Serologic responses and effectiveness of hepatitis A vaccination among human immunodeficiency virus–positive individuals during the outbreak of acute hepatitis A
Potential conflict of interest: Nothing to report.
Preliminary analyses of these data were presented as Abstract 582 at the 22nd Conference on Retroviruses and Opportunistic Infections, Seattle, WA, February 13-16, 2017.
Abstract
Outbreaks of hepatitis A virus (HAV) infection have been occurring among men who have sex with men in the Asia-Pacific region, the United States, and several European countries since June 2015 and recently among persons who are homeless and use illicit drugs in the United States. We evaluated the serologic responses and effectiveness of HAV vaccination in human immunodeficiency virus (HIV)–positive individuals during the outbreak in Taiwan. From June 1, 2015, to September 30, 2016, anti-HAV immunoglobulin G was prospectively determined among all HIV-positive individuals. We prospectively observed 1,533 HAV-seronegative, HIV-positive individuals (94.1% being men who have sex with men with a median cluster of differentiation 4 (CD4) count of 550 cells/μL) who were advised to receive two doses of HAV vaccine administered 6 months apart. Of them, 1,001 individuals (65.3%) received at least one dose of HAV vaccine during the study period and 532 (34.7%) declined to receive vaccine. The primary endpoints were serologic response at weeks 28-36 and acquisition of HAV infection during follow-up. The incidence rate of acute HAV infection was 3.7 and 99.3 per 1,000 person-years of follow-up in the vaccinated and unvaccinated groups, respectively, resulting in a vaccine effectiveness of 96.3%. At weeks 28-36, the seroconversion rates were 63.8% and 93.7% in the intention-to-treat and per-protocol analyses, respectively. The factors associated with seroconversion at weeks 28-36 were younger age (per 1-year decrease, adjusted odds ratio, 1.08; 95% confidence interval, 1.02-1.12) and undetectable plasma HIV RNA load (adjusted odds ratio, 3.19; 95% confidence interval, 1.32-7.68). Conclusion: During the outbreak of acute hepatitis A, two-dose HAV vaccination is effective at preventing HAV infection among HIV-positive individuals receiving combination antiretroviral therapy; our data highlight the importance of HAV serologic screening and vaccination to prevent outbreaks of acute hepatitis A in at-risk populations. (Hepatology 2018;68:22-31).
Abbreviations
-
- AHR
-
- adjusted hazard ratio
-
- AOR
-
- adjusted odds ratio
-
- cART
-
- combination antiretroviral therapy
-
- CD4
-
- cluster of differentiation 4
-
- CI
-
- confidence interval
-
- HAV
-
- hepatitis A virus
-
- HBV
-
- hepatitis B virus
-
- HCV
-
- hepatitis C virus
-
- HIV
-
- human immunodeficiency virus
-
- HR
-
- hazard ratio
-
- IDU
-
- injecting drug user
-
- IgG
-
- immunoglobulin G
-
- ITT
-
- intention-to-treat
-
- LOCF
-
- last observation carried forward
-
- MSM
-
- men who have sex with men
-
- PP
-
- per-protocol
-
- PVL
-
- plasma HIV RNA load
Hepatitis A virus (HAV) is transmitted fecally–orally and has been one of the major etiologies of foodborne diseases, accounting for 13.7 million illnesses and 28,000 deaths in 2010 worldwide.1 Effective prevention strategies, including improvement of sanitation and provision of HAV vaccination, have shifted several countries from high HAV endemicity to low endemicity.2, 3 However, any introduction of HAV into susceptible populations may potentially cause outbreaks, especially in those with risky behaviors. While most outbreaks were traced to contaminated foods, several outbreaks have been reported among men who have sex with men (MSM) and injecting drug users (IDUs) who were engaged in oral–anal sex and use of contaminated illicit drugs, respectively.4 Outbreaks of acute hepatitis A among those at-risk populations have been described since the 1980s and have reemerged across the Asia-Pacific region, the United States, and several European countries since 2015.4-7 In Taiwan, an unprecedented outbreak with more than 1,000 indigenous cases of acute hepatitis A occurred in 2015-2016, which was characterized by a high proportion of MSM and human immunodeficiency virus (HIV)–positive individuals.8-10 Furthermore, phylogenetic analysis demonstrated that the outbreak strains in Taiwan and The Netherlands were identical, suggesting that HAV might have been transmitted through sexual networks and international travel.11
