Basolateral CD147 induces hepatocyte polarity loss by E-cadherin ubiquitination and degradation in hepatocellular carcinoma progress
Potential conflict of interest: Nothing to report.
Supported by the National Natural Science Foundation of China (31571434 and 31401222) and the National Basic Research Program of China (2015CB553701).
Abstract
Hepatocytes are epithelial cells with highly specialized polarity. The disorder and loss of hepatocyte polarity leads to a weakness of cell adhesion and connection, the induction of epithelial–mesenchymal transition, and eventually the occurrence of hepatocellular carcinoma (HCC). Cluster of differentiation 147 (CD147), a tumor-related glycoprotein, promotes epithelial–mesenchymal transition and the invasion of HCC. However, the function of CD147 in hepatocyte depolarization is unknown. Here we identified that CD147 was basolaterally polarized in hepatocyte membrane of liver tissues and HepG2 cells. CD147 not only promoted transforming growth factor-β1–mediated hepatocyte polarity loss but also directly induced endocytosis and down-regulation of E-cadherin which contributed to hepatocyte depolarization. Overexpression of CD147 induced Src activation and subsequently recruited ubiquitin ligase Hakai for E-cadherin ubiquitination and lysosomal degradation, leading to decreases of partitioning defective 3 expression and β-catenin nuclear translocation. This signal transduction was initiated by competitive binding of CD147 with integrin β1 that interrupted the interaction between the Arg-Gly-Asp motif of fibronectin and integrin β1. The specific antibodies targeting integrin α5 and β1 reversed the decrease of E-cadherin and partitioning defective 3 levels induced by CD147 overexpression. In human liver tissues, CD147 polarity rates significantly declined from liver cirrhosis (71.4%) to HCC (10.4%). CD147-polarized localization negatively correlated with Child-Pugh scores in human liver cirrhosis (r = –0.6092, P < 0.0001) and positively correlated with differentiation grades in HCC (r = 0.2060, P = 0.004). HCC patients with CD147-polarized localization had significantly better overall survival than patients with CD147 nonpolarity (P = 0.021). Conclusion: The ectopic CD147-polarized distribution on basolateral membrane promotes hepatocyte depolarization by activation of the CD147–integrin α5β1–E-cadherin ubiquitination–partitioning defective 3 decrease and β-catenin translocation signaling cascade, replenishing a molecular pathway in hepatic carcinogenesis. (Hepatology 2018;68:317-332).
Abbreviations
-
- CD147
-
- cluster of differentiation 147
-
- DEN/PB
-
- diethylnitrosamine/phenobarbital
-
- ECM
-
- extracellular matrix
-
- EMT
-
- epithelial–mesenchymal transition
-
- HCC
-
- hepatocellular carcinoma
-
- KO
-
- knockout
-
- MRP2
-
- multidrug resistance–associated protein 2
-
- Par3
-
- partitioning defective 3
-
- RGD
-
- Arg-Gly-Asp
-
- TGF-β1
-
- transforming growth factor-β1
Hepatocytes have a unique polarization arrangement in which each of two adjacent cells form an apical membrane (i.e., bile canaliculus) and a sinusoidal membrane representing the basolateral plasma membrane.1 Establishment and maintenance of hepatocyte polarity are essential for normal cell physiology and liver tissue homeostasis, which require carefully orchestrated cooperation between cell adhesion molecules, polarity protein complexes, extracellular matrix (ECM), and intracellular protein trafficking machinery.2 Growing evidence suggests that loss of epithelial cell polarity leads to a reduction in cell adhesion and excessive proliferation, followed by induction of epithelial–mesenchymal transition (EMT) and finally promotion of tumor progression.3, 4 A number of proteins including junctional proteins and intracellular trafficking machinery proteins were determined to be associated with inherited liver diseases.5 However, the hepatocyte polarity–associated structural and functional components involved in acquired liver disease, particularly hepatocellular carcinoma (HCC), are rarely recognized.
