Farnesoid X receptor signaling activates the hepatic X-box binding protein 1 pathway in vitro and in mice
Potential conflict of interest: Nothing to report.
Supported by NIDDK R01 DK093807, The Max Goldenberg Foundation, The George Lockerbie Cancer Foundation and PSC Partners Seeking a Cure (to R.M.G.) and R01DK104656 (to L.W.).
Abstract
Bile acids are endogenous ligands of the nuclear receptor, farnesoid X receptor (FXR), and pharmacological FXR modulators are under development for the treatment of several liver disorders. The inositol-requiring enzyme 1α/X-box binding protein 1 (IRE1α/XBP1) pathway of the unfolded protein response (UPR) is a protective cellular signaling pathway activated in response to endoplasmic reticulum (ER) stress. We investigated the role of FXR signaling in activation of the hepatic XBP1 pathway. Mice were treated with deoxycholic acid (DCA), cholestyramine, GW4064, or underwent bile duct ligation (BDL), and hepatic UPR activation was measured. Huh7-Ntcp and HepG2 cells were treated with FXR agonists, inhibitor, small interfering RNA (siRNA), or small heterodimer partner (SHP) siRNA to determine the mechanisms of IRE1α/XBP1 pathway activation. DCA feeding and BDL increased and cholestyramine decreased expression of hepatic XBP1 spliced (XBP1s). XBP1 pathway activation increased in Huh7-Ntcp and HepG2 cells treated with bile acids, 6α-ethyl-chenodeoxycholic acid (6-ECDCA) or GW4064. This effect decreased with FXR knockdown and treatment with the FXR inhibitor guggulsterone. FXR agonists increased XBP1 splicing and phosphorylated IRE1α (p-IRE1α) expression. Overexpression of SHP similarly increased XBP1 splicing, XBP1s, and p-IRE1α protein expression. SHP knockdown attenuated FXR agonist-induced XBP1s and p-IRE1α protein expression. Co-immunoprecipitation (Co-IP) assays demonstrate a physical interaction between overexpressed green fluorescent protein (GFP)-SHP and FLAG-IRE1α in HEK293T cells. Mice treated with GW4064 had increased, and FXR and SHP null mice had decreased, basal Xbp1s gene expression. Conclusion: FXR signaling activates the IRE1α/XBP1 pathway in vivo and in vitro. FXR pathway activation increases XBP1 splicing and enhances p-IRE1α expression. These effects are mediated, at least in part, by SHP. IRE1α/XBP1 pathway activation by bile acids and pharmacological FXR agonists may be protective during liver injury and may have therapeutic implications for liver diseases. (Hepatology 2018;68:304-316).
Abbreviations
-
- 6-ECDCA
-
- 6α-ethyl-chenodeoxycholic acid
-
- ALT
-
- alanine aminotransferase
-
- ATF4/6
-
- activating transcription factor 4/6
-
- BDL
-
- bile duct ligation
-
- BIP/GRP78
-
- binding immunoglobulin protein/glucose-regulated protein, 78 kDa
-
- CHOP
-
- C/EBP-homologous protein
-
- Co-IP
-
- co-immunoprecipitation
-
- DCA
-
- deoxycholic acid
-
- DMSO
-
- dimethyl sulfoxide
-
- ER
-
- endoplasmic reticulum
-
- ERAD
-
- endoplasmic reticulum-associated protein degradation
-
- ERdj4/DNAJB9
-
- DnaJ (Hsp40) homolog, subfamily B, member 9/endoplasmic reticulum DNA J domain-containing protein 4
-
- FBS
-
- fetal bovine serum
-
- FXR
-
- farnesoid X receptor
-
- GAPDH
-
- glyceraldehyde 3-phosphate dehydrogenase
-
- GFP
-
- green fluorescent protein
-
- IP
-
- immunoprecipitation
-
- IRE1α
-
- inositol-requiring enzyme 1α
-
- NASH
-
- nonalcoholic steatohepatitis
-
- PBC
-
- primary biliary cholangitis
-
- PERK
-
- PKR-like ER kinase
-
- p-IRE1α
-
- phosphorylated IRE1α
-
- SHP
-
- small heterodimer partner
-
- siRNA
-
- small interfering RNA
-
- TCA
-
- taurocholic acid
-
- TCDCA
-
- taurochenodeoxycholic acid
-
- TDCA
-
- taurodeoxycholic acid
-
- UPR
-
- unfolded protein response
-
- XBP1
-
- X-box binding protein 1
-
- XBP1s
-
- XBP1 spliced
-
- XBP1u
-
- unspliced XBP1
-
- WT
-
- wild type
The unfolded protein response (UPR) is an adaptive cellular response to endoplasmic reticulum (ER) stress that maintains homeostasis by increasing protein processing capacity and attenuating protein translation.1-3 The UPR is important in many hepatic diseases including cholestasis, non-alcoholic fatty liver disease, alpha-1 antitrypsin deficiency, ischemia-reperfusion injury and viral hepatitis.4 However, the cellular mechanisms regulating the hepatic UPR remain incompletely understood. Three UPR pathways are activated through the transmembrane ER sensors: inositol-requiring enzyme 1α (IRE1α), PKR-like ER kinase (PERK), activating transcription factor 6 (ATF6), and the IRE1α/X-box binding protein 1 (XBP1) pathway is the evolutionarily most conserved. When ER stress occurs, IRE1α autophosphorylates and activates its endoribonuclease activity that is responsible for the unconventional splicing of XBP1 mRNA. IRE1α splices a 26-nucleotide sequence from unspliced XBP1 (XBP1u) mRNA and causes a translational frameshift, producing the transcriptionally active XBP1 spliced (XBP1s). XBP1s regulates downstream target genes, such as endoplasmic reticulum DNA J domain-containing protein 4 (ERdj4), to promote protein folding and ER-associated degradation (ERAD).5, 6 This pathway has been implicated in the pathogenesis, and as a protective response, in several forms of liver injury.7-9 In vitro studies demonstrate that the bile acid, deoxycholic acid (DCA), causes protein unfolding and aggregation.10 However, the molecular mechanisms by which cholestasis or bile acid signaling regulates the XBP1 pathway remain unexplored.
