Patient-reported outcomes in cirrhosis: A scoping review of the literature
Potential conflict of interest: Dr. Kanwal received grants from Merck and Gilead.
Supported by the National Institutes of Health through the Michigan Institute for Clinical and Health Research (KL2TR002241, to E.T.).
Abstract
Patients with cirrhosis seek improvement in their symptoms, functioning, quality of life, and satisfaction with the care they receive. However, these patient-reported outcomes (PROs) are not routinely measured for clinical care, research, or quality improvement. The members of the American Association for the Study of Liver Diseases Practice Metrics Committee, charged with developing quality indicators for clinical practice, performed a scoping review of PROs in cirrhosis. The aim is to synthesize a comprehensive set of PROs for inclusion into a standard patient-centered outcome set. We searched Medline, Embase, the Cumulative Index to Nursing and Allied Health Literature, PsycINFO, and the Cochrane Trial Library since inception, with final searches run between April 20 and June 1, 2017. Studies were included if they reported the construction and/or validation of a PRO instrument for patients with cirrhosis or if they assessed the clinical (case-mix) variables determining responses to established PRO scales. Eleven studies were selected that yielded 259 items specific to patients with cirrhosis. After removing duplicates, 152 unique items were isolated. These items were consolidated into seven domains: physical symptoms, physical function, mental health, general function, cognition, social life, and satisfaction with care. The seven domains included 52 subdomains (e.g., physical domain, abdominal pain subdomain). Twelve variables were identified that independently modified established PRO scales. These included clinical factors (severity of liver disease and its complications, medication burden, and comorbidities), specific PROs (cramps, pruritis), and surrogate outcome measures (falls, hospitalization). Conclusion: This scoping review identified and categorized a large existing set of PRO concepts that matter to patients with cirrhosis; these outcomes may now be translated into usable measures both for the assessment of the quality of cirrhosis care in clinical practice and to perform research from the patient's perspective. (Hepatology 2018;67:2375-2383).
Abbreviations
-
- HRQOL
-
- health-related quality of life
-
- PRO
-
- patient-reported outcome
The prevalence of cirrhosis in the United States has almost doubled in the last decade, resulting in a substantial rise in its associated morbidity and mortality.1, 2 As many as half of patients with cirrhosis develop clinical complications, including variceal hemorrhage, ascites, hepatic encephalopathy, and hepatocellular carcinoma. The health burden of cirrhosis is amplified by its dramatic impact on patients' health-related quality of life (HRQOL), resulting from a range of physical, psychological, and social stressors engendered by cirrhosis and its treatment.
Ensuring that patients with serious illness receive patient-centered care is a fundamental goal of medical care. For this care to be patient-centered, it must be aligned with what matters to the patients. Despite the realization that patient-reported outcomes (PROs) are important to patients, current quality improvement efforts rarely assess PROs. One explanation underlying this disconnect is lack of standardized PROs that may serve as benchmarks for quality measurement and improvement in cirrhosis.
To address this gap, and under the auspices of the Practice Metrics Committee of the American Association for the Study of Liver Diseases, we conducted a scoping review of PROs in cirrhosis. Our goal was to identify and summarize the PRO domains that may serve as candidates for a set of outcomes as part of quality improvement efforts in cirrhosis.
Materials and Methods
We performed a scoping review. A scoping review is a variant of a systematic review which aims to identify and map key concepts with a large body of evidence.3 The scoping review methodology can be more appropriate than a systematic review when the body of literature is large, complex, heterogeneous, and without a prior comprehensive review.4 We hypothesized that the literature would already contain significant information about PROs relevant to cirrhosis, but the article may not be explicitly labeled as such.
SEARCH STRATEGY
With the aid of an informationist with expertise in scoping reviews (J.E.S.), we searched six databases: Medline, OVID, Cumulative Index to Nursing and Allied Health Literature, Embase, PsycINFO, and Cochrane Library. No date restrictions were applied, and final searches were conducted between April 20 and June 1, 2017. The search strategy for each database is provided in the Supporting Information. Searches combined a block of terms specific to disease (i.e., cirrhosis and its complications, as well as each liver disease enumerated) with terminology specific to PROs (e.g., patient-reported measures, quality of life, and patient satisfaction) and with names of published PRO instruments used in liver disease (e.g., Short Form-36) abstracted from a prior systematic review.5 Sentinel articles were used to validate the search strategy and provide additional references through looking at reference lists and conducting citation tracking searches through the database SCOPUS. Articles meeting inclusion criteria were also used to citation-track using Scopus.