E-cadherin is one of the main components of adherens junctions and plays a key role in carcinogenesis and cancer development. Impaired expression of E-cadherin promotes hepatocellular carcinogenesis and is associated with a worse prognosis in humans.6 Moreover, several studies indicate that down-regulation of E-cadherin in liver cancer is caused by different mechanisms, including loss of heterozygosity, methylation of the E-cadherin promoter region, transcriptional repressors, and ubiquitin degradation.7, 8 We previously demonstrated that E-cadherin expression in liver tissues gradually decreases in the process of liver adjacent to hemangiomas–viral hepatitis–cirrhosis–well-differentiated HCC–moderately differentiated HCC–poorly differentiated HCC. An adhesion molecule, cluster of differentiation 147 (CD147), is negatively correlated with E-cadherin expression in human HCC.9
CD147 is a type I transmembrane glycoprotein and highly expressed in liver tumor cells. Our previous reports have demonstrated that a positive autoregulatory loop of transforming growth factor-β1 (TGF-β1)–CD147 signaling induces EMT and promotes the carcinogenesis and metastasis of HCC.10, 11 Other earlier studies reported a basolateral distribution of increased CD147 on hepatocyte plasma membrane in rat liver tissue by stimuli of metabolic activation.12 A sorting signal, leucine-252 in the C-terminal domain of CD147, has been identified in Madin-Darby canine kidney cells, which dictates its basolateral localization.13 CD147 carrying the sorting information contributes to polarized targeting of the monocarboxylate transporter 1/CD147 heterocomplex in the basolateral membrane of kidney cells.14 Very little is known regarding whether CD147 has the polarized localization in human hepatocytes and the pathways involved in hepatocyte depolarization during HCC development.
The goal of this study was to determine the role of the apical–basal cell polarity machinery in HCC progression, with a focus on the oncogene CD147. Here, we demonstrate that CD147 localizes at the basolateral membrane of human hepatocytes, which closely relates to HCC progression. We provide a signaling pathway of CD147–integrin α5β1–E-cadherin ubiquitination–partitioning defective 3 (Par3) decrease and β-catenin translocation, which mediates the hepatocyte polarity loss, to replenish the mechanism of HCC development.
Materials and Methods
ANTIBODIES AND REAGENTS
Antibodies against human CD147 was produced by our lab; Par3 (07-330) and integrin α5 (MAB1956Z) were obtained from Merck Millipore (Darmstadt, Germany); β-catenin (51067-2-AP), occludin (13409-1-AP), lamin B (66045-1-AP), α-tubulin (11224-1-AP), and Src (60315-1-AP) were purchased from Proteintech (Wuhan, China); phosphotyrosine (ARR8004) was obtained from Antibody Revolution (South San Francisco, CA); E-cadherin (ET1607-75) and β-actin (M1210-2) were purchased from Huabio (Hangzhou, China); multidrug resistance–associated protein 2 (MRP2, ab3373) and mouse CD147 (ab34016) were obtained from Abcam (Cambridge, UK); ZO-1 (sc-10804), integrin β1 (sc-13590), phosphorylated Src (Tyr416, sc-101802), and ubiquitin (sc-271289) were purchased from Santa Cruz Biotechnology (Dallas, TX); Na+/taurocholate cotransporting polypeptide (orb13624), mouse MRP2 (orb11075), and CBLL1/Hakai (orb2538) were obtained from Biorbyt (Cambridge, UK); E-cadherin (610182) was from BD Biosciences (Franklin Lakes, NJ); Alexa Fluor 555 phalloidin (A34055) was from Invitrogen (Carlsbad, CA); CellLight Lysosomes-RFP (C10597) was purchased from Cell Signaling Technology; Src family activator (sc-3052) was purchased from Santa Cruz Biotechnology; Src kinase inhibitor Src I-1, MG132, and chloroquine were from Sigma (St. Louis, MO); TGF-β1 (100-21-10VG) was obtained from Peprotech (Rocky Hill, NJ).
REAL-TIME RT-PCR
RNA was extracted using the Total RNA Kit II (Omega, Riverside, CA) and reverse-transcribed into complementary DNA by PrimeScript RT reagent kit (TaKaRaBio, Otsu, Japan). Single-stranded complementary DNA was amplified by quantitative RT-PCR using a SYBR Premix ExTaq kit (TaKaRaBio) on the Stratagene Mx3005P Real-Time PCR System (Agilent Technologies, Germany). Primer sequences are listed in Supporting Table S1.
PATIENT TISSUE SAMPLES
All human tissues including liver cirrhosis, HCC, and adjacent tumor were obtained during routine surgical procedures at the Xijing Hospital of Fourth Military Medical University (Xi'an, China). The histopathological diagnosis was based on the World Health Organization criteria by hematoxylin and eosin staining. Histological grade of tumor differentiation was determined according to the classification proposed by Edmondson and Steiner.15 Ethical approval was obtained from the Ethics Committee of the Fourth Military Medical University, and informed consent was obtained from each patient.