Bile acids are endogenous ligands of the nuclear receptor, farnesoid X receptor (FXR), and activation of hepatic FXR regulates the expression of many genes in the liver.11, 12 Many inhibitory effects on gene expression are attributed to FXR-mediated induction of small heterodimer partner (SHP), which is considered a universal transcriptional inhibitor.13 There is also SHP-independent gene regulation by direct transcriptional activation of FXR target genes.11, 12, 14, 15 FXR plays important roles in maintaining bile acid homeostasis, as well as regulating lipid and cholesterol metabolism.15, 16 Pharmacological FXR agonists are therapeutic in several experimental models of liver injury and are currently approved or in clinical trials for metabolic and cholestatic liver diseases. Interactions of the FXR pathway and the hepatic UPR, however, remain unexplored. The purpose of this study is to determine the role of bile acid and FXR signaling in regulation of the hepatic IRE1α/XBP1 pathway. These studies will enhance our understanding of the role of the UPR in liver diseases and may have an important therapeutic implication.
Materials and Methods
MATERIAL
Bile acids and cholestyramine were purchased from Sigma-Aldrich (St. Louis, MO). GW4064, (z)-guggulsterone and 6α-ethyl-chenodeoxycholic acid (6-ECDCA) (obeticholic acid) were purchased from Cayman Chemical (Ann Arbor, MI). FXR, SHP small interfering RNA (siRNA), and scramble siRNA were purchased from GE Dharmacon (Lafayette, CO). Lipofectamine RNAiMax and LTX transfection reagents were purchased from ThermoFisher (Waltham, MA). Antibodies against XBP1 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were from Proteintech (Chicago, IL), β-actin, FLAG, and green fluorescent protein (GFP) antibodies were from Sigma-Aldrich (St. Louis, MO), IRE1α antibody was from Cell Signaling Technology (Danvers, MA), and phosphorylated IRE1α (p-IRE1α) antibody was from Novus Biologicals (Littleton, CO). SHP antibody was from Santa Cruz Biotechnology (Dallas, TX).
ANIMAL EXPERIMENTATION
FVB/NJ, C57BL/6J and FXR (–/–) mice (8-10 weeks) were purchased from Jackson Laboratory (Bar Harbor, ME). SHP (–/–) mice colonies were maintained as reported.17 Mice were maintained under 12-hour light/dark cycles with unlimited access to regular chow and water until the first day of the study. For feeding experiments, male FVB/NJ mice received chow, chow supplemented with 0.3% (w/w) DCA, or chow supplemented with 2% (w/w) cholestyramine for up to 7 days. Male C57BL/6J mice were gavaged with GW4064 (150 mg/kg) as described.18 At the conclusion of each experiment, mice were fasted for 4 hours and livers were excised, flash-frozen in liquid nitrogen, and stored at −70°C. Whole blood was obtained from the right atrium by cardiac puncture. For mice used in bile acid analysis, liver, gallbladder, and small intestine were collected and minced in 100% ethanol. Bile duct ligation (BDL) in female C57BL/6J mice was performed by the Northwestern University Microsurgery Core. All protocols and procedures were approved by the Northwestern University Institutional Animal Care and Use Committee guidelines.
SERUM ALANINE AMINOTRANSFERASE ASSAY
Serum alanine aminotransferase (ALT) was measured using a spectrophotometric assay according to the manufacturer's protocol (Teco Diagnostics, Anaheim, CA).
ANALYSIS OF BILE ACID POOLS
Total bile acid pool size and composition were measured by high-performance liquid chromatography as described.19 Samples were spiked with glycocholic acid as an internal standard to control for extraction efficiency. Individual bile salt species were identified by their characteristic retention times and by using bile acid standards.
