Phenomenon of Endothelial Antibody Capture: Principles and Potential for Locoregional Targeting of Hepatic Tumors
See Editorial on page 1672.
Supported by Deutsche Krebshilfe (70111718).
Abstract
The systemic drug circulation represents a source of adverse effects during tumor targeting. We studied the binding efficacy of endothelium-specific antibodies after a very short contact with an antigen target, along with assessing the intravascular capture and targeting potential of these antibodies after locoregional injection. Fast-binding anti–CD 146 (clone ME-9F1) and anti-CD31 (clone 390) antibodies were selected based on histological analysis of their binding activity. The efficacy of antibody capture by hepatic endothelium under different conditions was analyzed using an isolated liver perfusion model. The local enrichment of R-phycoerythrin and 125I-conjugated antibody was studied in vivo in two hepatic tumor models using biodistribution, scintigraphic imaging, and fluorescence microscopy. Upon injection into the tumor-feeding artery, the antibody was immediately captured in the microvasculature during the first passage. At doses not exceeding the saturation level of endothelial epitopes, the capture efficacy was almost 90%. We showed that the efficacy of endothelial capture is controlled by factors such as antibody affinity, number of binding sites on the endothelium, and microvascular flow rate. The targeting potential of endothelial capture was experimentally proven in vivo using scintigraphic imaging and biodistribution analysis after locoregional intra-arterial injection of 125I-labeled antibodies in hepatic tumor models. Conclusion: The unique phenomenon of endothelial capture can broadly prevent systemic circulation of the antibody or antibody–drug conjugates applied by intravascular injection and may have specific relevance for targeting of hepatic tumors.
Traditionally, antibodies and their fragments are the prime examples of molecular targeting agents.1 Antibody–drug conjugates have greatly extended the targeting capacity of antibodies for clinical application.2 To facilitate transport to tumor sites, targeting monoclonal antibodies (mAbs) circulate in the blood. Because their penetration rate into the tissue is low,3 they persist in the bloodstream for several days4 and cause adverse effects of varying severity.5
In contrast to tumor cells, tumor-associated endothelial cells are directly accessible to circulating drugs. Therefore, the tumor vasculature is considered a direct target or attractive agent for drug delivery.6, 7 Among different vascular strategies, only vascular endothelium growth factor targeting with bevacizumab or ramucirumab has become an approved tool for the treatment of some cancer types. However, these drugs confer only a moderate survival advantage.8, 9
Locoregional application of the targeting agent into the tumor-feeding artery increases the local intravascular concentration of this drug during the first intravascular passage, which enhances local intratumoral concentration and improves chemotherapy and radiotherapy outcomes.10, 11 However, any existing tool including hepatic arterial infusion12 cannot get around the step of systemic drug circulation, leading to adverse effects and limiting the therapeutic concentration of targeting drugs.5 The only locoregional methods, such as transarterial chemoembolization13 and selective internal radiotherapy,14 use microparticles which embolize blood vessels and can prevent circulation of targeting drugs.
In the present study, we analyze the binding efficacy of endothelium-specific antibodies after a very short contact time with antigen and the translational potential of these antibodies using a single intravascular passage.
