Both NASH and ASH share common pathophysiological processes, with sterile inflammation being a key phenomenon.2 However, in spite of advances, many aspects of the pathophysiology of steatohepatitis and its relationship with sterile inflammation remain a mystery.4 The sterile inflammatory response in the liver is a complex process, which involves multiple cell types and signaling pathways. Resident and incoming macrophages are the main players in the initiation and propagation of inflammation, but hepatocytes, sinusoidal endothelial and hepatic stellate, and cells also have key roles. In addition, increased production of pro-inflammatory cytokines such as interleukin (IL)-1β and IL-18 contribute to the inflammatory process. Notably, production of these cytokines is activated by intracellular multiprotein complexes collectively termed “inflammasomes,” which are able to sense damage-associated molecular patterns released by injured hepatocytes or pathogen-associated molecular patterns, which can originate from the gut in a setting of impaired intestinal permeability.5 The most studied inflammasome is the nucleotide-binding oligomerization domain–like receptor 3, which appears to play a relevant role in both NASH and ASH.5 Of note, it is increasingly being appreciated that an intimate and complex crosstalk between inflammatory and metabolic pathways exists. On the one hand, activation of immune cells determines marked metabolic changes (e.g., alterations in insulin signaling, macrophage reprogramming with switching from oxidative phosphorylation to aerobic glycolysis); on the other hand, some nutrients, metabolites, or cell products (e.g., palmitic acid, mitochondrial DNA) are able to directly activate the inflammatory program. This molecular crosstalk shapes liver damage and drives disease progression in NASH and likely also in ASH.6
Other important players in the regulation of the hepatic inflammatory response are members of the hypoxia inducible factor (HIF) family of proteins, in which hypoxia inducible factor 1 alpha (HIF-1α) is the well-studied member.7 HIF-1α is a transcription factor that regulates more than 800 genes, including heme oxygenase-1, vascular endothelial growth factor, glucose transporters and glycolytic enzymes, which are critical for the adaptive cellular responses to acute injury. However, chronic activation of HIF-1α may result in pathological effects in the liver. Of note, activation of HIF-1α-related pathways has been reported to occur in several models of acute and chronic liver disease as well as in liver samples from ASH and NASH.7 Although this may be regarded as a cellular adaptation to changes in oxygen tension in the inflamed hepatic microenvironment, pro-inflammatory cytokines can also activate HIF-1α in an oxygen-independent manner. Thus, it has been suggested that HIF-1α inhibition could prevent and eventually ameliorate chronic liver disease.
Several molecules have been identified and evaluated to modify sterile inflammation in steatohepatitis. A recent study by Ouyang et al. in Cell Metabolism8 showed than the old cardiotonic digoxin protects from liver inflammation and damage in the setting of experimental liver damage. The authors convincingly show that digoxin reduces the severity of steatosis, inflammation, and hepatocellular damage in different experimental settings including acute (LPS/D-galactosamine and thioacetamide model) and chronic (high-fat diet [HFD] and Lieber-Decarli ethanol liquid diet plus single ethanol binge mouse model) models of sterile inflammatory injury. Interestingly, in the HFD NASH model, the authors demonstrated prevention and attenuation of liver damage by digoxin. Digoxin improved oxidative stress in these models through maintaining cellular redox homeostasis. Of note, digoxin suppresses reactive oxygen species production from hepatocytes and immune cells in both in vivo and in vitro settings. Additionally, the authors explored pathways modified by digoxin using microarrays of liver tissues and found that HIF-1α-related pathways were downregulated by digoxin and further confirmed that digoxin suppresses HIF-1α activation and downstream signature genes in the inflamed liver as well as in isolated immune cells. Furthermore, the authors demonstrated that digoxin modulates sterile inflammation by suppression of pyruvate kinase M2 (PKM2)-promoted HIF-1α transcription. PKM2 is a key metabolic regulator present predominantly in activated myeloid cells. Dimeric PKM2 can translocate to the nucleus, where it will interact with HIF-1α and regulate expression of numerous pro-glycolytic enzymes, which are a critical determinant of the Warburg effect consisting of increased anaerobic glucose use by cells under high energy and biosynthetic demands. In addition to regulating HIF-1α, PKM2 may also boost IL-1β induction, thus playing a role in amplification of inflammatory phenomena. Using proteomic screening, Ouyang et al. showed that digoxin binds PKM2 and reduces binding of histones to PKM2, resulting in chromatin remodeling and downregulation of HIF-1α transactivation. Interestingly, these effects are independent of PKM2 kinase activity and are achieved at doses below those required for a cardiac effect. Together, these data identify PKM2 as a mediator of liver sterile inflammation and provide molecular insights for the observed effects of digoxin in attenuating liver injury from ASH and NASH. However, additional mechanisms may be at play, as recently shown by Wang et al.,9 who demonstrated that digoxin may also promote autophagolysosomal activity through activation of the transcription factor EB, which results in amelioration of metabolic syndrome in mice.
The study by Ouyang et al. has several notable strengths. It gives both clinically relevant and mechanistic data regarding the effect of cardiac glycosides in sterile inflammation secondary to multiple models of liver injury. In addition, the identification of potential therapeutic targets in a disease that lacks effective pharmacologic treatment is paramount. Furthermore, the identification of an approved drug that can inhibit this pathway could facilitate a rapid transition to the clinic. However, more studies are needed in humans to assess the long-term effect and clinically relevant outcomes.
Digoxin is one of the oldest drugs used today; it increases ionotropism and decreases chronotropism through partial inhibition of the Na+/K+ ATPase on the plasma membrane of myocytes. Additionally, digoxin has been shown to exercise a myriad of other functions including inhibition of HIF-1α pathway activation. These and other noncardiac effects of digoxin have led to the suggestion of potentially new therapeutic roles for digoxin and other cardiac glycosides in various diseases. Indeed, its potential toxicity has limited its clinical use. Recent studies assessing the effects of new digoxin formulations (i.e., liver-tropic biodegradable, biocompatible nanoparticles) have also shown hepatoprotective effects in models of diet-induced obesity9 and open the possibility for liver-directed therapy, thus avoiding digoxin's adverse effects (Fig. 1).
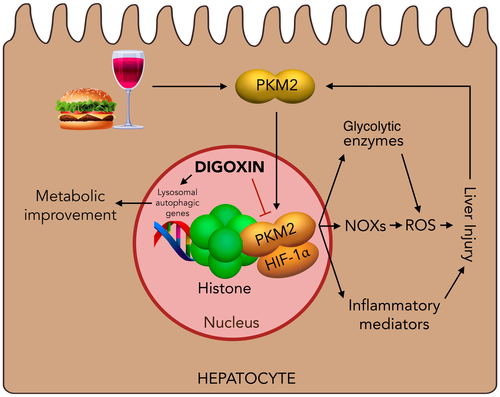
In summary, Ouyang et al. have elegantly demonstrated a role of PKM2 in hepatic sterile inflammation through promotion of HIF-1α transactivation and how digoxin can exercise hepatoprotective actions though interference of this pathway. Indeed, further study of the effects of digoxin in NASH and ASH is warranted.
Potential conflict of interest
Nothing to report.
Acknowledgment
This work was partially supported by grants from the Fondo Nacional de Ciencia y Tecnología de Chile (FONDECYT grant 1150327 to M.A.); grant PIA/Basal PFB12 from the Comisión Nacional de Investigación, Ciencia y Tecnología; and by an AASLD/LIFER from the AASLD Foundation (to J.P.A.).