CELL CULTURE AND siRNA TRANSFECTION
HepG2 cells (ATCC, Manassas, VA) were cultured in Eagle's minimal essential medium with 10% fetal bovine serum (FBS) and maintained at 37°C in 5% CO2. Lenti-X HEK293T cells (Takara Bio., Mountain View, CA) were cultured in Dulbecco's modified Eagle's medium with 10% FBS and 4 mM of glutamine. Huh7 cells stably expressing rat sodium-taurocholate polypeptide (Huh7-Ntcp) were kindly provided by Dr. Simon Hohenester (University of Munich, Munich, Germany) and were grown in minimal essential medium containing 10% FBS, 1% nonessential amino acids, 2 mM of glutamine, 1 mM of sodium pyruvate, 100 units/mL of penicillin, 100 μg/mL of streptomycin, and 0.25 μg/mL of amphotericin B.20 Cells were grown to 80% confluence and serum-deprived for 24 hours before treatment with taurocholic acid (TCA), taurodeoxycholic acid (TDCA), taurochenodeoxycholic acid (TCDCA), 6-ECDCA, GW4064, or vehicle. In guggulsterone studies, cells were pretreated with 50 μM of guggulsterone for 1 hour before receiving either dimethyl sulfoxide (DMSO) or 6-ECDCA. For siRNA transfection studies, HepG2 cells were transfected with 7.5 pmol of FXR siRNA, 9.5 pmol of SHP, or scramble siRNA using Lipofectamine RNAiMax for 48 hours before GW4064 treatment, according to the manufacturer's protocol.
LUCIFERASE REPORTER ASSAY
The XBP1-luc reporter vector was kindly provided by Dr. Albert Koong (Stanford University, Palo Alto, CA) to assay the atypical splicing of the XBP1 mRNA.21 Huh7-Ntcp or HepG2 cells were transfected with 0.45 μg of XBP1-luc reporter gene and 0.05 μg of Pol III-Renilla luciferase vector as a control for transfection efficiency using Lipofectamine LTX transfection reagent per the manufacturer's instruction. After a 48-hour transfection, cells were treated with 6-ECDCA, GW4064, or DMSO for 8 hours. For SHP overexpression study, Huh7-Ntcp or HepG2 cells were cotransfected with a total of 2.0 μg of vector using pcDNA3 or human GFP-SHP (0.0-2.0 μg) expression plasmid22 with or without 0.45 μg of XBP1-luc reporter gene vector and 0.05 μg of Pol III-Renilla luciferase vector for 48 hours. At the end of the experiment, cells were harvested in passive reporter lysis buffer. Firefly and Renilla luciferase activities were measured with a dual-luciferase reporter assay kit (Promega, Madison, WI). All experiments were performed in triplicate. The luciferase activity was calculated as the ratio of Firefly/Renilla luminescence.
RNA EXTRACTION AND REAL-TIME QUANTITATIVE PCR
Total RNA was extracted from frozen liver or cell culture using TRIzol reagent according to the manufacturer's protocol (ThermoFisher, Waltham, MA). Real-time quantitative PCR (qPCR) was performed as described.7 All primers were synthesized by Integrated DNA Technology (Coralville, CA).
Co-immunoprecipitation (Co-IP)
HEK293T cells were transfected with pcDNA3, human SHP-GFP, or human FLAG-IRE1α23 plasmids using Calfectin reagent (SignaGen Laboratories, Rockville, MD) according to the manufacturer's protocol. Forty-eight hours posttransfection, cells were rinsed with cold phosphate-buffered saline, scraped into tubes, centrifuged, and pellets flash frozen in liquid nitrogen before –70°C storage. For immunoprecipitation (IP) experiments, pellets were lysed in 150 mM of NaCl, 20 mM of Tris, and 1 mM of ethylenediaminetetraacetic acid (NTEN) buffer containing protease and phosphatase inhibitors, cleared by centrifugation, and protein concentration estimated. Lysates (750 μg) were precleared with Protein A/G beads then immunoprecipitated overnight, 4°C, with 2 μg of SHP or 1 μg of FLAG antibodies. Immunoprecipitates were captured with Protein A/G beads and washed extensively with NTEN buffer, before elution by heating in 2× loading buffer. NTEN buffer alone was used as a negative control. Eluates and reserved lysate (input) were electrophoresed and transferred to polyvinylidene fluoride membranes and probed with indicated antibodies.
WESTERN IMMUNOBLOTTING
Protein homogenates from frozen livers or cultured cells were isolated and used with western immunoblotting as described.7 Nuclei preparations were performed using a nuclear extraction kit (Caymen Chemical, Ann Arbor, MI) according to the instructions of the manufacturer.