MOUSE MODELS
Diethylnitrosamine/phenobarbital (DEN/PB)-induced HCC and hepatocyte-specific CD147 knockout (KO) mouse models were established as described.11, 16
STATISTICAL ANALYSIS
Data are expressed as mean ± SD and were obtained from three independent experiments, using GraphPad Prism v5.0 software (GraphPad Software, La Jolla, CA) and SPSS 23.0 (SPSS, Inc., Chicago, IL). One-way analysis of variance and t tests were performed to compare mean values. Pearson's correlation test was used to assess the correlation of CD147 mRNA levels in HCC tissues with other molecules. Spearman's correlation was used to analyze the correlation between CD147 and Par3 expression by immunohistochemistry. Tests for association between CD147-polarized localization and clinical pathologic variables were computed using the χ2 test or Fisher's exact test. Overall survival was calculated using Kaplan-Meier analysis and the log-rank test. P < 0.05 was considered significant.
Additional materials and methods are included in the Supporting Information.
Results
TGF-β1 PROMOTES HEPATOCYTE POLARITY LOSS
EMT, which could be induced by TGF-β1, is typically characterized by the loss of cell–cell adhesion and apical–basal cell polarity, as well as the increased motility of cells.17 To observe the change of hepatocyte polarity, we firstly investigated the cellular response to TGF-β1 stimulation in HepG2 cells. Compared with the control group, the cell–cell adhesion gradually weakened and the TGF-β1-treated cells gradually became discrete with time (Supporting Fig. S1A). Under the stimulation of TGF-β1 for 96 hours, immunofluorescence of MRP2 showed that the hepatocyte polarity index was significantly decreased (P < 0.001; Supporting Fig. S1B). These results indicated that TGF-β1 decreases hepatocyte polarity. Surprisingly, not only was the expression of CD147 increased but also the cellular localization of CD147 was altered with TGF-β1 treatment (Supporting Fig. S1C,D). Then we explored the expression of CD147 and polarity-related genes in response to TGF-β1 stimulation in HepG2 and Huh-7 cell lines. The mRNA and protein levels of CD147 were up-regulated under TGF-β1 stimulation. Concurrently, E-cadherin and Par3 expression levels were down-regulated (Supporting Fig. S1E,F).
CD147 IS BASOLATERALLY POLARIZED IN HEPATOCYTES
With DEN/PB-induced HCC progression, CD147 was up-regulated in liver tumors compared to normal liver (M0).16 Interestingly, immunohistochemical analysis showed that CD147 had a polarized localization on hepatocytes at the initial phase (M1–M4), then changed to nonpolarization with the progress of HCC (M5–M12) (Fig. 1A). It has been reported that CD147 has a basolateral targeting signal (i.e., a single leucine) of the cytoplasmic tail recognized by kidney cell lines.13 CD147 colocalized with the basolateral marker Na+/taurocholate cotransporting polypeptide but not with the apical marker MRP2 or F-actin in mouse liver tumor tissues and HepG2 cells, demonstrating that CD147 was basolaterally polarized in hepatocyte membrane (Fig. 1B). We further proved that CD147 colocalized with adherens junction markers E-cadherin and β-catenin but not with tight junction markers ZO-1 and occludin (Fig. 1C). Immunofluorescence analysis showed that in HepG2 spheroids the apical protein marker MRP2 and the basolateral protein marker integrin β1 were localized to the distinct regions of the cell membrane with no colocalization, indicating that the HepG2 spheroids have adopted a form of polarization. Then we confirmed that CD147 colocalized with integrin β1 but not with MRP2 and F-actin, proving that CD147 indeed was localized in the basolateral membrane of hepatocytes (Fig. 1D). As we have reported, the positive loop of TGF-β1–CD147 promoted EMT and HCC progression.9, 11 With TGF-β1 treatment, the hepatocyte polarity index notably decreased in CD147-overexpressing cells (HepG2–CD147) compared with that in HepG2 cells (P < 0.01; Fig. 1E). Likewise, western blotting showed that CD147 overexpression promoted the TGF-β1-induced decrease of E-cadherin and Par3 (Fig. 1F). The results collectively demonstrated that CD147 accelerates the TGF-β1-induced hepatocyte polarity loss.