STUDY SELECTION
Studies were included if they reported the construction/validation of a PRO instrument for patients with cirrhosis or if they assessed the clinical variables that determined responses to established PRO scales and reported granular data-specific domains/responses within the scales. As above, we defined PRO as patients' own assessment of their health status including symptoms, reports of daily functioning, quality of life, and satisfaction with health care. For example, if a study on quality of life provided details on these domains, it was included; but if it only reported summary scores from a previously developed instrument (such as Short Form-36), then it was excluded.
We only included English-language publications. We excluded studies focusing on liver transplant, including those pertaining to patients awaiting liver transplant or recollecting their condition prior to transplantation. We also excluded studies of children; of patients with acute liver failure, noncirrhotic chronic liver disease (including treatment for hepatitis C), or hepatocellular carcinoma; and that focused on pre–liver transplant or post–liver transplant experiences.
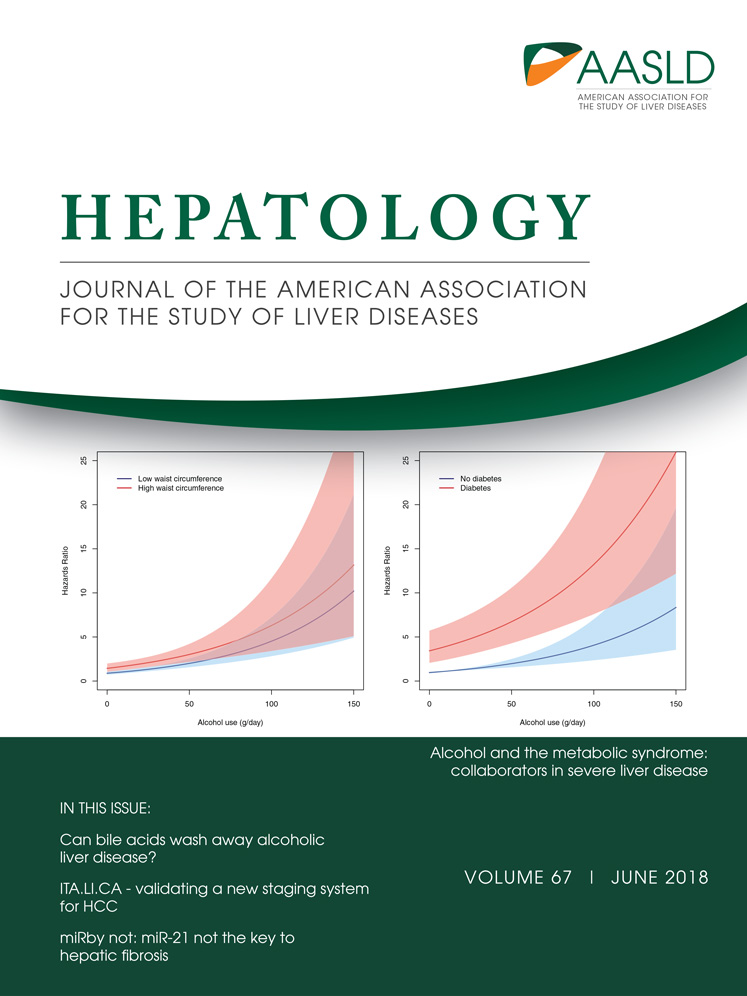
Seven reviewers independently screened titles and abstracts for eligibility. Each title was reviewed by at least two reviewers. The articles excluded by title, abstract, and after full-text review were recorded. Each reviewer selected the full-text studies he or she judged to be relevant or unclear. These studies were included in a final grouping where selection conflicts between the two index reviewers were further adjudicated by two arbitrators (E.B.T. and M.V.) where unresolved conflicts were settled by a third arbitrator (F.K.).
DATA EXTRACTION AND ANALYSIS
Included studies were summarized according to their population, aims, methods, and key findings. All survey items and determinants of PRO responses were aggregated and categorized by two reviewers (E.B.T., M.V.). Verbatim duplicates were excluded. Discrepancies with respect to inclusion and categorization were adjudicated by a third review (F.K.).