HAV vaccination is recommended for those with increased risks of exposure, such as MSM, IDUs, and travelers.12, 13 In response to the outbreaks, HAV vaccination campaigns targeting MSM and IDUs had been launched to curb the epidemic of acute hepatitis A in epidemic areas.4 Following HAV vaccination, >95% of individuals seroconverted and 90% or more reduction in clinical cases of acute hepatitis was observed.14, 15 Albeit highly immunogenic and effective in the general population, suboptimal serologic response to HAV vaccination in moderately to severely immunodeficient HIV-positive individuals has been shown in several studies. Notably, a meta-analysis estimated an overall serologic response rate of 64% to HAV vaccination among HIV-positive individuals.16 The findings were confirmed in clinical trials. Moreover, antibody levels apparently waned over time among HIV-positive individuals.17-19 Although the immunogenicity of HAV vaccination has been explored in HIV-positive individuals, the clinical effectiveness of HAV vaccination in those patients has rarely been evaluated in the setting of ongoing outbreaks.4 In this prospective cohort study, we investigated the serologic responses and vaccine effectiveness of HAV vaccination and elucidated the associated factors with seroprotection among HIV-positive individuals during a large outbreak of acute hepatitis A in Taiwan.
Participants and Methods
SETTING AND STUDY POPULATION
The outbreak of acute hepatitis A has been occurring since June 2015 in Taiwan, with a total of 1,440 indigenous cases reported as of September 30, 2017, and >50% of the patients receiving medical care in northern Taiwan.20 Up to 70% and 60% of the case patients were MSM and HIV-positive individuals, respectively. A campaign of two-dose HAV vaccination was launched at designated hospitals for HIV care since September 2015; thereafter, the Taiwan Centers for Disease Control implemented a subsidized single-dose HAV vaccination program to individuals aged 40 years or less who were HIV-positive or newly diagnosed with syphilis or gonorrhea on October 1, 2016.8, 9
This prospective cohort study was conducted at a university hospital in metropolitan Taipei. During the outbreak period, all of the HIV-positive individuals were screened for anti-HAV immunoglobulin G (IgG), and those testing negative for anti-HAV antibody were advised to receive two doses of HAV vaccine, administered 6 months apart.
From June 1, 2015, to September 30, 2016, HIV-positive adults aged at least 19 years who tested negative for anti-HAV IgG were included in this study. The study period was selected based on the date when the outbreak began and before the Taiwan Centers for Disease Control started to implement the one-dose free-vaccine subsidy program. Patients were excluded from the study if they had undergone primary HAV vaccination before June 2015 or had the signs or symptoms of acute hepatitis A (fever, nausea, vomiting, anorexia, diarrhea, malaise, upper abdominal pain, and jaundice) at baseline. Because determination of HAV antibody and provision of HAV vaccination were part of the public health response to the ongoing outbreak of acute hepatitis A, the study was not considered as research requiring approval by the Research Ethics Committee. Written informed consent was waived.
HAV VACCINATION
HAV-seronegative, HIV-positive adults were advised to undergo two-dose HAV vaccination at their own expense during the study period. However, the limited access to HAV vaccines in nonendemic countries led to vaccine shortages during outbreaks.5, 21, 22 Delivery of two-dose HAV vaccines was separated by at least 6 months and used HAVRIX 1440 enzyme-linked immunosorbent assay units (GlaxoSmithKline, Biologicals, Rixensart, Belgium), which was substituted by VAQTA 50 units (Merck and Co., Inc., West Point, PA) since May 19, 2016, due to a shortage of HAVRIX. After vaccination, follow-up of anti-HAV IgG was determined between 4 and 24 weeks; afterward, the second dose of HAV vaccine was administered, followed by the determinations of anti-HAV IgG at weeks 28-36 and 48. The vaccinees were followed from baseline until occurrence of incident acute hepatitis A, death, loss to follow-up, or the end of this study on September 30, 2017, whichever occurred first. The unvaccinated group included patients declining to receive HAV vaccine from June 2015 to September 2016, and follow-up of anti-HAV IgG was determined at weeks 24 and 48 to identify cases of incident asymptomatic HAV infection. The nonvaccinees were followed from baseline until occurrence of incident acute hepatitis A or asymptomatic HAV infection, death, loss to follow-up, receipt of the free-of-charge HAV vaccine provided by the Taiwan Centers for Disease Control after October 1, 2016, or the end of this study on September 30, 2017, whichever occurred first.