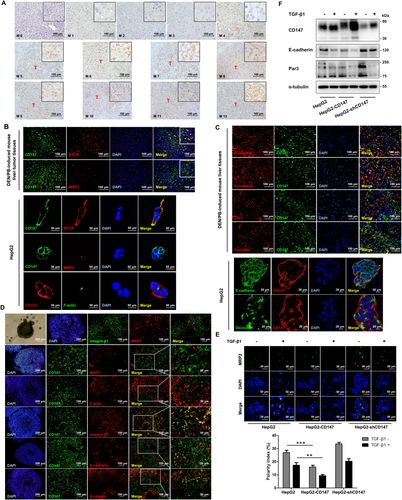
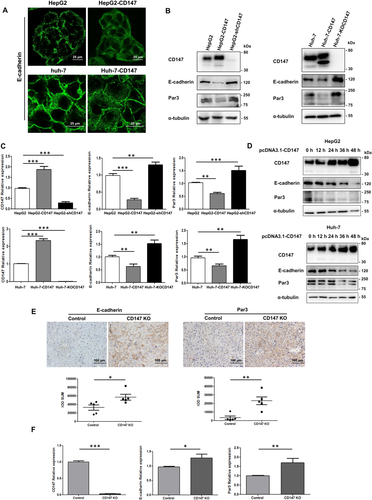
OVEREXPRESSION OF CD147 DECLINES E-CADHERIN AND Par3 LEVELS
In Fig. 1E, the immunofluorescence of MRP2 also showed that CD147 overexpression could directly decrease the polarity index (P < 0.001). To elucidate the molecular mechanisms of CD147 in promoting hepatocyte polarity loss, we constructed three stable cell lines with the properties of CD147 overexpression, knockdown, and knockout. Immunofluorescence showed an obvious endocytosis of E-cadherin in CD147 overexpression cells (Fig. 2A). Western blotting and RT-PCR analysis revealed that CD147 overexpression allowed an attenuation of E-cadherin and Par3 expression. Both CD147 knockdown and CD147 KO up-regulated E-cadherin and Par3 levels (Fig. 2B,C). Using a transient transfection system in which CD147 overexpression gradually increased with time, E-cadherin and Par3 expression was observed to decrease accordingly (Fig. 2D). Next, five pairs of hepatocyte-specific CD147 KO mice and control littermates were used to confirm these results. With DEN/PB induction, the incidence and number of liver tumor nodules in hepatocyte-specific CD147 KO mice are delayed compared to control mice, and we demonstrate that CD147 KO in hepatocytes strongly suppresses HCC progression.11 Immunohistochemical analysis of liver tumor tissues showed that CD147 KO suppressed the decrease of E-cadherin and Par3 (Fig. 2E). Furthermore, mRNA analysis revealed the same results (Fig. 2F). Taken together, CD147 overexpression down-regulates E-cadherin and Par3 expression, indicating a contribution to the disorder of hepatocyte polarity.
CD147 RECRUITS HAKAI AND UBIQUITINATES E-CADHERIN FOR LYSOSOMAL DEGRADATION
It has been reported that E-cadherin ubiquitination degradation, mediated by E3 ubiquitin ligase Hakai, is a unique mechanism for destruction of cell–cell adhesion in EMT.18, 19 Because CD147 has been proven to induce EMT, we wondered whether CD147 decreased the level of E-cadherin also by the ubiquitination pathway, thus inducing the loss of polarity. Firstly, we transiently expressed hemagglutinin-ubiquitin in HepG2 and Huh-7 cells. Compared with vector control cells, CD147 overexpression enhanced the association of E-cadherin with Hakai and ubiquitin (Fig. 3A). To elucidate the E-cadherin degradation pathway in CD147-overexpressing cells, next we used MG132 (an inhibitor of proteasomal degradation) and chloroquine (an inhibitor of lysosomal degradation). Western blotting showed that chloroquine treatment, but not MG132, prevented E-cadherin decrease and ubiquitination (Fig. 3B). Furthermore, immunofluorescent staining showed that the distribution of E-cadherin was limited on the plasma membrane and the colocalization of E-cadherin with the lysosome marker Lysosomes-RFP was attenuated with chloroquine treatment (Fig. 3C). Taken together, CD147 overexpression enhances E-cadherin ubiquitination by recruiting Hakai for lysosomal degradation.