Materials and Methods
ISOLATED MOUSE LIVER PERFUSION
Intrahepatic infusions in C57BL/6 mice (Charles River Laboratories, Sulzfeld, Germany) were performed using saline or modified Krebs-Henseleit buffer (Sigma-Aldrich, Deisenhofen, Germany: 118 mM sodium chloride, 4.7 mM potassium chloride, 1.2 mM magnesium sulfate, 1.2 mM monopotassium phosphate, 11.1 mM D-glucose) supplemented with 1% bovine serum albumin (Roth, Karlsruhe, Germany) and 25 mM sodium bicarbonate (Grüssing, Filsum, Germany). After euthanasia, the abdominal cavity was opened. A small catheter (0.6 mm) was inserted into the portal vein and connected to a syringe infusion pump (WPI, Sarasota, FL). The blood was removed from the liver by an infusion of 1 mL of saline or modified Krebs-Henseleit buffer at 1 mL/minute. The liver was subsequently removed from the abdomen using microsurgery and placed into a small Teflon chamber. The perfusion was started with 100 μL of antibody solution of the respective concentration, followed by perfusion with 0.9 mL of saline or modified Krebs-Henseleit buffer at the flow rate of choice to wash the unbound antibody from the liver. All unconjugated and R-phycoerythrin (RPE)–conjugated mAb clones were purchased from Biolegend (San Diego, CA). The solution was collected and used for determination of antibody concentration using fluorimetry (FluoStar Optima; BMG Labtech, Ortenberg, Germany) or enzyme-linked immunosorbent assay (ELISA) (rat IgG ELISA; Thermo Fisher Scientific, Waltham, MA). To prevent significant decrease of fluorescent intensity of saline-dissolved antibody, the antibody concentration was measured within 45 minutes after sample preparation. For “whole-mount” microscopy after perfusion with RPE-conjugated antibodies, the liver was placed on a coverslip and endothelium-bound fluorescent antibody was visualized using fluorescence microscopy (Axio Observer.Z1; Zeiss, Jena, Germany) equipped with a monochromatic light source (Colibri modules 470 nm and 555 nm). All images were collected and processed using ZEN software (ZEN 2011; Zeiss). The intraportal pressure during perfusion with different flow rates was measured using a system which includes a pressure monitoring set (Edwards Lifesciences, Irvine, CA) and a self-built electronic device with a customized assessment and analysis software.
In some experiments, the liver was perfused using intermittent “perfusion–subsequent antibody collection” cycles with a 0.2 mL/minute flow rate. In other experiments, different buffer volumes from 10 μL to 16 mL containing the same antibody amount (2 μg) were perfused with a 1 mL/minute flow rate.
IMAGE-BASED DESCRIPTIVE AND QUANTITATIVE ANALYSIS OF IMMUNOFLUORESCENCE STAINING
The following RPE-conjugated antibodies were used: anti–CD309 (clone 89B3A5), anti-CD144 (clone BV13), anti-CD105 (clone MJ7/18), anti-CD31 (clone 390), anti-CD146 (clone ME-9F1), and anti-CD34 (clones RAM34, MEC14.7) (all mAbs from Biolegend). Tissue slides (7 μm thickness, frozen sections) were stained by one-step direct immunofluorescence.
For image-based descriptive analysis, mouse tumor or liver tissue was stained for 15 minutes or 5 seconds, respectively, with 2 μg/mL RPE-conjugated antibody. Endothelium-bound fluorescent antibody was then visualized as described above.
Image-based quantitative analysis of immunofluorescence used for determination of half-maximal effective concentration (EC50) values was performed by staining 12 slides of the respective tissue with an appropriate series of antibody dilutions for 15 minutes or 5 seconds. Endothelium-bound fluorescent antibody was then visualized and imaged as described above. Each high-resolution immunofluorescent image (11.3 mm2) contained at least 100 antibody-labeled blood vessels, which were marked using the ZEN software. Each value was corrected for background and expressed as mean fluorescence intensity. For the calculation of EC50, the mean fluorescence intensity values were further analyzed with the customized SCTMult software (version 1.3.0.1; W.G.). The analysis for each incubation time and tissue was repeated thrice for clones ME-9F1, 390, and MJ7/18 and twice for clones 89B3A5 and BV13.
ENDOTHELIAL CELL ISOLATION AND ANTIBODY BINDING
Hepatic endothelial cells were isolated from the liver of C57BL/6 mice (Charles River; 10-14 weeks) by collagenase digestion and magnetic separation (Miltenyi Biotec, Bergisch Gladbach, Germany), as described.15
For maximum antibody binding studies, isolated cells were incubated with an excessive amount (2 μg/mL) of RPE-labeled antibodies for 30 minutes at 37 °C, washed using cold phosphate-buffered saline, and analyzed using fluorimetry (FluoStar Optima). The amount of bound antibody per single cell was calculated. For each investigated antibody the experiment was repeated 1-3 times.