In accordance with the national HIV treatment guidelines, HIV-positive individuals returned for assessment of virological, immunological, and clinical status every 3-6 months.8 HIV-related medical services have been provided free of charge at the designated hospitals, including combination antiretroviral therapy (cART), monitoring of CD4 cell count and plasma HIV RNA load (PVL), and serologies of HAV, hepatitis B virus (HBV), hepatitis C virus (HCV), and syphilis at baseline and during follow-up.
OUTCOMES
The primary endpoints were serologic response at weeks 28-36 of HAV vaccination and acquisition of HAV infection during the follow-up. Acquisition of HAV infection included acute hepatitis A and asymptomatic HAV infection. Acute hepatitis A, defined as the presence of clinical symptoms, elevated aminotransferases or jaundice, and positive anti-HAV immunoglobulin M, was a nationally notifiable disease. Asymptomatic HAV infection was defined as seroconversion to anti-HAV IgG positivity without clinical signs and symptoms prior to HAV vaccination. The history of HAV vaccination was inquired and verified in the electronic medical records in all individuals with asymptomatic HAV infection to exclude vaccine-induced immunity. The secondary endpoint was serologic response at week 48 of HAV vaccination. In the vaccinated group, the serologic response was seroconversion to anti-HAV IgG positivity after HAV vaccination and estimated in the intention-to-treat (ITT) analysis with missing-equals-nonresponse. Sensitivity analyses were performed with the ITT analysis using the last observation carried forward (LOCF) approach and per-protocol (PP) analysis. The LOCF method imputed missing data on anti-HAV IgG follow-up at weeks 28-36 and 48 by the test results after week 24 of vaccination. In 554 HAV-seronegative, HIV-positive patients who received the first dose of HAV vaccine and had both determinations of anti-HAV antibody titers 4-12 weeks after vaccination and follow-up of anti-HAV antibody titers immediately before the administration of the second dose, the rate of seroconverted vaccinees with waning immunity before administration of the second dose of HAV vaccine was low (1.3%, 7/554); therefore, the ITT analysis using the LOCF approach was appropriate for estimating the serologic response.
LABORATORY INVESTIGATIONS
Serum anti-HAV IgG was determined with the use of a chemiluminescence immunoassay (ARCHITECT HAVAb-IgG; Abbott Diagnostics, Wiesbaden, Germany). In patients with clinical manifestations of acute hepatitis A, anti-HAV immunoglobulin M was determined with the use of a chemiluminescence immunoassay (ARCHITECT HAVAb-IgM; Abbott Diagnostics). HBV surface antigen and HCV antibody were determined at baseline and annually using an enzyme immunoassay (Abbott Laboratories, Abbott Park, IL). CD4 count and PVL were quantified by flow cytometry (BD FACS Calibur; Becton Dickinson, CA) and the Cobas AmpliPrep/Cobas TaqMan HIV-1 test (version 2.0; Roche Molecular Systems) with a lower detection limit of 20 copies/mL, respectively. Serologic tests for syphilis were performed using the rapid plasma reagin test (BD Macro-VueTMRPR Card tests) and the Treponema pallidum particle agglutination test (FTI-SERODIA-TPPA; Fujirebio Taiwan Inc., Taoyuan, Taiwan), and rapid plasma reagin titer was followed every 3-6 months. Coinfection with syphilis was regarded as a surrogate marker for risky sexual behavior.23 Patients were diagnosed as having syphilis if they developed clinical manifestations of primary and secondary syphilis or a 4-fold increase in a rapid plasma reagin titer with a reactive T. pallidum particle agglutination assay.24
STATISTICAL ANALYSIS
We assessed clinical characteristics across groups by descriptive statistics. Categorical variables were analyzed using Fisher's exact test or the chi-squared test. Continuous variables were compared using the Wilcoxon-Mann-Whitney test. The incidences of acute HAV infection in the vaccinated and unvaccinated groups were estimated with the Kaplan-Meier method. To avoid bias introduced by attributing the period waiting to receive vaccination to the vaccinated group rather than the unvaccinated group, undergoing vaccination was evaluated as a time-dependent variable to allow for the change over time in each individual. Cox regression analysis with the time-dependent covariate was applied for elucidating the predictors of acquiring HAV infection.25 Vaccine effectiveness was measured by the percentage reduction in the risk of acquiring HAV infection among the vaccinated group relative to the unvaccinated group. A logistic regression model was used to determine the associations between serologic response and predictor variables, with adjustments made for time-updated variables, such as CD4 count and PVL. Ninety-five percent confidence intervals (CIs) of odds ratios or hazard ratios (HRs) were computed to estimate the effects of each variable. All tests were two-tailed, and a P value <0.05 was considered significant. Statistical analyses were performed using STATA software version 12.0 (Stata Corporation, College Station, TX).