Results
POPULATION CHARACTERISTICS IN INCLUDED STUDIES
Of 5,647 studies screened, 11 studies met our inclusion criteria (Fig. 1). For a description of the included studies, see Table 1. Of these 11 studies, four were from the United States,6-9 two from Denmark,10, 11 and one each from China, Taiwan, Japan, Spain, and Italy.12-16 Overall, these studies enrolled 2,299 (range 11-544) patients. The stage of liver disease was available for 1,550 (67%), of whom 586 (38%) were Child A and 964 (62%) were Child B-C. Granular details of cirrhosis etiology were available for 1,268 (55%) patients, of whom 643 (51%) had viral hepatitis and 360 (28%) had alcoholic liver disease.
| Reference | Population Evaluated | Aims | Method | Key Findings |
|---|---|---|---|---|
| Younossi et al.9 | 133 Ambulatory patients: CTP A (32%), CTP B (23%), CTP C (11%), no cirrhosis (34%). Cholestatic disease (23%), “hepatocellular” (77%). | Develop disease-specific HRQOL instrument. |
Semistructured interviews to select 156 candidate instrument items in cohort of 60 patients. Determined importance of each item in separate cohort of 75 patients for reduction to 29 items. |
Identified six domains: abdominal symptoms, fatigue, systemic symptoms, activity, emotional function, and worry. Scores worsen with CTP class. |
| Gralnek et al.7 | 221 ambulatory patients: CTP A (29%), CTP B (52%), CTP C (19%). Viral (51%), alcohol (16%), cholestatic (12%). | Develop disease-specific HRQOL instrument. | Semistructured interviews. Psychometric analysis. Content validation. |
36 items detailing activities of daily living, concentration, memory, sexual functioning, sleep, loneliness, hopelessness, quality of social interaction, health distress, and self-perceived stigma. |
| Marchesini et al.13 | 544 patients (ambulatory and admitted): CTP A (38%), CTP B (38%), CTP C (24%). Viral (64%), alcohol (29%). |
Determine clinical variables associated with HRQOL (SF-36 and NHP). |
Cross-sectional survey responses. Results compared to age-matched and sex-matched healthy controls. | The two most important clinical variables associated with HRQOL were CTP and muscle cramps. The presence of HE, pruritus, ascites, diuretics, hospitalization, and comorbidities affected HRQOL subscales. |
| Chatrath et al.6 | 150 ambulatory patients: CTP A (29%), CTP B (47%), CTP C (24%). Viral (42%), alcohol (22%). | Determine impact of cramps on HRQOL (using CLDQ). | Cross-sectional survey responses and clinical assessment. | Cramp severity correlates with CLDQ score. Cramps associated with worse scores in all CLDQ subscales but worry. |
| Tsai et al.12 | 49 hospitalized patients: CTP A (6%), CTP B (43%), CTP C (51%). Alcohol (59%), viral (35%). | Determine frequency of physical and psychological symptoms. | Semistructured interviews. Rank ordering by frequency. Content validation. | Physical and psychological symptoms are highly correlated. Abdominal symptoms and worry were the most severe physical and psychological domains, respectively. |
| Román et al.15 | 118 ambulatory patients: CTP A (62%), CTP B (34%), CTP C (4%). Alcohol (62%), viral (36%). |
Determine relationship between falls and HRQOL (using SF-36). |
Cross-sectional survey responses and clinical assessment for cognitive dysfunction. | Physical components of HRQOL are most associated with cognitive dysfunction and hyponatremia. Mental components of HRQOL are most associated with falls. |
| Vaughn-Sandler et al.8 | 149 ambulatory patients: CTP A (58%), CTP B (36%), CTP C (5%). Viral (34%), alcohol (12%). | Determine prevalence and impact of stigma. | Cross-sectional survey responses to questions on stigma abstracted from multiple surveys with opportunity for free-text response. | Stigma is associated with lower QOL, depression, lower likelihood of seeking medical care, and less social support. |
| Onishi et al.14 | 175 ambulatory patients evaluated before and after ascites therapy: CTP A (12%), CTP B (50%), CTP C (38%). | Develop ascites-specific symptom scale. | Semistructured interviews with psychometric analysis. Longitudinal assessment for changes following treatment. | Seven-question scale that correlates with severity of ascites, SF-36 scores, and response to treatment. |
| Mikkelsen et al.10, 11 | 11 ambulatory patients with alcoholic cirrhosis and HE. | To identify conditions that limit or support coping with physical and psychological problems. | Semistructured interviews before and after a coping and physical activity group intervention. | Patients feel responsible to and seek acknowledgment/positive attitudes from their clinicians, find it difficult to seek help for alcohol relapse. The intervention improves patient assessments of self-control, community, and strength. |