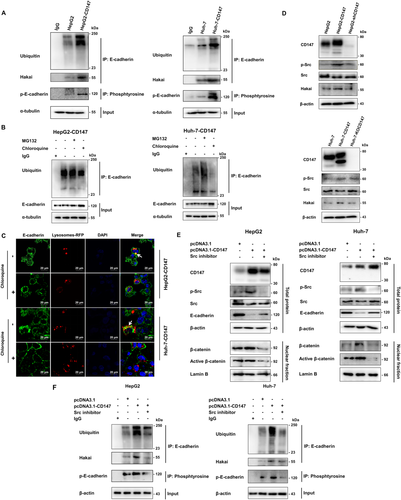
CD147 PROMOTES Src ACTIVATION THAT REGULATES E-CADHERIN UBIQUITINATION DEGRADATION
Overexpression of CD147 enhances pY416-Src expression in HepG2 and Huh-7 cells20 and leads to the translocation of β-catenin into the nucleus.11 Moreover, several studies have indicated that Src kinase phosphorylates the E-cadherin cytoplasmic domain, followed by stabilization of the binding of Hakai to E-cadherin for its subsequent endocytosis and degradation.21, 22 Under Src activation, E-cadherin expression was down-regulated in both HCC cell lines (Supporting Fig. S2). Moreover, CD147 overexpression promoted Src phosphorylation and Hakai expression (Fig. 3D). Based on these results, we made an assumption that CD147 overexpression activated Src and induced E-cadherin tyrosine phosphorylation, then enhanced Hakai recruitment for E-cadherin ubiquitination and β-catenin translocation into the nucleus. Importantly, our results demonstrated that E-cadherin expression was markedly enhanced and β-catenin expression of the nucleus was suppressed in CD147-overexpressing cells by Src inhibition (Fig. 3E). Notably, the activation of β-catenin and the down-regulation of E-cadherin caused by CD147 overexpression required Src activation. The tyrosine phosphorylation of E-cadherin increased in the CD147-overexpressing cells; however, it was greatly attenuated by Src inactivation (Fig. 3A,F). Next, we found that the E-cadherin ubiquitination and the association of E-cadherin and Hakai were both significantly decreased with Src inhibitor treatment in CD147-overexpressing cells (Fig. 3F), implying that E-cadherin phosphorylation induced by Src activation promotes its interaction with Hakai for ubiquitination. Overall, Src activation is involved in the regulation of β-catenin activity and E-cadherin expression, which is mediated by CD147.
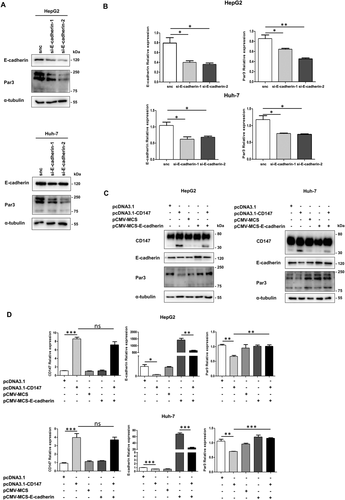
CD147 DECREASES Par3 LEVEL THROUGH E-CADHERIN DOWN-REGULATION
Then we explored the relation between E-cadherin and Par3. By silencing E-cadherin expression with small interfering RNA in HepG2 and Huh-7 cells, the protein and mRNA expression of Par3 was significantly reduced (Fig. 4A,B). We next wondered whether CD147 down-regulated Par3 expression by E-cadherin reduction. Compared with CD147-overexpressing cells, the decline of Par3 was destroyed by E-cadherin overexpression, as demonstrated by western blotting (Fig. 4C) and RT-PCR (Fig. 4D). The results suggested that the CD147-mediated decrease of the Par3 level is through E-cadherin.
INVOLVEMENT OF INTEGRIN α5β1 IN CD147-INDUCED E-CADHERIN AND Par3 DOWN-REGULATION
Integrins are the major ECM receptor family that consists of 18 α-subunits and 8 β-subunits in mammals. Most integrin ligands are known to be components of the ECM including collagen, fibronectin, and laminin.23 The fibronectin receptor integrin α5β1 primarily binds to fibronectin to anchor cells to maintain epithelial polarity and mediate downstream signaling.24 The Arg-Gly-Asp (RGD) motif in the central cell-binding domain of fibronectin was an important site of cell recognition for integrins through the metal ion–dependent adhesion site of the integrin β1 subunit. We previously demonstrated that the interaction of CD147 with the integrin β1 metal ion–dependent adhesion site motif activates the downstream signaling pathway, subsequently enhancing the malignant properties of HCC cells, and that the RGD motif can inhibit this interaction.25 In order to clarify which upstream integrin molecule is responsible for CD147-mediated signaling pathway activation, we analyzed all of the integrin subunits and CD147 mRNA expression in HCC tissues compared with adjacent tissues and their correlation using a public microarray data set (GSE22058). The mRNA levels of CD147 and integrins α2, α5, α6, α7, α11, αE, αV, β1, β4, and β5 in tumor tissues were significantly higher than those of adjacent tissues, whereas the levels of integrins α1, α4, α9, αD, α2B, β2, and β8 in tumor tissues were lower (Supporting Fig. S3). In HCC tissues, CD147 mRNA levels were positively correlated with integrins α2 (r = 0.2079, P = 0.0421) and α5 (r = 0.2388, P = 0.0191; Supporting Fig. S4). We then focused on the roles of integrins α5 and β1. Coated fibronectin enhanced expression of both E-cadherin and Par3, which could be reduced by CD147 overexpression (Fig. 5A). However, after adding RGD peptide, the decline of E-cadherin and Par3 levels in CD147-overexpressing cells was reversed (Fig. 5B). The results suggested that the interaction between CD147 and integrin β1 interrupts the interaction between fibronectin and integrin β1, which perturbs the ECM homeostasis. Western blotting analysis showed that blocking integrins α5 and β1 with specific antibodies resulted in restoration of E-cadherin and Par3 levels (Fig. 5C). Taken together, the ectopic expression of CD147 competitively binds with integrin α5β1, thus activating Src and subsequently inducing E-cadherin ubiquitination degradation, leading to a Par3 decrease (Fig. 5D).