Results
Between June 1, 2015, and September 30, 2016, 1,629 HIV-positive individuals tested negative for anti-HAV IgG. Of these, 93 individuals having previously received HAV vaccines and three patients diagnosed with acute hepatitis A at baseline were excluded from the study. Among the 1,533 included individuals, 1,001 (65.3%) received at least one dose of HAV vaccine (vaccinated group) and 532 (34.7%) did not (unvaccinated group) (Fig. 1). As of the end of the study on September 30, 2017, 965 of 1,001 vaccinated patients (96.4%) had completed the two-dose vaccine series, and all vaccinated patients had completed 48 weeks of follow-up after vaccination.
Table 1 shows the characteristics of included individuals. They were mostly MSM (94.1%) with a median age of 34 years, and 88.0% had been receiving cART with a median baseline CD4 count of 550 cells/μL. In the vaccinated group, the median interval from HAV seronegativity to the first dose of HAV vaccination was 5 weeks (interquartile range, 1-13); at vaccination, cART coverage, median CD4 count, and the proportion of undetectable PVL had increased from 89.6% at baseline to 96.9%, from 554 to 574 cells/μL, and from 77.5% to 84.4%, respectively. Compared with the unvaccinated group, the vaccinated group tended to be older and MSM, more likely to have received cART with higher CD4 counts and proportion of undetectable PVL, and less likely to be seropositive for HCV. While about one quarter of the individuals developed syphilis during the follow-up, there were no significant differences in the percentages of chronic HBV infection and syphilis between the two groups (Table 1).
| Vaccinated (n = 1,001) | Unvaccinated (n = 532) | P | |
|---|---|---|---|
| Demographics | |||
| Age, median (IQR), years | 35 (30-41) | 33 (28-39) | <0.01 |
| Male sex, n (%) | 983 (98.2) | 511 (96.1) | 0.01 |
| MSM, n (%) | 951 (95.0) | 492 (92.5) | 0.05 |
| HBsAg positivity, n (%) | 103 (10.3) | 43 (8.1) | 0.16 |
| Anti-HCV positivity, n (%) | 61 (6.1) | 56 (10.5) | <0.01 |
| Receiving immunosuppressant,a n (%) | 6 (0.6) | 4 (0.8) | 0.72 |
| Receiving cART at baseline, n (%) | 897 (89.6) | 452 (85.0) | 0.01 |
| Receiving cART at vaccination, n (%) | 970 (96.9) | NA | — |
| Clinical parameters | |||
| CD4 count at baseline, median (IQR) | 554 (420-731) | 540 (348-727) | 0.02 |
| <200 cells/μL, n (%) | 46 (4.6) | 50 (9.4) | <0.01 |
| >350 cells/μL, n (%) | 853 (85.2) | 396 (74.4) | <0.01 |
| PVL at baseline, median (IQR) | UDb (UD-UD) | UD (UD-145) | <0.01 |
| <20 copies/mL, n (%) | 776 (77.5) | 363 (68.2) | <0.01 |
| >5 log10 copies/mL, n (%) | 40 (4.0) | 32 (6.0) | 0.08 |
| CD4 count at vaccination, median (IQR) | 574 (442-748) | NA | — |
| <200 cells/μL, n (%) | 26 (2.6) | NA | — |
| >350 cells/μL, n (%) | 882 (88.1) | NA | — |
| PVL at vaccination, median (IQR) | UD (UD-UD) | NA | — |
| <20 copies/mL, n (%) | 845 (84.4) | NA | — |
| >5 log10 copies/mL, n (%) | 4 (0.4) | NA | — |
| Syphilis during follow-up, n (%) | 276 (27.6) | 145 (27.3) | 0.90 |
- a Included concurrent use of chemotherapy and immunomodulation agents.