For antibody uptake and release studies, isolated cells were cultured in a monolayer in IV-μ ibidi chambers (Ibidi, Martinsried, Germany) with MCDB-131 medium (CCPro, Oberdorla, Germany) supplemented with 10% fetal calf serum (CCPro) for 4-5 days. The cells were then incubated for 15 minutes at 37°C with RPE-conjugated ME-9F1 mAb (2 ng/mL) and Alexa Fluor 488-conjugated 390 mAb (5 ng/mL; both from Biolegend). After the incubation, the antibody solution was removed and, after washing with phosphate-buffered saline, replaced with medium. Finally, antibody uptake and retention by isolated cells were analyzed using image-based fluorimetry and fluorescence microscopy (Zeiss) as described above. Selected time points for imaging were 0, 2, 4, and 24 hours.
INJECTIONS IN VIVO, REAL-TIME MICROSCOPY, AND SCINTIGRAPHIC IMAGING
Animal experiments were performed in accordance with international rules and approved by the local committee for animal care (Regierungspräsidium Karlsruhe). Mice (C57BL/6; Charles River) were kept in standard cages under a 12-hour dark–light cycle with ad libitum access to water and standard animal food. Pancreatic cancer (Panc02)16 and hepatocellular cancer (Hep55.1C)17 were produced in male mice (10-14 weeks). For this aim, animals were anesthetized by isofluran inhalation. Tumor cells (1 to 1.5 × 106) resuspended in 5-10 μL of phosphate-buffered saline were injected into defined liver segments (left anterior and posterior segments or right middle segment)18 using a 20-μL syringe (Hamilton, Bonaduz, Switzerland). Experiments were performed 12-14 days after inoculation. The animals were anesthetized with intraperitoneal injections of 40 mg/kg ketamine S (Pfizer, Berlin, Germany) and 10 mg/kg xylazine (Bayer, Leverkusen, Germany).
For the speed measurement of tumor blood vessel filling, 400 μg of fluorescein-labeled albumin were intravenously injected in a Panc02 mouse tumor model in vivo. For the descriptive analysis and quantification of fluorescein-albumin in the tumor vessels, real-time microscopy was applied (Zeiss) for 120 seconds.
In order to access the tumor-feeding artery for locoregional intra-arterial antibody injections, all branches of the hepatic artery were ligated except the one feeding the tumor-bearing liver, usually the left anterior and posterior or right middle segment. Depending on two major variants of local vascular anatomy in C57BL/6 mice, a 34G needle (Hamilton) was inserted into the superior pancreaticoduodenal or the gastric artery, moved forward into the hepatic artery, and used for injection of RPE-conjugated or 125I-conjugated antibody. For fluorescence microscopy 200 ng/mouse of mAb in 5 μL was injected; 1 ng/g body weight or 5 ng/g body weight in 5 or 10 μL was applied for scintigraphy in Panc02 and Hep55.1C tumor models, respectively, for ME-9F1 mAb and in the Panc02 tumor model for isotype mAb. Microscopic or scintigraphic analyses were performed 10-15 minutes after injection. Fluorescence “whole-mount” microscopy was performed as described above.
For scintigraphy, ME-9F1 mAb was conjugated with 125I. Radiolabeling was achieved using the chloramine-T method as described,19 and the protein was purified on a size exclusion chromatography cartridge (BioRad, Hercules, CA). The animal or the dissected tissue was placed on a gamma imager (Biospace Lab, Paris, France) equipped with a high-energy collimator, and images were recorded over 10 minutes. Each experiment was reproduced 2-3 times. For biodistribution analysis, tissues were weighed and analyzed using a gamma-counter (Berthold Technologies, Bad Wildbad, Germany). The local delivery of 125I-conjugated antibody was finally expressed as a percentage of total injected dose per gram of tissue weight.