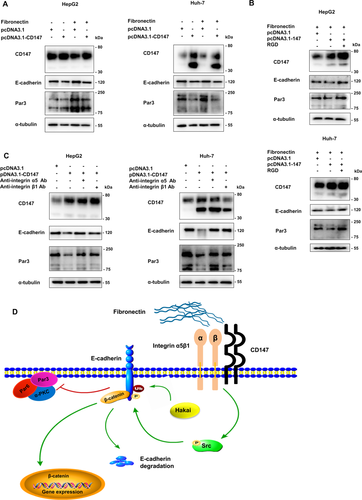
ASSOCIATION OF CD147 WITH E-CADHERIN AND Par3 EXPRESSION IN HUMAN HCC TISSUES
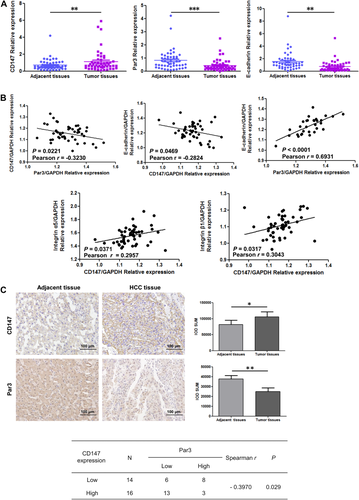
To confirm the relation among these genes in HCC, we analyzed the mRNA expression in 50 pairs of clinical samples, HCC and paired adjacent tissues. The expression of Par3 and E-cadherin was demonstrated to be lower; CD147 was higher in human HCC specimens than adjacent noncancerous specimens by RT-PCR (Fig. 6A). Moreover, data analysis of HCC tissues showed significant negative correlations between CD147 and Par3 (r = –0.3230, P = 0.0221) and between CD147 and E-cadherin (r = –0.2824, P = 0.0469) and positive correlations between CD147 and integrin α5 (r = 0.2957, P = 0.0371) and between CD147 and integrin β1 (r = 0.3043, P = 0.0317) (Fig. 6B). Simultaneously, E-cadherin was positively associated with Par3 mRNA levels in human HCC (r = 0.6931, P < 0.0001). To further verify the association between CD147 and Par3 expression, immunohistochemical analysis of 30 cases of HCC tissues was performed and indicated that CD147 expression was negatively associated with Par3 expression (r = –0.3970, P = 0.029; Fig. 6C).