- b UD, <20 copies/mL.
- Abbreviations: HBsAg, hepatitis B surface antigen; NA, not applicable; UD, undetectable.
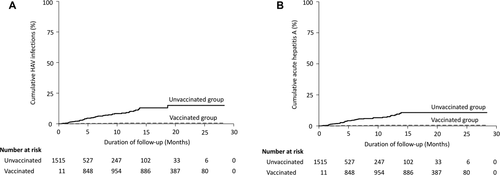
During the follow-up, 65 patients (4.2%) acquired HAV infections, 5 (7.7%) in the vaccinated group and 60 (92.3%) in the unvaccinated group. Among the 60 nonvaccinees with HAV infections, 50 (83.3%) had acute hepatitis A and 10 (16.7%) had asymptomatic seroconversion, with 7 having elevations of aminotransferases. The total follow-up duration was 1,365 and 604 person-years for the vaccinated group and unvaccinated group, respectively. The incidence rates of acute HAV infection were 3.7 per 1,000 person-years of follow-up in the vaccinated group and 99.3 per 1,000 person-years of follow-up in the unvaccinated group (HR, 0.04; 95% CI, 0.02-0.10) (Fig. 2A), while the respective incidence rates of acute hepatitis A were 3.7 and 82.8 per 1,000 person-years of follow-up (HR, 0.05; 95% CI, 0.02-0.14) (Fig. 2B). Notably, all of the five vaccinees with acute hepatitis A only received a single dose of HAV vaccine, with a median time from vaccination to incident acute hepatitis A of 3 months (interquartile range, 1-6). Two of the five vaccinees with anti-HAV IgG follow-up before acquiring acute hepatitis A did not develop seroprotective antibody after receiving a single dose of HAV vaccine. With regard to the nonvaccinees with acute hepatitis A, the median time from baseline to incident HAV infections was 4 months (interquartile range, 2-7).
The characteristics of individuals with and those without acute HAV infections are summarized in Supporting Table S1. All of the patients with acute HAV infections were MSM with a median age of 31 years, and >50% of them had concurrent syphilis. Table 2 demonstrates the results of the Cox regression analysis for factors associated with acquiring acute HAV infections and acute hepatitis A. In multivariable analysis, HIV-positive individuals who received HAV vaccine had significantly reduced risk for acquisition of HAV infection (adjusted HR [AHR], 0.04; 95% CI, 0.02-0.10) and acute hepatitis A (AHR, 0.05; 95% CI, 0.02-0.14), which resulted in a vaccine effectiveness of 96% (95% CI, 90%-98%) and 95% (95% CI, 86%-98%) for preventing acute HAV infection and acute hepatitis A, respectively. In contrast, acquiring syphilis during the follow-up was associated with occurrence of acute HAV infection (AHR, 3.87; 95% CI, 2.34-6.42) and acute hepatitis A (AHR, 4.04; 95% CI, 2.33-7.02).