STATISTICAL ANALYSIS
Statistical analysis was performed with SPSS software (IBM, New York, NY). Data are shown as mean ± standard deviation. To study differences between the groups, a t test was used. P < 0.05 was considered significant.
Results
SELECTED ENDOTHELIUM-SPECIFIC ANTIBODY CLONES BIND EFFECTIVELY IN HISTOLOGICAL SLIDES OF TUMOR AND NORMAL LIVER TISSUE USING VERY SHORT (5-SECOND) INCUBATION TIME
The minimal injection time required for filling of all microvascular blood vessels after systemic injection was determined as the minimal time of first intravascular passage. This time was studied using intravital microscopy and systemic injection of fluorescein-labeled albumin in a hepatic mouse tumor model. Almost all microvascular tumor blood vessels were filled within 5-10 seconds (Fig. 1A,B).
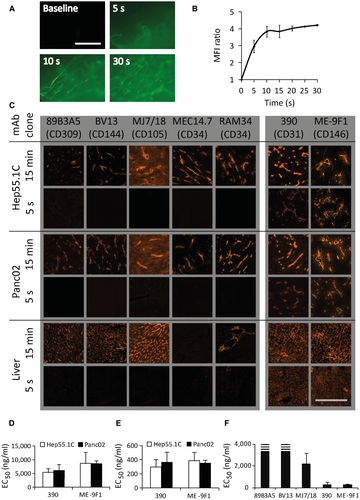
Next, to analyze antibody binding in both normal and malignant tissues in this short period, we compared the binding capability of several endothelium-specific antibodies on histological slides of mouse pancreatic and liver cancer as well as samples of normal liver tissue. All mAb clones stained endothelium on histological slides using an incubation time of 15 minutes at a concentration of 2 μg/mL (Fig. 1C). Only clones ME-9F1 and 390 showed detectable labeling after incubation for only 5 seconds (Fig. 1C). To quantify their binding capacity, we measured the EC50 using image-based fluorimetry of immunofluorescence staining (Fig. 1D-F). The EC50 values for the two clones (RPE-labeled ME-9F1 and 390 mAbs) ranged from 270 to 387 ng/mL after 15 minutes of incubation, whereas EC50 values for the other clones were much higher or even exceeded the detectable level (Fig. 1D-F). The EC50 of the same antibody clone was not significantly different between different tissue types (pancreatic cancer, hepatic cancer, liver).
SELECTED ANTIBODY CLONES CAN BE ALMOST COMPLETELY CAPTURED BY ENDOTHELIUM DURING THE FIRST INTRAVASCULAR PASSAGE
The capture of several endothelium-specific mAb clones during the first intravascular passage was quantitatively analyzed in an isolated mouse liver model. For most experiments, the standard flow rate of 1 mL/minute, which corresponds to a physiological intraportal pressure of 10 mm Hg, was used (Supporting Table S1). If the liver was perfused with an antibody dose (12.8 μg/liver) corresponding to the conventional clinical dosage (approximately 8 mg/kg), antibodies showed a detectable capture during the intravascular passage. Depending on the mAb clone (Fig. 2A), the extraction efficiency varied from 4% to 18%. Unexpectedly, if the liver was perfused with a low antibody dose (0.2 μg/liver, approximately 0.125 mg/kg), 80%-90% of the total amount of antibody was captured during the single intravascular passage (Fig. 2A).
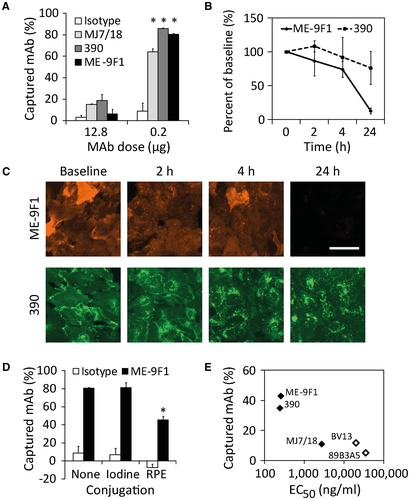
An analysis of maximal antibody-binding capacity of isolated hepatic endothelial cells showed comparable results for different mAb clones, which varied from 1.9 to 2.9 fg/cell (Supporting Fig. S1). After cellular uptake, the fluorescence signal decreased continuously, with half-life times varying from 4 to 24 hours, depending on the mAb clone (Fig. 2B,C).