STATISTICAL ANALYSIS
Each experiment was repeated a minimum of three times. Data are shown as means ± SEM. Comparison between two groups was performed by Student t test. Statistical significance was defined as P values of less than 0.05.
Results
DCA FEEDING INCREASES BILE ACID POOL AND HEPATIC XBP1 PATHWAY EXPRESSION
It has pbeen reported that feeding cholic acid to mice increases hepatic Xbp1s expression, presumably attributed to the presence of ER stress.24 We hypothesized that Xbp1s induction may be mediated, at least in part, by FXR signaling activation of p-IRE1α with a resultant increase in hepatic XBP1s expression. We fed mice with a diet containing 0.3% DCA for 0, 1, 3, and 7 days to enrich the bile acid FXR ligands. Nuclear expression of XBP1s protein was increased after 1 and 3 days of DCA feeding. Hepatic nuclear XBP1s was not detectable at baseline or at 7 days (Fig. 1A). p-IRE1α was not detected at any time point because of low level of expression in wild-type (WT) mice. Figure 1B demonstrates that hepatic gene expression of Xbp1s and its downstream target, ERdj4, increased 6-fold and 1.5-fold, respectively, in DCA-fed mice compared to chow-fed controls (P < 0.05). Figure 1C demonstrates that DCA feeding for 1 day resulted in a 2-fold increase in the total bile acid pool (P < 0.001) and significant increases in the FXR bile acid ligands, TCDCA, TCA, and TDCA. TCDCA and TDCA contents were 1.2 ± 0.3 and 1.8 ± 0.6 μmol/100g mouse, respectively, in DCA-fed mice, whereas both bile acids were nondetectable in chow-fed mice (P < 0.05). TCA increased 7-fold in DCA-fed mice, being 52.0 ± 3.6 versus 7.3 ± 1.6 μmol/100g mouse (P < 0.001). Serum ALT levels were similar in the mice fed chow or DCA for 1 day and increased at days 3 and 7 (Supporting Fig. S1); all liver histology was normal. Hepatic gene expression of the UPR genes, Atf4, Atf6, BIP/GRP78, binding immunoglobulin protein/glucose-regulated protein, 78 kDa (Bip), and C/EBP-homologous protein (Chop), did not increase at any time points, and, in fact, gene expression of Atf4 and Atf6 decreased at 7 days (Fig. 1B). This suggests that bile acid feeding may activate the liver XBP1s pathway.
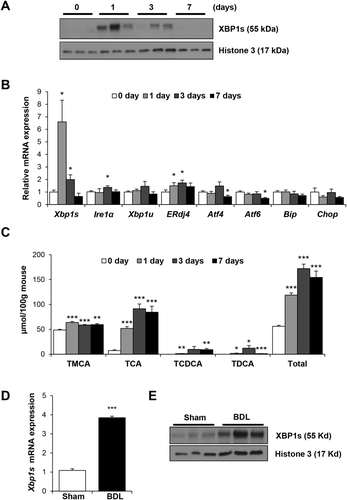
BDL INCREASES HEPATIC XBP1s EXPRESSION
In order to determine whether the IRE1α/XBP1 pathway is activated in another model of cholestasis, BDL or sham laparotomy was performed on C57BL/6J mice for 48 hours. Serum ALT and alkaline phosphatase (ALP) were higher in the BDL group (ALT, 734 ± 412 vs. 28 ± 3 U/L; ALP, 251 ± 66 vs. 83 ± 6 U/L; P < 0.05 in BDL vs. sham, respectively). Figure 1D demonstrates that 48 hours after BDL, hepatic Xbp1s mRNA increased 4-fold (P < 0.001), and Fig. 1E shows that XBP1s nuclear protein increased significantly.
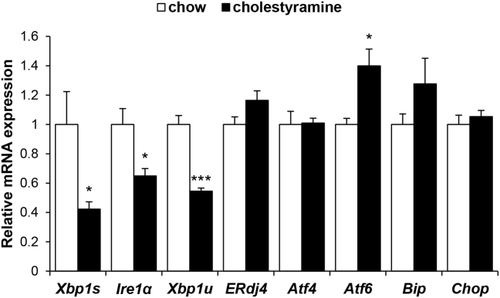
REDUCING BILE ACID POOL WITH CHOLESTYRAMINE DECREASES HEPATIC XBP1 PATHWAY EXPRESSION
Because expansion of the bile acid pool with DCA feeding may potentially cause ER stress, we fed mice a diet containing 2% (w/w) cholestyramine for 7 days to reduce the bile acid pool and measured the effect on liver IRE1α/XBP1 signaling. Figure 2 demonstrates that feeding cholestyramine for 7 days resulted in significant reductions of basal gene expression of Xbp1s, Xbp1u, and Ire1α by 58%, 46%, and 35%, respectively (P < 0.05), whereas basal ERdj4 expression did not change. Baseline p-IRE1α and XBP1s protein levels were not detected. Hepatic gene expression of Atf4, Atf6, Bip, and Chop was not diminished by cholestyramine feeding, and, in fact, Atf6 expression increased slightly at 7 days.