| Ying et al.16 | 464 ambulatory patients: unknown CTP and etiology. | To develop the LC-PROM, a cirrhosis-specific PRO scale that addresses all physical symptoms, psychological feelings, daily activities, and therapeutic status. | Semistructured interviews with 10 patients, followed by item refinement by another 10 patients. Item reduction performed in a sample of 200 subjects (150 with cirrhosis). Test performance in 464 patients with cirrhosis and 112 controls. | LC-PROM consists of 55 items which include questions to obtain information about treatment satisfaction, compliance, and drug side effects. |
- Abbreviations: CLDQ, Chronic Liver Disease Questionnaire; CTP, Child-Turcotte-Pugh; HE, hepatic encephalopathy; LC-PROM, Liver Cirrhosis Patient-Reported Outcome Measure; NHP, Nottingham Health Profile; QOL, quality of life; SF-36 = Short-Form-36.
DESCRIPTION OF STUDY AIMS AND METHODS
HRQOL or PRO Scale Development
Four studies—by Younossi et al.,9 Gralnek et al.,7 Onishi et al.,14 and Zhang et al.16—aimed to create disease-specific scales. Three of these studies only included patients with cirrhosis. The fourth study included patients with as well as those without cirrhosis; however, the majority (66%) had cirrhosis in this study. One study14 focused exclusively on patients with ascites. Each study used a deliberate, stepwise approach to determine the pertinent domains and questions; these included semistructured patient interviews to develop themes, separate interviews to narrow and refine the questions, and an evaluative cohort to demonstrate final results which included an assessment of responsiveness to therapy (Table 1).
Qualitative Analysis
Four studies described the results of qualitative analyses where patients were asked open-ended questions about their experiences. In three cases, these were performed in focus groups with semistructured interviews. Tsai et al. interviewed patients and asked them to rank-order the most important physical and psychological components of cirrhosis.12 Over the course of two articles,10, 11 Mikkelsen et al. described the results of patient interviews focusing on the conditions that limit or support coping with physical and psychological problems before and after a group-therapy intervention. Vaughn-Sandler et al. mailed surveys with questions and opportunities for free-text responses to capture the rate and impact of feelings of stigma.8
Impact of Clinical Variables on PRO Domains
Cross-sectional survey studies by Marchesini et al.,13 Chatrath et al.,6 and Román et al.15 evaluated the impact of clinical variables on domains of established PRO scales including the Chronic Liver Disease Questionnaire, Short Form-36, and Nottingham Health Profile, respectively. Román et al. were specifically interested in the impact of falls and cognitive dysfunction, and Chatrath et al. focused exclusively on the impact of muscle cramps. Marchesini et al. explored several clinical variables and used regression techniques to determine the factors independently associated with suboptimal PROs.
CATEGORIZED STUDY FINDINGS
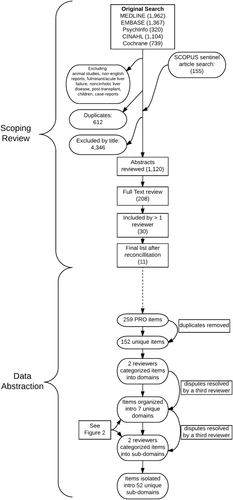
Overall, the 11 studies yielded 259 items specific to patients with cirrhosis. After removing duplicates, 152 unique items were isolated (Supporting Information). Each could be categorized into one of seven broader domains (physical symptoms, physical function, mental health, general function, cognition, social life, and satisfaction with care) as well as 52 subdomains (Fig. 2). The physical health domain had the most subdomains (e.g., abdominal pain, appearance, dizziness). Examples of the mental health domain included depression and alcohol abuse, the function domain included items regarding career and daily activities, the social life domain dealt with stigma and isolation, the satisfaction with care domain touched on items relating to medication side effect concerns and the quality of the patient–doctor relationship, and, finally, the cognition domain included items on concentration and forgetfulness, among others. In many cases, multiple items referred to the same subdomain; e.g., multiple questions covered material pertinent to abdominal pain, bloating, and sexual function. There were many ways of asking about, for example, the abdominal pain or itching subdomains of physical health. In the Supporting Information, we provide representative example items for each subdomain, e.g., “I was annoyed at abdominal pain and indigestion” (abdominal pain from Zhang et al.16) and “How much have you been troubled by itching during the last two weeks?” (pruritis from Younossi et al.5).