ANTIBODY LABELING/MODIFICATION CAN REDUCE THE EFFICACY OF ENDOTHELIAL CAPTURE
To verify the vascular distribution of the captured antibody, isolated livers were perfused with antibodies conjugated with RPE or a radioactive isotope of iodine (125I). As described,20 protein modification can change affinity, resulting in altered binding properties. In the present study, no capture of isotype mAb was detected. Moreover, the intensity of the RPE signal was even increased after perfusion, which resulted in slightly negative values of calculated endothelial capture of isotype mAb (Fig. 2D). For ME-9F1 mAb, both RPE and 125I conjugates showed a high efficacy of endothelial capture, although conjugation with the 250-kDa protein (RPE), but not with the small isotope (iodine), significantly reduced endothelial capture efficacy (Fig. 2D). Of interest, the EC50 of antibody binding correlated well with the capture efficacy results in the isolated liver perfusion model (Fig. 2E). In subsequent experiments, RPE-labeled antibodies were used for fluorimetric analyses and microscopic imaging. 125I-labeled antibodies were used for scintigraphic imaging and biodistribution studies.
EFFICACY OF ENDOTHELIAL CAPTURE IS DEPENDENT ON THE FLOW VELOCITY BUT NOT ON THE ANTIBODY DOSE
Several variables are potentially important for endothelial capture efficacy, including flow velocity, local antibody concentration, and the duration of injection. Using RPE-labeled antibodies, we studied the influence of these variables on endothelial capture and found that capture of three different antibodies depended markedly on flow velocity: lower flow rate was consistently accompanied by higher endothelial capture efficacy (Fig. 3A). Increasing exposure time to endothelial antigens using flow reduction from 1 to 0.5 mL/minute significantly increased the endothelial capture efficacy (P < 0.05). We found no significant increase in endothelial capture after further flow reduction from 0.5 to 0.2 mL/minute (P > 0.05), despite an apparent tendency (Fig. 3A). Thus, the initially observed endothelial capture reduction caused by antibody RPE labeling (Fig. 2D) was compensated.
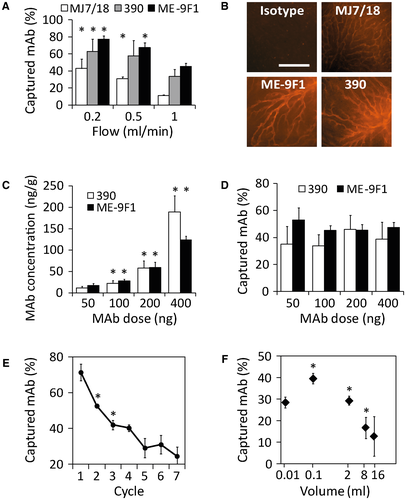
With the highest perfusion rate (1 and 0.5 mL/minute), qualitative whole-mount microscopy after isolated liver perfusion experiments showed homogeneous labeling of microvascular blood vessels. At 0.2 mL/minute, labeling of blood vessels was less homogeneous (Fig. 3B).
Liver perfusion with increasing antibody doses resulted in a significant (P < 0.05) increase in antibody concentration in the tissue (Fig. 3C). Surprisingly, the efficacy of endothelial capture did not significantly change (Fig. 3D).
REPEATED INJECTIONS OR LONG DURATION OF ANTIBODY INJECTION REDUCE EFFICACY OF ENDOTHELIAL CAPTURE
In subsequent experiments, we performed intermittent isolated liver perfusion with 100 ng of RPE-labeled ME-9F1 mAb (0.2 mL/minute). Measures of the efficacy of endothelial capture after every perfusion cycle showed progressive reduction (Fig. 3E). We identified another interesting feature in further experiments in which the isolated liver was perfused with 2 μg of RPE-conjugated ME-9F1 or isotype mAb diluted in different volumes from 10 μL to 16 mL (Supporting Table S1). The highest antibody capture was achieved after an injection of 100 μL, whereas long-duration antibody infusion showed a significant gradual decrease of antibody capture (Fig. 3F).