| HAV Infection (Acute Hepatitis A and Asymptomatic Acute HAV Infection) | Acute Hepatitis A | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariable | Multivariable | Univariable | Multivariable | |||||
| HR (95% CI) | P | HRa (95% CI) | P | HR (95% CI) | P | HRa (95% CI) | P | |
| Exposure | ||||||||
| HAV vaccinationb | 0.04 (0.02-0.10) | <0.01 | 0.04 (0.02-0.10) | <0.01 | 0.05 (0.02-0.14) | <0.01 | 0.05 (0.02-0.14) | <0.01 |
| Baseline characteristics | ||||||||
| Age, per 1-year increase | 0.96 (0.93-0.99) | 0.02 | 0.98 (0.95-1.01) | 0.22 | 0.95 (0.92-0.99) | 0.01 | 0.97 (0.94-1.01) | 0.15 |
| MSMc | — | — | — | — | — | — | — | — |
| HBsAg positivity | 0.79 (0.32-1.97) | 0.61 | 1.11 (0.44-2.81) | 0.82 | 0.74 (0.27-2.06) | 0.57 | 1.07 (0.38-3.01) | 0.90 |
| Anti-HCV positivity | 1.32 (0.57-3.06) | 0.52 | 0.85 (0.37-1.99) | 0.71 | 1.58 (0.68-3.69) | 0.29 | 1.05 (0.44-2.46) | 0.92 |
| Receiving cART at baseline | 0.74 (0.35-1.55) | 0.42 | 2.33 (0.98-5.53) | 0.06 | 0.73 (0.33-1.62) | 0.44 | 2.16 (0.85-5.50) | 0.11 |
| CD4 count at baseline, per 10 cells/μL increase | 0.99 (0.98-1.00) | 0.10 | 0.99 (0.98-1.00) | 0.25 | 0.99 (0.98-1.00) | 0.18 | 1.00 (0.98-1.01) | 0.36 |
| Undetectable PVL at baseline | 0.49 (0.30-0.81) | 0.01 | 0.73 (0.40-1.32) | 0.30 | 0.51 (0.29-0.89) | 0.02 | 0.77 (0.40-1.47) | 0.42 |
| Syphilis during follow-up | 3.86 (2.36-6.32) | <0.01 | 3.87 (2.34-6.42) | <0.01 | 4.12 (2.40-7.07) | <0.01 | 4.04 (2.33-7.02) | <0.01 |
- a The HRs are the estimates of the effect of covariates on acquisition of HAV infection and acute hepatitis A, adjusted for HAV vaccination, age, being MSM, HBsAg positivity, anti-HCV positivity, receiving cART, CD4 count and undetectable PVL at baseline, and syphilis during follow-up using Cox regression analysis.
- b Underwent HAV vaccination during the study period.
- c All patients with HAV infection and acute hepatitis A were MSM.
- Abbreviation: HBsAg, hepatitis B surface antigen.
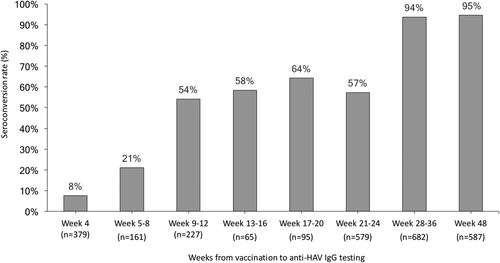
The rates of seroconversion to anti-HAV IgG positivity after vaccination with follow-up intervals are depicted in Fig. 3. Before the second dose of HAV vaccine, 1,001 vaccinees had 1,506 follow-up tests of anti-HAV IgG and the overall seroconversion rate was estimated to be 41.0% (617/1,506). In the majority of vaccinees, anti-HAV IgG was measured at weeks 9-12 and weeks 21-24, which concurred with the intervals between routine outpatient visits. The seroconversion rate was only 7.7% (29/379) at 4 weeks after the first dose of vaccination, which increased over time to 21.1% (34/161) at weeks 5-8, 54.2% (123/227) at weeks 9-12, 58.5% (38/65) at weeks 13-16, 64.2% (61/95) at weeks 17-20, and 57.3% (332/579) between week 21 and the administration of the second dose of HAV vaccine.
In the ITT analysis, the seroconversion rates following the administration of the second dose of HAV vaccine were 63.8% (639/1,001) and 55.4% (555/1,001) at weeks 28-36 and 48, respectively. In the ITT analysis using the LOCF approach, the seroconversion rates were 71.6% (717/1,001) and 88.4% (885/1,001) at weeks 28-36 and 48, respectively. In the PP analysis, the seroconversion rates further increased to 93.7% (639/682) and 94.5% (555/587) at weeks 28-36 and 48, respectively.
Table 3 shows the results of multivariable analysis, which demonstrates the association between seroconversion at weeks 28-36 with younger age (per 1-year decrease, adjusted odds ratio [AOR], 1.08; 95% CI, 1.02-1.12) and undetectable PVL at vaccination (AOR, 3.19; 95% CI, 1.32-7.68). Immunogenicity to HAV vaccination could be further improved by receiving HAV vaccine at a CD4 count >350 cells/μL compared with <350 cells/μL (AOR, 9.04; 95% CI, 4.70-17.39) and >500 cells/μL compared with <500 cells/μL (AOR, 3.76; 95% CI, 1.97-7.19).