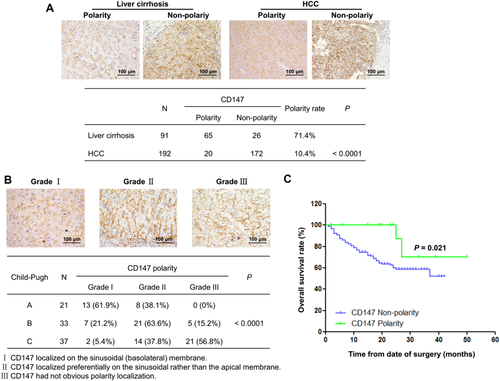
CLINICAL SIGNIFICANCE OF CD147-POLARIZED LOCALIZATION IN HUMAN LIVER CIRRHOSIS AND HCC PROGRESSION
To explore the major role of CD147-polarized localization, we collected 91 human liver cirrhosis and 192 HCC tissues with CD147-positive expression, which were analyzed by immunohistochemistry. The detailed clinical pathological characteristics of all HCC patients in the study cohort are listed in Supporting Table S2. According to CD147 localization patterns, patients were divided into two groups: CD147 polarity and CD147 nonpolarity. Statistical analysis showed that CD147 polarity rates were 71.4% in cirrhosis and 10.4% in HCC, indicating that the polarity rate of CD147 in HCC was significantly lower than that in liver cirrhosis (P < 0.0001; Fig. 7A). Then the 91 patients with liver cirrhosis were classified into categories A, B, and C by the Child-Pugh grade. Meanwhile, the CD147 distribution patterns were stratified into grades I, II, and III, with grade I representing the best CD147-polarized localization. The CD147 polarity rates stratified with polarity grades negatively correlated with Child-Pugh grade in human liver cirrhosis (r = –0.6092, P < 0.0001) (Fig. 7B). Moreover, we found a positive relation between CD147-polarized localization and tumor differentiation grade in 192 HCC cases (r = 0.2060, P = 0.004) (Table 1). Overall survival analysis showed that HCC patients with CD147-polarized localization had significantly better survival than patients with CD147 nonpolarity (P = 0.021) (Fig. 7C). Thus, CD147-polarized localization could serve as a good prognostic marker for HCC patients.
| CD147 | n | HCC Differentiation Grade | P | |||
|---|---|---|---|---|---|---|
| High | Moderate | Poor | Undifferentiation | |||
| Polarity | 20 | 1 (5%) | 14 (70%) | 5 (25%) | 0 (0%) | 0.004 |
| Nonpolarity | 172 | 12 (7%) | 53 (30.8%) | 105 (61%) | 2 (1.2%) | |
Discussion
It has been reported that the asymmetric distribution of proteins and lipids to distinct membrane domains maintains the apical–basal polarity of hepatocytes. Two intercellular adhesion complexes, known as adherens junctions and tight junctions, contribute to the formation and the maintenance of apical and basolateral domains. Formation of the adherens junctions leads to assembly of the tight junction. Alterations of adhesion and polarity complex proteins which impaired intercellular junctions are associated with depolarization, loss of differentiated characteristics, enhanced epithelial cell proliferation, and the acquisition of invasive potential. It is reported that the deficiency of liver serine palmitoyltransferase activity in early life interrupts the adherens junctions and promotes tumorigenesis.26 In this study, we found that the ectopic CD147-polarized distribution on adherens junctions disrupts hepatocyte polarity homeostasis and associates with the progress of liver disease.
The constituents of adherens junctions are based on the E-cadherin and β-catenin complex, which connects the actin cytoskeleton of neighboring cells. Tumor progression is often associated with the loss of E-cadherin function and the transition to a more motile and invasive phenotype. A meta-analysis discloses that reduced E-cadherin expression indicates a poor prognosis for patients with HCC.27 Here, we demonstrated that the E-cadherin level decreased in HCC tumor tissues compared with paired adjacent tissues (Fig. 6A), which was in accordance with the analysis of microarray data set GSE22058 (Supporting Fig. S3). Our previous study showed that CD147 down-regulated E-cadherin expression and that CD147 expression was negatively related to E-cadherin in human HCC tissues.9 However, it is not fully understood how CD147 negatively regulates E-cadherin. In epithelial cells, Hakai interacts with the Src kinase phosphorylated E-cadherin, inducing ubiquitination and endocytosis of the E-cadherin.22, 28 One of the key findings in this study is that CD147 overexpression induces endocytosis of E-cadherin (Fig. 2A) and mediates the degradation of ubiquitinated E-cadherin through the lysosomal pathway (Fig. 3). The E-cadherin/β-catenin complex at the cell membrane suppresses the motility and invasiveness of tumor cells. Disruption of adherens junctions can lead to the nuclear translocation and increased transcriptional activity of β-catenin.29 Our research demonstrated that CD147 overexpression stimulated Src activation, which also increased β-catenin nuclear translocation (Fig. 3E). That is, we provide evidence that CD147 is elevated by stimuli (e.g., TGF-β1) and will localize on the basolateral domain around adherens junctions, disrupting the E-cadherin/β-catenin complex, resulting in damage to the adherens junctions and the promotion of EMT.