ENDOTHELIAL CAPTURE ENRICHES LABELED ANTIBODY IN TUMOR/PERITUMORAL TISSUE IN VIVO
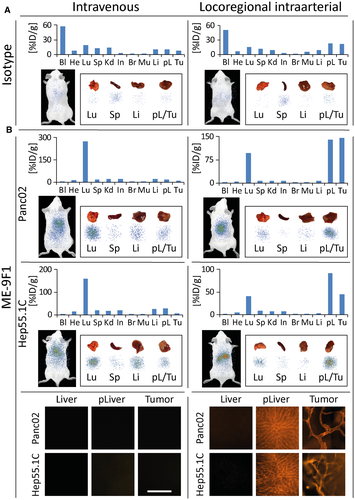
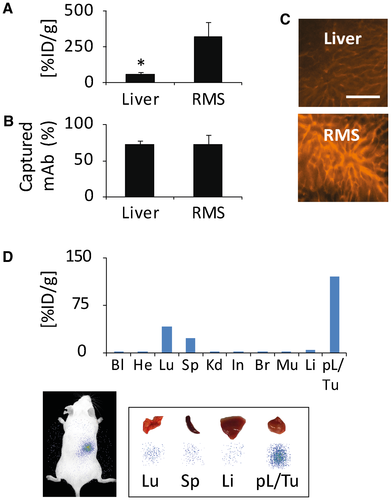
To analyze the selective enrichment of antibody in the tumor, we injected low doses of RPE-conjugated and 125I-conjugated ME-9F1 or isotype mAb either intravenously or locoregionally into the artery feeding tumor-bearing liver segments (left anterior and posterior segments; Supporting Table S2). After injections of isotype mAb, its highest concentration was found in the blood circulation (Fig. 4A). Although the locoregional intra-arterial injection of isotype mAb resulted in a 2.9-fold increase of antibody enrichment, the tissue mAb concentration remained very low (Fig. 4A). In contrast to isotype mAb, however, ME-9F1 mAb was completely extracted from the circulation, and only trace concentrations were detected in the blood after both intravenous and locoregional intra-arterial injection (Fig. 4B). Intravenous application of ME-9F1 mAb led to preferential antibody capture in the lung, whereas the intrahepatic and intratumoral enrichment was very low (Fig. 4B). After locoregional intra-arterial injection, ME-9F1 mAb was immediately captured by the tumor and peritumoral liver during the first transvascular passage, in both Panc02 and Hep55.1C models. The respective tumor-specific accumulation was detected by whole-body and tissue scintigraphic imaging (Fig. 4B). Only a small fraction of antibody passed through the tumor-bearing tissue and was subsequently captured in the lung (Fig. 4B). Very low antibody concentrations were found in the tumor-distant liver (Fig. 4B). At the microscopic level, an exclusive and homogeneous labeling of the perfused microvasculature in tumor and peritumoral liver was found.
ENDOTHELIAL CAPTURE–BASED LOCAL ANTIBODY CONCENTRATION INCREASES WITH DECREASING TISSUE MASS IN VIVO
We then compared the antibody delivery for perfusion of either the whole liver or one small liver segment, namely the right middle segment18 (Supporting Table S2). The local antibody delivery was highly increased after perfusion of a small tissue volume (Fig. 5A,C), although the fraction of antibody capture was not significantly different between perfusion of bigger and smaller tissue masses (Fig. 5B,C). Furthermore, injection of 125I-conjugated ME-9F1 mAb into the tumor-bearing small liver segment (right middle segment) resulted in a higher local antibody concentration when compared to antibody distribution in large (left anterior and posterior) liver segments (Fig. 5D; Supporting Table S2). The tumor/peritumoral tissue was well contrasted in scintigraphic imaging (Fig. 5D).