| Univariable | Multivariable | |||
|---|---|---|---|---|
| OR (95% CI) | P | ORa (95% CI) | P | |
| Baseline characteristics | ||||
| Age, per 1-year increase | 0.94 (0.91-0.98) | <0.01 | 0.93 (0.89-0.98) | <0.01 |
| Male sexb | — | — | — | — |
| MSM | 0.45 (0.06-3.39) | 0.44 | 0.16 (0.02-1.38) | 0.10 |
| Weight, per 1-kg increase | 1.00 (0.98-1.03) | 0.76 | 1.02 (0.99-1.06) | 0.21 |
| Current smoking | 1.62 (0.76-3.45) | 0.21 | 1.63 (0.72-3.69) | 0.24 |
| HBsAg positivity | 0.38 (0.18-0.81) | 0.01 | 0.52 (0.23-1.19) | 0.12 |
| Anti-HCV positivity | 0.53 (0.18-1.57) | 0.25 | 0.47 (0.14-1.61) | 0.23 |
| Receiving immunosuppressantb | — | — | — | — |
| Receiving cART at vaccination | 0.93 (0.12-7.16) | 0.94 | 0.58 (0.06-5.74) | 0.64 |
| Nadir CD4 count, per 10 cells/μL increase | 1.05 (1.03-1.07) | <0.01 | 1.02 (0.99-1.05) | 0.18 |
| CD4 count at vaccination, per 10 cells/μL increase | 1.04 (1.03-1.06) | <0.01 | 1.02 (0.99-1.05) | 0.16 |
| Undetectable PVL at vaccination | 2.86 (1.44-5.71) | <0.01 | 3.19 (1.32-7.68) | 0.01 |
| Follow-up parameters | ||||
| Time-updated CD4 count, per 10 cells/μL increase | 1.04 (1.02-1.06) | <0.01 | 1.01 (0.98-1.04) | 0.45 |
| Undetectable PVL at each testing | 1.95 (0.87-4.38) | 0.11 | 1.41 (0.53-3.74) | 0.49 |
| Syphilis during follow-up | 0.89 (0.45-1.78) | 0.75 | 0.77 (0.35-1.69) | 0.52 |
- a The ORs are the estimates of the effect of covariates on serologic response at weeks 28-36 of vaccination, adjusted for age, sex, being MSM, weight, smoking, HBsAg positivity, anti-HCV positivity, nadir CD4 count, CD4 count and undetectable PVL at vaccination, time-updated CD4 count and undetectable PVL, and syphilis during follow-up using a logistic regression model.
- b All vaccinees without seroconversion at weeks 28-36 of HAV vaccination were male and not taking immunosuppressants.
- Abbreviations: HBsAg, hepatitis B surface antigen; OR, odds ratio.
Discussion
In this study investigating the serologic responses and clinical effectiveness of HAV vaccination during a large outbreak of acute hepatitis A among MSM, we found that the serologic response to HAV vaccination among HIV-positive individuals was delayed with a seroconversion rate of around 60% before the second dose of HAV vaccine; however, the response rate could further increase to >90% after the second dose. Despite the delayed and suboptimal serologic response, HAV vaccination was still clinically effective at preventing acute HAV infections. Improved surrogates of immune status, such as higher CD4 counts and suppressed PVL, enhanced the immunogenicity to the two-dose vaccine series.
Previous studies consistently suggested that the immunogenicity of HAV vaccination among HIV-positive individuals was impaired, even in the cART era. In a randomized controlled trial enrolling participants with a baseline median CD4 count of 355 cells/μL and undetectable PVL, the seroconversion rates on the two-dose schedule at week 28 were 69% and 72% in the ITT and PP analyses, respectively.17 In a prospective observational study including subjects undergoing two-dose HAV vaccination who had a baseline cART coverage rate of 67%, mean CD4 count of 538 cells/μL, and mean PVL of 2.5 log10 copies/mL, the seroconversion rates at week 48 were 76% and 82% in the ITT and PP analyses, respectively.18 Because improved immunologic and virologic characteristics were well-recognized factors associated with seroconversion, the immune response to HAV vaccine will be further improved when cART is currently recommended regardless of CD4 count.4, 26 Our patients had a higher baseline cART coverage rate (88%) and median CD4 count (550 cells/μL), which may have contributed to the seroconversion rate of >90% at weeks 28-36 and 48 in the PP analysis.