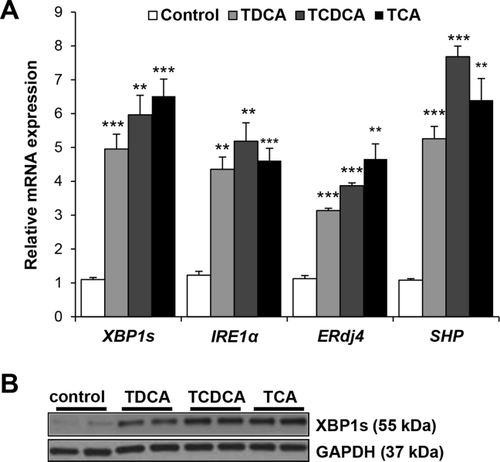
BILE ACIDS ACTIVATE IRE1α/XBP1 PATHWAY IN VITRO
Because the hepatic XBP1 pathway was activated by expansion of the bile acid pool and in response to BDL, and basal gene expression of the XBP1 pathway was reduced by contraction of the bile acid pool, we next investigated the in vitro effects of bile acids on the XBP1 pathway. Initial experiments demonstrated that treating Huh7-Ntcp cells for 6 hours with 0- to 100-μM concentrations of TDCA, TCDCA, and TCA increased XBP1s protein expression in a dose-dependent manner, with XBP1s protein expression increases evident at 10-μM bile acid concentration (Supporting Fig. S2). Therefore, we treated Huh7-Ntcp cells for 6 hours with 10 μM of TDCA, TCDCA, TCA, or vehicle and determined the effect on the XBP1 pathway. Gene expression of XBP1s, ERdj4, and IRE1α all increased in response to treatment with each bile acid, and SHP expression increased 5- to 8-fold (Fig. 3A). XBP1s protein expression similarly increased, with greater effects occurring in response to the FXR ligands, TCDCA and TCA (Fig. 3B). Therefore, the bile acid species that were enriched in vivo by DCA feeding also activate the IRE1α/XBP1 pathway in vitro.
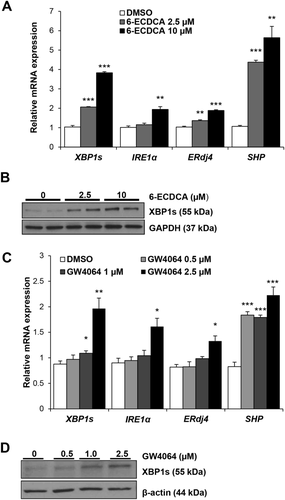
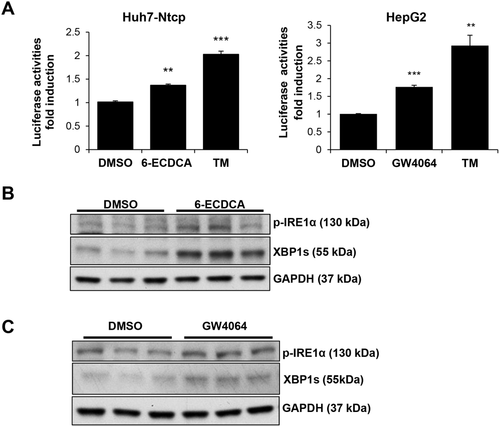
FXR AGONISTS ACTIVATE IRE1α/XBP1 PATHWAY IN VITRO
Because bile acids are endogenous ligands of the nuclear receptor, FXR, we then treated Huh7-Ntcp cells and HepG2 cells with high-affinity pharmacological FXR agonists to determine the effects of FXR signaling on the XBP1 pathway. Figure 4A,B demonstrates that treating Huh7-Ntcp cells with the high-affinity FXR agonist, 6-ECDCA (0-10 μM), for 4 hours increased gene expression of XBP1s, ERdj4, and IRE1α, as well as XBP1s protein expression, in a dose-dependent manner. Figure 4C,D shows that treating HepG2 cells with the FXR ligand, GW4064 (0.0-2.5 μM), for 6 hours similarly increased both gene expression of XBP1s, ERdj4, and IRE1α and protein expression of XBP1s in a dose-dependent manner. No changes of XBP1s expression occurred within 2 hours of treatment in either cell type (Supporting Fig. S3A,B). As expected, both FXR agonists increased gene expression of SHP (Fig. 4A,C and Supporting Fig. S3C,D).