Multiple studies evaluated whether specific variables were associated with significant differences in the scores of PRO scales such as the Short Form-36, Nottingham Health Profile, and Chronic Liver Disease Questionnaire (Table 2). Twelve variables were identified based on the review of these studies. These included clinical factors (etiology and severity of liver disease, cognitive dysfunction and hepatic encephalopathy, ascites, comorbidities, medication burden, loop diuretics, hospitalization) and patient-reported symptoms (cramps, pruritis, falls)
| Scale/Study | Domains Affected by Clinical Variables | Clinical Variables | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ascites | Comorbidity | Severity of Disease |
Cognitive Dysfunction |
Pill Burden |
Disease Etiology |
Falls |
Hospital Admits |
HE |
Loop Diuretic |
Muscle Cramps |
Pruritis | ||
| SF-36 | General Health | • | – | – | • | – | X | • | – | • | – | • | • |
| Vitality | • | – | • | • | – | X | • | – | • | • | • | • | |
| Physical Roles | • | – | • | • | • | X | • | • | • | • | • | • | |
| Physical Functioning | • | – | • | • | • | X | • | • | • | • | • | • | |
| Emotional Roles | • | – | – | • | • | X | • | – | • | • | • | • | |
| Mental Health | • | – | – | • | – | X | • | – | – | • | • | • | |
| Social Functioning | – | – | • | • | – | X | • | – | – | – | • | • | |
| Bodily Pain | • | – | – | – | • | X | • | • | – | • | • | – | |
| NHP | Energy | • | – | • | X | • | X | X | • | • | – | • | • |
| Sleep | • | – | – | X | – | X | X | – | • | – | • | • | |
| Pain | – | – | – | X | • | X | X | – | – | – | • | • | |
| Emotional Reactions | • | • | – | X | – | X | X | – | – | – | • | • | |
| Social Isolation | • | • | – | X | – | X | X | – | – | – | • | • | |
| Physical Mobility | • | • | • | X | • | X | X | • | • | • | • | • | |
| CLDQ | Fatigue | X | X | X | X | X | X | X | X | X | X | • | X |
| Activity | X | X | X | X | X | X | X | X | X | X | • | X | |
| Systemic Symptoms | X | X | X | X | X | X | X | X | X | X | • | X | |
| Abdominal Symptoms | X | X | X | X | X | X | X | X | X | X | • | X | |
| Emotional Function | X | X | X | X | X | X | X | X | X | X | • | X | |
| Vaughn-Sandler et al.8 | Stigma | X | X | X | X | X | • | X | X | X | X | • | X |
- •, statistically significant association; –, lack of an association; X, the impact of this variables was not assessed for that particular scale/study.
- Abbreviations: CLDQ, Chronic Liver Disease Questionnaire; HE, hepatic encephalopathy; NHP, Nottingham Health Profile; SF-36, Shortform-36.
Discussion
In this scoping review, we systematically identified 152 distinct PROs reported by patients with cirrhosis. We then distilled them into a set of seven major domains and 52 subdomains. These data from 11 studies reflect the experiences of 2,299 patients from seven countries across the spectrum of disease etiology and severity. Whereas any one study's results may not generalize to another context, this scoping review provides a comprehensive list of PROs that are applicable to most patients with cirrhosis seen in different health care settings.
