AKTions by Cytoplasmic lncRNA CASC9 Promote Hepatocellular Carcinoma Survival
Funding
Supported by the National Institute on Aging Intramural Research Program, National Institutes of Health.
See Article on page 1817
Abbreviations
-
- CASC9
-
- cancer susceptibility candidate 9
-
- HCC
-
- hepatocellular carcinoma
-
- and lncRNA
-
- long noncoding RNA
Hepatocellular carcinoma (HCC) is the most common form of liver cancer and is the fifth most common type of cancer worldwide. Given that hepatocellular carcinogenesis arises through complex changes in genetic, epigenetic, and signaling programs, HCCs exhibit widely heterogeneous molecular signatures.1 Despite this diversity, HCC cells share robust cell survival and proliferative phenotypes, and hence these traits have been targeted in preclinical and early clinical studies.2
Noncoding RNAs (ncRNAs) comprise most (> 95%) transcripts encoded by the mammalian genome. They play key regulatory roles in physiologic processes, such as cell division, differentiation, and stress and immune responses, and have also been implicated in many diseases including cancer. Among the vast class of ncRNAs, several long noncoding RNAs (lncRNAs, defined as being longer than 200 nucleotides) with tumor suppressive or oncogenic function in solid tumors have been identified over the past decade.3 For example, lncRNAs HOTAIR, MALAT1, H19, MEG3, GAS5, and XIST have been found to be dysregulated in a wide range of malignancies.3 Evidence has also begun to emerge that lncRNAs are capable of regulating different aspects of HCC, such as cell proliferation, apoptosis, epithelial-to-mesenchymal transition, invasion, and metastasis.4 However, little is known about the mechanisms in which lncRNAs regulate carcinogenesis in HCC. Even less is known about the molecular partners, such as the RNA-binding proteins and the microRNAs, which modulate the function of HCC lncRNAs.
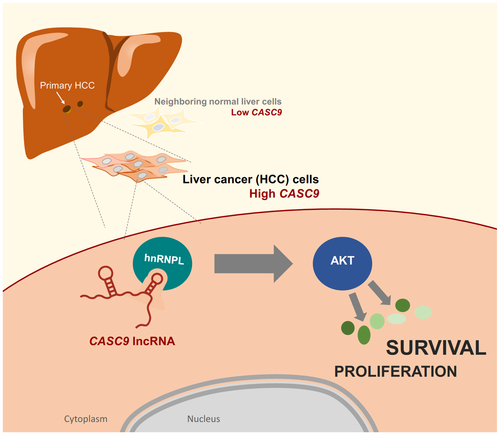
In this issue of Hepatology, Klingenberg et al.5 report a comprehensive collection of viability-associated lncRNAs in HCC. The authors identified and studied in molecular detail a prominent cytoplasmic lncRNA, CASC9 (cancer susceptibility candidate 9), which was preferentially abundant in HCC compared with normal liver. CASC9 had recently been shown to promote tumorigenesis in the esophagus, stomach, lung, and nasopharynx; in keeping with these findings, CASC9 was also significantly induced in HCCs and correlated inversely with patient survival.5 Moreover, a series of loss-of-function studies revealed that higher expression of CASC9 was strongly associated with major phenotypes observed in liver tumorigenesis, such as excessive proliferation and loss of susceptibility to apoptosis. However, in contrast to earlier studies reporting that CASC9 was primarily nuclear in other models of carcinogenesis, Klingenberg et al.5 found that CASC9 was largely cytoplasmic in HCC. Interestingly, quantitative proteomic analysis using SILAC (stable isotope labeling by amino acids in cell culture) revealed that a major role of CASC9 was to activate the cytoplasmic kinase AKT, as well as the AKT-related network of signaling molecules, including upstream and downstream AKT regulators (e.g., PI3K, mTOR, PTEN, PDK1/2). This striking observation was particularly interesting in the context of HCC, as these signaling pathways were frequently altered in HCC oncogenic profiles that correlated with poor overall survival.6, 7
Further biochemical analyses revealed that CASC9 associated with the RNA-binding protein HNRNPL to form a functional lncRNP (long noncoding ribonucleoprotein) complex in the cytoplasm. The specific mechanism in which AKT is phosphorylated (and hence activated) by CASC9-HNRNPL was not elucidated in biochemical detail in this investigation. Nonetheless, CASC9 constitutes an exciting addition to a small but growing class of cytoplasmic lncRNAs capable of orchestrating the activity of major signaling pathways. Other examples include lncRNAs LINK-A, Lnc-DC, NKILA, and Lethe, which were found to be modulated by lncRNAs capable of altering the phosphorylation or abundance of certain signaling proteins such as BRK, STAT3, NF-κB, and LRRK2.8 In this context, the activation of AKT by CASC9-HNRNPL represents the first example of a cytoplasmic lncRNP affecting HCC signaling.
Finally, in addition to the novel molecular features of CASC9-HNRNPL, Klingenberg et al.5 highlight several attractive aspects of CASC9 as a therapeutic target in HCC. First, CASC9 enhances HCC cell survival and proliferation by activating AKT, a major kinase “hub” through which many cell survival cascades are realized. Thus, interventions to lower CASC9 to inactivate AKT would be expected to dampen cell survival and mitogenic pathways widely and robustly. The impact of CASC9 on HCC cell survival and proliferation was reflected in the broad spectrum of mRNAs and proteins differentially expressed when CASC9 was silenced.5 The diverse gene expression programs affected by CASC9 abundance likely reflected the fact that the downstream targets of AKT, both direct and indirect, included many transcription factors (e.g., FOXO, NFκB, TP53, HIF1A) and translational regulators (e.g., TSC1/2, mTOR). Second, devising interventions to reduce the levels of a lncRNA (in this case CASC9) are expected to be more successful than interventions to increase the levels of a regulatory RNA in cells; the former tend to be straightforward, even if reductions are incomplete, whereas for the latter, delivery methods and control of magnitude/timing of expression can be problematic. Third, lncRNAs residing in the cytoplasm, as is the case for CASC9 in HCC, are more easily targeted for suppression by antisense oligonucleotides or RNA-silencing technologies than lncRNAs residing in protected compartments (e.g., nuclei or mitochondria).
In summary, the HCC lncRNA CASC9 was found to have a novel molecular partner, HNRNPL, reside in the cytoplasm, and promote cell survival and proliferation by enhancing AKT function (Fig. 1). Accordingly, therapies aimed at reducing CASC9 levels in HCC warrant thorough consideration.
Potential conflict of interest
Nothing to report.
Acknowledgment
This work was supported in its entirety by the National Institute on Aging Intramural Research Program, National Institutes of Health.