FXR INHIBITION AND KNOCKDOWN DECREASE FXR AGONISTS-ACTIVATED XBP1s PROTEIN EXPRESSION
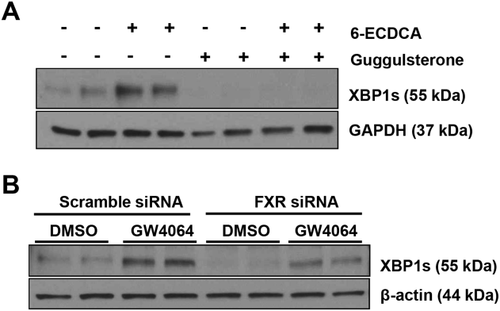
We subsequently investigated the effects of the FXR inhibitor, guggulsterone, and FXR knockdown on both basal and FXR agonist-induced XBP1s protein expression. Huh7-Ntcp cells were treated with 10 μM of 6-ECDCA or vehicle, in the presence or absence of 50 μM of guggulsterone. Figure 5A demonstrates that both basal and 6-ECDCA-induced XBP1s protein expression were blocked by pretreatment with guggulsterone. In fact, protein expression of XBP1s in 6-ECDCA and guggulsterone cotreated cells remained below the basal XBP1s expression levels of untreated controls. Given that inhibitors may lack specificity, and to further confirm the inhibitory effect of guggulsterone, we used HepG2 cells transfected with FXR siRNA (or scramble siRNA) to demonstrate that FXR knockdown similarly decreased both basal and GW4064-induced increases in XBP1s protein expression (Fig. 5B). Supporting Fig. S4A confirms that FXR siRNA treatment reduced expression of FXR mRNA by over 70%.
FXR AGONISTS INDUCE XBP1 SPLICING AND IRE1α PHOSPHORYLATION
The atypical splicing of XBP1 into its active spliced form by IRE1α is a major regulatory mechanism for the IRE1α/XBP1 pathway.6, 25 We utilized an XBP1 splice activity luciferase-reporter gene construct (XBP1-luc) to measure the effect of FXR agonists on XBP1 splicing. Figure 6A demonstrates that treatment of Huh7-Ntcp and HepG2 cells with either 10 μM of 6-ECDCA or 2.5 μM of GW4064 for 8 hours increased XBP1 splicing compared to DMSO vehicle-treated cells (P < 0.01). DMSO-treated cells had identical luciferase activity to untreated cells.
The phosphorylated form of IRE1α (p-IRE1α) is the active form that splices Xbp1u into Xbp1s. Thus, we measured the expression of p-IRE1α in Huh7-Ntcp and HepG2 cells treated with 6-ECDCA and GW4064, respectively. Figure 6B shows that the expression of p-IRE1α in Huh7-Ntcp cells increased as early as 2 hours after the administration of 6-ECDCA, and Fig. 6C shows that p-IRE1α expression in HepG2 cells increased by 4 hours after treatment with GW4064. There were no increases in p-IRE1α expression at earlier time points in either cell types (Supporting Fig. S3A,B). These data indicate that FXR agonist-induced XBP1s expression was associated with increases in both p-IRE1α expression and XBP1 splice activity. Of note, the gene expression of polo-like kinase 3 (PLK3) was not changed at 1 or 2 hours after 6-ECDCA or GW4064 treatment, indicating that increased p-IRE1α expression was not associated with PLK3 gene induction.
SHP IS ASSOCIATED WITH FXR AGONIST-INDUCED ACTIVATION OF IRE1α/XBP1 PATHWAY
Because many FXR signaling effects are mediated by SHP, and SHP mRNA expression was up-regulated as early as 30 minutes or 1 hour in GW4064-treated HepG2 cells or 6-ECDCA-treated Huh7-rNTCP cells, respectively (Supporting Fig. S3C,D), we next overexpressed human SHP in HepG2 cells to determine the effect on the activation of IRE1α/XBP1 pathway. Figure 7A demonstrates a dose-response curve of transfections (0-2 μg) overexpressing SHP using a GFP-tagged SHP plasmid. SHP overexpression resulted in a dose-dependent increase of the XBP1-luc reporter luciferase activity, indicative of increased splicing activity. Figure 7B further shows that this was associated with increased XBP1s and p-IRE1α protein expression.
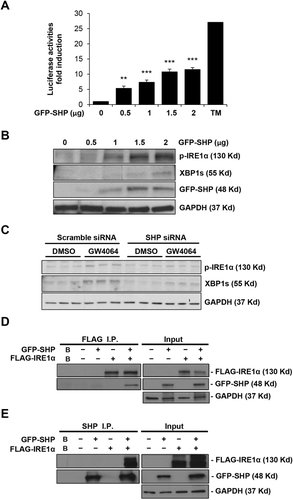
To further confirm the specific role of SHP in IRE1α/XBP1 pathway activation, we performed SHP knockdown experiments using SHP siRNA. Figure 7C demonstrates that SHP knockdown decreased GW4064-induced XBP1s and p-IRE1α protein expression in HepG2 cells. Supporting Fig. S4B confirms that SHP siRNA treatment reduced expression of SHP mRNA by 79%. These data suggest that FXR agonist-induced IRE1α/XBP1 pathway activation is, at least in part, mediated by SHP.