These data extend the literature on PROs in cirrhosis in two principal ways. First, our goal was to identify a broad range of PROs that matter to our patients. Many disease-specific HRQOL instruments (e.g., Chronic Liver Disease Questionnaire9 and Liver Disease Quality of Life7) have already been developed for patients with cirrhosis. Other nonspecific tools, such as the Patient-Reported Outcomes Measurement Information System, have been validated in populations with cirrhosis.17 Indeed (and not surprisingly), the broader domains that we identified map well with the domains in existing HRQOL instruments. Our review also found many additional items and subdomains that were not included in previous HRQOL tools. Whereas HRQOL instruments reliably capture the patient's values, preferences, and responses to therapeutic interventions, they do not necessarily speak to other PRO domains such as symptom status, function, satisfaction with care, and adherence to medication.18 Furthermore, in contrast to the development of psychometric indices, for the purpose of quality improvement, it is important to identify measures that are sufficiently granular to inform specific therapeutic needs (i.e., as relates to frailty or sexual dysfunction) and are responsive to therapeutic interventions (such as control of ascites).
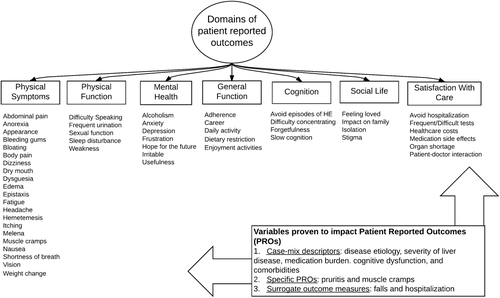
Second, our review also identified 12 variables that had a statistically significant and clinically meaningful impact on established HRQOL scales. Although some of these factors were PROs (pruritis, cramps), most were clinician-reported features of cirrhosis that significantly impacted PROs. The latter may either serve as surrogate outcome measures (such as falls, hospitalization) or variables for case-mix risk adjustment of PROs (such as the etiology of liver disease and the presence of comorbid conditions).
To build on this study, we suggest two next steps. One, these PROs can serve as important measures for clinical care that many physicians may want to explore with their patients. Answers to many questions that may often go unasked, about, for example, falls, pruritis, or sexual function, have the capacity to modify clinical management in meaningful ways. Two, these PROs will serve as potential candidates for outcome-based quality measures. These items will serve as candidate outcome measures for an upcoming two-round modified Delphi panel process for ranking and selection by a group of content experts. Thereafter, a specification process will follow to convert these findings into discrete measures with clear numerators, denominators, and denominator exclusions that can be implemented into clinical care and followed longitudinally. A similar PRO identification process was recently employed by the International Consortium for Health Outcomes Measurement group.19, 20 As this group has identified, new reporting standards may increase the demands for data collection on frontline providers. Implementation is an unresolved challenge for the growing experience with PRO sets.19-21 In addition to the work on PRO identification, implementation efforts designed with sensitivity to clinician workflow are necessary. The optimal quality measure is clinically meaningful, standardized, and easily measured while neither overburdening the frontline clinician (and patient) nor increasing the costs of health care delivery. Realizing the goal of integrating a set of PRO assessments in routine clinical practice will require designing items with responses that are easily abstracted from existing data and leveraging the functionality of electronic health records. In Fig. 3, we summarize the work to date and outline the remaining steps needed to achieve our goals.
These data must be interpreted in the context of the study design. First, given the inclusion criteria for the selected studies, these data apply to pretransplant adult patients with cirrhosis; generalizability to posttransplant or pediatric patients is unclear. Second, the list of 52 subdomains may be reduced further if some factors are found to be duplicative or equivalent by patients. For example, the term “bleeding episodes” may suffice for hematemesis, melena, and epistaxis. Third, the relative importance of specific domains is certain to change with the patient's clinical context. The patient with Child A cirrhosis may benefit from surveys that focus on specific domains and subdomains such as mental health (sexual function, alcohol, and stigma), whereas those with Child C may need to be asked different questions (such as adherence, isolation, and caregiver burden).
In conclusion, the symptoms and preferences expressed by patients with cirrhosis are equal in importance to their clinical outcomes. Well-defined quality indicators based on processes of care have been defined to facilitate improved clinical outcomes. Similar indicators are lacking for PROs. This scoping review identified a large existing set of PRO concepts, which may now be translated into usable measures to support quality improvement in cirrhosis.