In contrast to a high seroconversion rate of >95% at week 4 of HAV vaccination in the general population, our study revealed the impaired capability to mount an early serologic response to HAV vaccination among HIV-positive individuals.14, 15 Anti-HAV IgG antibodies were barely measurable until 3-6 months after the first dose of HAV vaccination, and a longer time needed to develop protective immunity to HAV vaccine has also been observed in studies conducted among immunocompromised patients.17, 27 In a randomized controlled trial, HIV-positive participants had increased serologic response from 39% at week 4 to 47% at week 24 before the second dose of HAV vaccination.17 In prospective observational studies, the seroconversion rate of transplant recipients and patients with rheumatoid arthritis was only around 10% at week 4, which increased to 19%-33% at week 24 before the second dose of HAV vaccination.27
The poorer and delayed serologic response to HAV vaccination among the HIV-positive individuals raises concerns about the effectiveness of the HAV vaccine at preventing HAV infection in the population, particularly in the setting of acute hepatitis A outbreaks. The effectiveness of HAV vaccination had been shown among children in endemic countries and MSM in the outbreak setting.28, 29 Despite the periodic outbreaks of acute hepatitis A among MSM and IDUs, HIV-positive patients with acute hepatitis A were seldom reported on a large scale until this unprecedented outbreak in Taiwan.4 Furthermore, the reemerging outbreaks recently have constrained vaccine supply in several nonendemic countries. 5, 21, 22 In this study, one dose of HAV vaccine was shown to be effective at preventing acute HAV infection during an outbreak with a reduction rate of 96%. Two doses of HAV vaccine could further increase the effectiveness with a reduction rate of 100%. These findings were similar to that observed in the Israeli universal toddlers' vaccination program, which reached a 95% reduction in hepatitis A incidence and no incident cases being noted after two doses of HAV vaccine.28
While it is recommended that two doses of HAV vaccine be administered at least 6 months apart, five patients acquired acute hepatitis A after the first dose and failure to develop seroprotective HAV antibody was noted in two patients who had follow-up of serologic response.12, 13 Our findings suggest the need for modified vaccination schedules to facilitate serologic response during an outbreak, especially earlier administration of the second dose. Previous studies augmented the serologic response to HAV vaccine by adding a booster dose at week 4 between the two doses of the standard schedule.17, 18 The accelerated schedule, with administration of combined HAV and HBV vaccine at 0, 7, and 21 days and 6-12 months, rapidly induces seroprotection and is recommended for those with imminent travel plans to endemic regions.30 Both modified vaccination schedules may be preferable during an outbreak of HAV infection, but more studies are warranted.
Strengths of this study include a large number of individuals and high HAV vaccine coverage to allow better estimates of serologic response and vaccine effectiveness in the outbreak setting. However, the observational nature of our study results in several limitations. First, the characteristics of the included individuals were not balanced. The decision to receive HAV vaccines might depend on the awareness of acute hepatitis A, the perception of the risk, and the socioeconomic status and immune status of the HIV-positive individuals. Although the lower vaccine coverage and higher risk for HAV infection in HIV/HCV-coinfected patients may result in overestimating vaccine effectiveness, HAV vaccination remained effective in 117 HCV-coinfected patients and 1,416 HCV-uninfected patients (86% and 97%, respectively) (data not shown). Second, the delivery of HAV vaccines and anti-HAV IgG measurements was at regular time intervals according to clinical care practices but not fixed time points; hence, the serologic response estimates should be interpreted with caution. Third, two types of HAV vaccines were used due to a vaccine shortage, leading to uncertain serologic and clinical responses to vaccine schedules using different combinations of vaccines among HIV-positive individuals. Fourth, the HAV antibody detection method used in the study was unable to distinguish between antibodies produced from vaccination and natural infection, although HAV infection might be diagnosed on the basis of clinical manifestations and follow-up of liver function tests. Finally, the follow-up duration of this study was short. The vaccine-induced seroprotection may wane over time in HIV-positive individuals, and the long-term effectiveness warrants further investigations.19
In conclusion, we found that HAV vaccination was highly effective at preventing acute HAV infection during the outbreak, despite the delayed serologic response among HIV-positive individuals. Our results support HAV serologic screening and vaccination for those at-risk HIV-positive populations in the era of cART scale-up.