Discussion
In the present study, we evaluated the intravascular capture of endothelium-specific antibodies. Our results showed that selected antibody clones can be almost completely captured by the endothelium during the first intravascular passage when applied at low doses. We designated this process “endothelial capture” (or “endocapt”). Although increased local drug enrichment after locoregional injection seems like an obvious outcome, only partial capture of an injected drug during a first intravascular passage can usually be expected. For example, previous clinical studies reported that the local concentration of tumor-targeting peptide was approximately 3-fold higher after hepatic arterial infusion than after intravenous injection.21 The present study demonstrated that the intrahepatic and intratumoral concentration of the isotype antibody was 2.9-fold higher after intra-arterial injection than after intravenous application, although the antibody enrichment remained very low. In contrast to the uptake of nonspecific antibody, the capture of selected endothelium-specific antibodies during the first intravascular passage was almost 90%, which represents a unique phenomenon. This capture would effectively prevent the entrance of the targeting drug (antibody or conjugates) into the systemic circulation in vivo. As shown here, the antibody was distributed in accordance with local microcirculatory conditions and the structure of the microvascular tree. Of interest, the endocapt efficacy (relative local antibody enrichment) did not change after application of different antibody amounts, while the saturation of endothelial epitopes was not achieved. Thus, for effective endocapt, the antibody can be applied in a flexible dose but should not exceed a certain level. The application of high antibody doses will lead to saturation of binding sites on endothelium and blunt the high efficacy of endocapt. The saturation of endothelial epitopes can be rapidly achieved; for example, with the ME-9F1 mAb, this saturation amounts to approximately 2 μg/g tissue in the mouse liver.22
The current findings show that a flow reduction of only 50% results in substantial improvement of endocapt of antibody–drug conjugates. This improvement could be important in cases of reduced antibody binding after modifications. Translation of this finding into clinical application could be achieved by use of intravascular balloon dilation, which is already a routine method for flow reduction in radiological investigations.23 In the current work, strong flow reduction to 0.2 mL/minute led to an irregular labeling of blood vessels, which may reflect inhomogeneous perfusion in different areas of the vasculature under strongly reduced vascular pressure.
Our findings also showed that repeated injections or long duration of antibody injection substantially reduced the efficacy of endocapt. We propose that with an increasing number of perfusion cycles, the amount of free binding sites on endothelium will decrease step by step, providing the most likely reason for the progressive reduction in endocapt. The diminishment of endocapt efficacy after prolonged injection time may have resulted from a local concentration gradient from the center to the periphery of the blood vessel, arising after rapid antibody immobilization on endothelium. The higher antibody concentration could have led to a steeper concentration gradient, which would be expected to result in faster lateral movement of antibody molecules and an increase in the probability of antibody–endothelium contact.
Both experiments demonstrate the importance of injection timing. The maximum efficacy of endocapt can be expected after only a single bolus injection. However, here, if the estimated time of bolus infusion was less than 1 second (corresponding to an infusion of 10 μL), the antibody could not reach a sufficient number of microvessels, making this approach less effective than the bolus infusion of 100 μL. As shown in the present study and confirmed elsewhere,24 the minimal filling time of the microvascular tree in mice is 5-10 seconds. This filling time must be species-specific because of different heart rate and organ volume.
Several previous studies focused on the immunotargeting of pulmonary and cerebral vasculature using endothelium-specific antibodies such as anti-CD31 and anti-CD54 mAbs in rodent and pig models.25-28 Using isolated organ perfusion and in vivo applications, these studies demonstrated a high potential of locoregional application for local tissue enrichment of antibody or antibody–drug conjugates. The authors also anticipated the relevance of application parameters and assumed that the slower infusion may augment the local antibody uptake.25
We believe that the method of endocapt has a very high potential for translation. Conventional clinical tools use systemic circulation to transport the targeting antibody to the tumor site (Fig. 6A). This unavoidable step leads to sustained drug delivery through the whole body, causing side effects and limiting the target dose. At the tumor site, the endothelial barrier and stroma form an additional obstacle between antibody and tumor cells that impedes the targeting.