A conserved group of core polarity proteins assemble into complexes to control and maintain cell polarity, consisting of Crumbs complex (Crumbs–Pals1–Patj), Par complex (Par3–Par6–aPKC), and Scribble complex (Scribble–Lgl–Dlg). Par3 provides anchorage to assemble the Par complex at the apical–lateral border, by binding Par6 and recruiting Par6-associated proteins and interacts with Tiam1 for tight junction formation.30 The previous research found that Par3 expression in human HCC tissues is down-regulated,31 which is similar to our observation (Fig. 6A,C). Par3 depletion or down-regulation drives the progression of esophageal squamous cell carcinoma.32 Loss of Par3 inhibits E-cadherin junction stability and decreases cell–cell cohesion to promote breast invasion and metastasis.33 The Par3 loss deregulates Rac1 activity to activate Jun N-terminal kinase–dependent proliferation and tumor growth in mammary tumors.34 Very little is known about the pathways that regulate loss of Par3 in carcinoma. In this study, another important finding is that we identified an upstream regulation of E-cadherin for Par3 expression in HCC. E-cadherin down-regulation directly reduced Par3 expression, which could be mediated by CD147 (Fig. 4). Epithelial cells orient their basal surface through the adhesion of integrin receptors to the ECM, and they extend membrane protrusions to contact neighboring cells, thus allowing the formation of cohesive layers of cells.35 The integrin receptors for components of the ECM, including integrin α1β1 (receptor of laminin and collagen), integrin α5β1 (receptor of fibronectin), and integrin α9β1 (receptor of tenascin), are expressed on the surfaces of hepatocytes in order to maintain normal cell-matrix communication. Once ECM deposition or integrin expression pattern change leads to the destruction of integrin–ECM, HCC is eventually promoted.23 Our previous studies found that CD147 was associated with integrins α3β1 and α6β1 and activated focal adhesion kinase–phosphoinositide 3-kinase–Ca2+ and focal adhesion kinase–paxillin signaling pathways to enhance tumor invasion and metastasis.36, 37 CD147 modulates cell–cell adhesion, cell–matrix adhesion, and migration by interaction with integrins, suggesting that CD147 and integrin participated in cell polarity. There is a considerable body of evidence that links signals downstream of integrin–ECM adhesions to the regulation of E-cadherin-mediated adhesions.38 TMPRSS4 induced EMT and invasion by activation integrin α5 signaling.39 CCN2 enhanced keratinocyte adhesion and migration through integrin α5β1.40 In this study, we demonstrated that CD147 has a positive correlation with integrin α5β1, which provides an occasion of interaction (Fig. 5B). The hetero-interaction between CD147 and integrin β1 competitively repressed the fibronectin–integrin β1 interaction and active downstream signaling to down-regulate E-cadherin and Par3 expression (Fig. 5). Therefore, basolaterally ectopic overexpression of CD147 disrupts the association between integrin α5β1 and fibronectin, resulting in the loss of cell steady state to promote HCC progress.
Several attempts, targeting cell polarity and adhesion components, have been made at the experimental level with interesting and promising results. Par6 protein appears to be a promising target in both early and late stages of breast cancer.41 aPKC is a new prognostic indicator and has been proposed as a novel therapeutic target for the treatment of gastric cancer.42 Restoring E-cadherin expression contributes to enhance the antitumoral effect of thymidine kinase/ganciclovir cancer gene therapy in pancreatic cancer models.43 We previously generated an antibody targeting CD147, named HAb18/metuximab,44 and a chimeric human–murine immunoglobulin G1 antibody cHAb18.45 The radioisotope 131I-labeled metuximab F(ab′)2 (trade name Licartin) was approved by the Chinese Food and Drug Administration for liver cancer treatment (approval no. S20060064). HAb18/metuximab, chimeric cHAb18, and Licartin significantly decreased the tumor metastasis of HCC tumor-bearing mice.45, 46 In a series of clinical trials, the combination of Licartin with liver transplantation,47 transcatheter arterial chemoembolization,48, 49 or radiofrequency ablation50 showed an improved clinical efficacy in HCC therapy. In this study, we elucidated that CD147-induced E-cadherin degradation mediates hepatocyte polarity loss, which demonstrates an important mechanism of HCC development and might facilitate our understanding of clinical CD147-targeted HCC therapy by restoring E-cadherin expression and reversing hepatocyte depolarization.
In summary, CD147 basolateral overexpression disrupted hepatocyte polarity to enhance HCC progress through (1) the destruction of ECM–integrin steady state, (2) promoting the endocytosis and down-regulation of adhesion molecular E-cadherin and the nuclear translocation of β-catenin, (3) the reduction of polarized protein Par3. Pharmaceutical intervention of CD147 and its related intracellular polarity pathway may provide a promising strategy to maintain hepatocyte polarity, thus preventing HCC development.
REFERENCES
Author names in bold designate shared co-first authorship.