Although SHP acts as a transcriptional repressor localized primarily in the nucleus, SHP also localizes and interacts with proteins in other cellular compartments.26 SHP is highly expressed in microsome-enriched subcellular fraction.27 Because IRE1α is an ER resident protein, we investigated a potential interaction between SHP and IRE1α which may lead to the activation of the IRE1α/XBP1 pathway. SHP and IRE1α were overexpressed in HEK293T cells with GFP-SHP and FLAG-IRE1α plasmids. Co-IP experiments demonstrate a physical interaction between SHP and IRE1α using IP assays with a FLAG (Fig. 7D) or SHP antibody (Fig. 7E). These data suggest that SHP could possibly activate IRE1α/XBP1s through a nontranscriptional mechanism.
FXR/SHP SIGNALING REGULATES HEPATIC XBP1 EXPRESSION IN VIVO
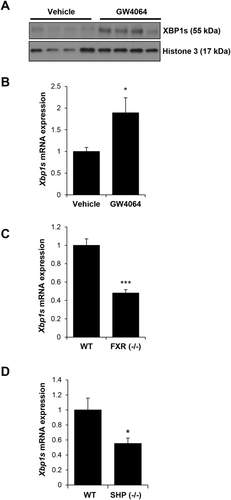
In order to determine the in vivo effects of FXR/SHP signaling on hepatic XBP1s expression, we administered GW4064 (150 mg/kg, 16 hours) to WT mice. Hepatic XBP1s protein was minimally detected at baseline, but increased in response to GW4064 administration (Fig. 8A). Hepatic Xbp1s gene expression also increased in response to GW4064 (Fig. 8B). Hepatic Xbp1u gene expression did not alter after GW4064 treatment (Supporting Fig. S5). We also examined baseline expression of hepatic Xbp1s gene expression in FXR (–/–) and SHP (–/–) mice. Hepatic gene expression of Xbp1s was reduced by 52% in FXR (–/–) mice and 45% in SHP (–/–) mice compared to WT mice (Fig. 8C,D). Because baseline hepatic XBP1s protein expression was not detected, reductions could not be observed.
Discussion
Bile acids are endogenous FXR ligands and hepatic FXR signaling regulates many liver metabolic, transport, and injury pathways.28, 29 The high-affinity FXR agonist, 6-ECDCA, has hepatoprotective effects including reduction in inflammation and fibrosis, improving biliary transport, regulating hepatic glucose and lipid metabolism, and regulating antiapoptotic pathways.30-33 6-ECDCA has been approved for the treatment of primary biliary cholangitis (PBC) and is currently being studied in clinical trials for other cholestatic, genetic, and metabolic liver diseases. However, interactions of bile acid and pharmacological FXR signaling on activation of the IRE1α/XBP1 pathways remain unexplored. In fact, there are few data demonstrating nuclear receptor regulation of any UPR pathways.
We demonstrate that activating FXR signaling by bile acids or FXR agonists induces XBP1s expression in vivo and in vitro. Mice fed a diet enriched in the FXR bile acid agonist, DCA, have increased hepatic expression of XBP1s and its downstream target, ERdj4. These changes occur concomitantly with increases of bile acid pool and content of the FXR bile acid ligands, TCDCA, TCA, and TDCA. After 1 day of DCA feeding, there was no histological or biochemical evidence of liver injury, suggesting that the early activation of the XBP1s pathway may be attributed to factors other than liver injury. In fact, the gene expression of other UPR pathways remained unchanged or decreased at all time points studied. Previous data demonstrated that mice fed 0.5% cholic acid for a week have a 9-fold increase in hepatic Xbp1s, although the UPR genes, Atf6 and Chop, also increased.24 Differences in gene expression may be attributed to their use of a higher dose of a different bile acid and/or difference in mouse strain background. The hydrophobic bile acid, DCA, has been demonstrated to causes protein unfolding and aggregation in vitro11; therefore, we do not exclude the possibility that other UPR pathways can be activated with DCA feeding at other time points or doses. Of note, the hydrophilic bile acid, TUDCA, can act as a chemical chaperone and potentially reduce ER stress.34 In addition, we also demonstrated the hepatic XBP1s expression similarly increases in a BDL model of cholestasis.