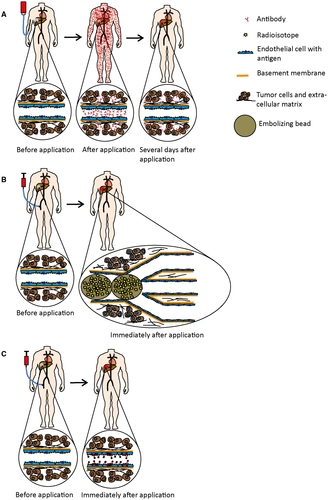
Locoregional approaches such as transarterial chemoembolization and selective internal radiotherapy29 use beads that are injected through tumor-feeding branches of the hepatic artery. Beads are distributed in the microvascular tree according to local microcirculatory conditions and embolize small afferent blood vessels, usually >20 μm in diameter. These beads do not penetrate into capillaries and are inhomogeneously distributed in a spot-like fashion within the tumor tissue (Fig. 6B).30, 31 The principle of endocapt-based targeting may represent an excellent alternative or addition to the above methods.
The use of radioimmunoconjugates may represent a promising application for both imaging and therapy (Fig. 6C). As shown here, antibody labeling with a small isotope such as iodine does not affect endocapt. In contrast to chemotherapeutic drugs, even a low antibody concentration may allow achievement of the effective local drug concentrations required for radioisotope-based imaging and therapy. The application dose can be calculated according to expected intratumoral antibody enrichment, namely the “tumor/normal tissue” ratio, which can be determined using dosimetry and may be specific for individual antibody clones. In contrast to selective internal radiotherapy, the endocapt may result in the deep and homogeneous distribution of the targeting nuclide throughout the microvascular network, including the smallest blood vessels (Fig. 6C). If reasonable, embolization could subsequently be performed.
In this study, the local antibody concentration was negatively correlated with the tissue mass, which was perfused after locoregional intra-arterial injection, indicating that relative antibody accumulation increased with decreasing tissue mass. The translational relevance of this finding is that a higher effective concentration of the targeting drug must be expected if a lower tissue mass/volume is chosen for locoregional application.
According to the biodistribution data, only a small fraction of antibody passed through the tumor-bearing tissue and was subsequently captured in the lung. The mass of the mouse lung is quite small (Supporting Table S2), so this capture resulted in a low but notable concentration. Because the lung tolerates even a high treatment load well,14 a low concentration cannot be considered as a limiting factor. For antibody selection, the EC50 of antibody binding on histological slides was determined. The binding was not dependent on the tissue type but correlated well with the capture efficacy results in the isolated liver perfusion model. This correlation indicates that determination of the EC50 of antibody binding in histological slides can be used as a predictive parameter for assessing endothelial capture for specific mAb clones. It has been shown that the cellular integration can lower the accessibility of antigens and subsequently reduce the antibody binding.32, 33 We propose that the quantification of antibody binding (EC50) in histological slides reflects both the antibody affinity and potential changes of antigen availability in the cellular context.
In summary, the present study represents an experimental application of the concept of endothelial antibody capture, using radioactively labeled antibodies in vivo. We believe that this unique phenomenon will provide considerable advantages for targeting hepatic tumors because locoregional application through the hepatic artery is routine for this tumor localization.
Acknowledgment
We thank Claudia Bernardi-Neuwirth for her excellent assistance, especially with cell isolation procedures. We further thank Karin Leotta for her excellent technical assistance with molecular imaging and the biodistribution studies.
Potential conflict of interest
Nothing to report.
REFERENCES
Author names in bold designate shared co-first authorship.
Abbreviations
-
- EC50
-
- half-maximal effective concentration
-
- endocapt
-
- endothelial capture
-
- mAb
-
- monoclonal antibody
-
- RPE
-
- R-phycoerythrin