We fed mice cholestyramine for 7 days to diminish the bile acid pool. Cholestyramine is a relatively inert bile acid binding resin that is not absorbed, so it is unlikely to cause hepatic ER stress or liver injury. Baseline gene expression of hepatic Ire1α, Xbp1s, and Xbp1u all decrease with cholestyramine feeding, although ERdj4 remains unchanged. ERdj4 is also regulated by IRE1α-independent pathways that may account for this lack of suppression.35 In contrast to the IRE1α/XBP1 pathway, hepatic expression of the UPR genes, Atf4, Atf6, Bip, and Chop are not reduced. We subsequently treated Huh7-Ntcp cells with several bile acids and detected in vitro activation of the IRE1α/XBP1 pathway, similar to that observed in vivo. These effects occur at a lower bile acid concentration than those often used for in vitro bile acid toxicity studies.20, 36, 37
To further determine that these effects are mediated, at least in part, by FXR signaling, we used two distinct high-affinity FXR agonists (6-ECDCA and GW4064) and two different liver cell lines (HepG2, Huh7-Ntcp) to demonstrate that the IRE1α/XBP1 pathway was activated in a dose-dependent manner. FXR agonists have multiple hepatoprotective effects, and it is unlikely the FXR agonists induce ER stress. This further supports that IRE1α/XBP1 pathway activation is FXR mediated, rather than secondary to generalized ER stress. We next determined that both basal and FXR agonists-induced expression of XBP1s protein is blocked by the FXR antagonist, guggulsterone, and by FXR knockdown, further demonstrating the specificity of FXR regulation.
The major regulation of the IRE1α/XBP1 pathway is by the atypical splicing of the unspliced form of XBP1 to XBP1s by p-IRE1α. We utilized a luciferase reporter construct to demonstrate that FXR agonists increase XBP1 splicing. The endoribonuclease activity of p-IRE1α is the only enzymatic activity known to splice XBP1, and therefore the luciferase reporter construct is specific for p-IRE1α splicing of XBP1.38, 39 We demonstrated that FXR agonists simultaneously increase p-IRE1α expression, providing a putative mechanism for the increased splicing activity. Many FXR functions are mediated by SHP and both FXR agonists induce SHP mRNA expression, preceding IRE1α phosphorylation. We demonstrated the role of SHP in activating p-IRE1α and XBP1s by SHP overexpression and knockdown experiments. Unfortunately, we were unable to detect increased p-IRE1α expression in our in vivo models, probably attributed to low level of expression at the time points studied. We cannot exclude the possibility that p-IRE1α was increased at other time points or that XBP1s was regulated through other mechanisms in addition to p-IRE1α activation. Hepatic gene expression of Xbp1u did not change after GW4064 treatment in mice. Analysis of genome-wide chromatin IP sequencing of FXR binding data shows potentially weak binding of FXR to the XBP1 immediate promoter region and at the junction of second exon-intron,40 although the physiological significance remains to be determined. We cannot exclude that possibility of additional transcriptional regulation of XBP1 by FXR.
Recent studies have shown that IRE1α signaling can be regulated independent of ER stress through protein interactions.23 Although SHP is generally considered as a transcriptional repressor, it has now been shown to interact with other proteins and regulate functions throughout the cell.27, 41-43 We demonstrate that SHP and IRE1α can interact and this interaction may be responsible for the increased p-IRE1α and XBP1s expression that occurs with FXR agonist stimulation. FXR shares many common target genes with LRH-1. A recent study has shown that nuclear receptor LRH-1 initiates an ER stress resolution pathway by inducing PLK3 expression, which phosphorylates ATF2.44 We did not observe any change in PLK3 gene expression with FXR agonist treatment, although regulation could also be posttranscriptional.
FXR agonists and FXR-mediated IRE1α/XBP1 pathway activation may be particularly beneficial during pathologic states of cholestasis when bile acid concentrations increase. Pharmacologic FXR agonists have beneficial anti-inflammatory, anti-cholestatic and anti-fibrotic effects on the liver; as well as beneficial effects on glucose and lipid metabolism.45, 46 Our data demonstrate that FXR agonists may also be hepatoprotective due to an interaction with the IRE1α/XBP1 pathway. However, we do not exclude the possible involvement of the PERK and ATF6 pathways.
The pathogenesis of PBC has been associated with ER stress,47 and a recent clinical trial demonstrated that obeticholic acid (6-ECDCA) is beneficial in patients with PBC who had inadequate responses to ursodeoxycholic acid.48 Dysregulation of XBP1 has also been shown to be important in the pathogenesis of nonalcoholic steatohepatitis (NASH),49 and obeticholic acid may improve liver histology in patients with noncirrhotic NASH.50 The pathogenesis of PBC and NASH are multifactorial, and FXR-mediated activation of the IRE1α/XBP1 pathway provides a pathway for targeting treatments of these and other forms of liver diseases.
Many hepatic diseases cause ER stress and the UPR is an essential compensatory mechanism to resolve ER stress in the liver. We demonstrate that FXR signaling activates the IRE1α/XBP1 pathway in the liver, at least in part, by SHP signaling. These data will enhance our understanding of IRE1α/XBP1 pathway regulation in both normal and pathological states in the liver and may have important therapeutic implications.
Acknowledgment
We thank Xin Yi Yeap and the Northwestern University Microsurgury Core (Zheng Jenny Zhang, Director) for their assistance with the bile duct ligation procedure.
REFERENCES
Author names in bold designate shared co-first authorship.




