Impact of hepatitis C virus polymorphisms on direct-acting antiviral treatment efficacy: Regulatory analyses and perspectives
Potential conflict of interest: Nothing to report.
The views expressed in this report are those of the authors and do not necessarily represent official policy of the FDA.
Abstract
Several highly effective, interferon-free, direct-acting antiviral (DAA)-based regimens are available for the treatment of chronic hepatitis C virus (HCV) infection. Despite impressive efficacy overall, a small proportion of patients in registrational trials experienced treatment failure, which in some cases was associated with the detection of HCV resistance-associated substitutions (RASs) at baseline. In this article, we describe methods and key findings from independent regulatory analyses investigating the impact of baseline nonstructural (NS) 3 Q80K and NS5A RASs on the efficacy of current United States Food and Drug Administration (FDA)-approved regimens for patients with HCV genotype (GT) 1 or GT3 infection. These analyses focused on clinical trials that included patients who were previously naïve to the DAA class(es) in their investigational regimen and characterized the impact of baseline RASs that were enriched in the viral population as natural or transmitted polymorphisms (i.e., not drug-selected RASs). We used a consistent approach to optimize comparability of results across different DAA regimens and patient populations, including the use of a 15% sensitivity cutoff for next-generation sequencing results and standardized lists of NS5A RASs. These analyses confirmed that detection of NS3 Q80K or NS5A baseline RASs was associated with reduced treatment efficacy for multiple DAA regimens, but their impact was often minimized with the use of an intensified treatment regimen, such as a longer treatment duration and/or addition of ribavirin. We discuss the drug resistance-related considerations that contributed to pretreatment resistance testing and treatment recommendations in drug labeling for FDA-approved DAA regimens. Conclusion: Independent regulatory analyses confirmed that baseline HCV RASs can reduce the efficacy of certain DAA-based regimens in selected patient groups. However, highly effective treatment options are available for patients with or without baseline RASs. (Hepatology 2018;67:2430-2448).
Abbreviations
-
- AASLD
-
- American Association for the Study of Liver Diseases
-
- DAA
-
- direct-acting antiviral
-
- DCV
-
- daclatasvir
-
- EASL
-
- European Association for the Study of the Liver
-
- EBR/GZR
-
- elbasvir and grazoprevir
-
- FDA
-
- United States Food and Drug Administration
-
- GLE
-
- glecaprevir
-
- GT
-
- genotype
-
- HCV
-
- hepatitis C virus
-
- IDSA
-
- Infectious Diseases Society of America
-
- IFN
-
- interferon
-
- LDV
-
- ledipasvir
-
- NDA
-
- new drug application
-
- NGS
-
- next-generation sequencing
-
- NS
-
- nonstructural
-
- Peg
-
- pegylated
-
- PIB
-
- pibrentasvir
-
- PrOD
-
- ombitasvir/paritaprevir/ritonavir + dasabuvir
-
- RASs
-
- resistance-associated substitutions
-
- RBV
-
- ribavirin
-
- SMV
-
- simeprevir
-
- SOF
-
- sofosbuvir
-
- SVR12
-
- sustained virological response at week 12
-
- VEL
-
- velpatasvir
-
- VOX
-
- voxilaprevir
In recent years, a revolution in chronic hepatitis C virus (HCV) treatment has taken place with the availability of interferon (IFN)-free, direct-acting antiviral (DAA)-based regimens. Several combination DAA regimens are now approved in the United States and provide highly effective, IFN-free (and, in many cases, ribavirin [RBV]-free) treatment options for all major HCV genotypes (GTs) and patient populations. Although many clinical trials have demonstrated exceptionally high rates of treatment success, for some DAA regimens suboptimal efficacy has been observed for certain HCV GTs/subtypes and/or patient subpopulations. Treatment failure with DAA-based regimens is often associated with the selection of HCV variants with reduced drug susceptibility and possibly reduced susceptibility to other DAAs in the same class. Therefore, a major goal of the United States Food and Drug Administration (FDA) review of HCV therapies has been to determine the patient and viral characteristics that influence treatment outcome and identify approaches that minimize the risk of treatment failure.
Early in the development of HCV DAAs, it was recognized that DAA resistance-associated substitutions (RASs) predominate as natural polymorphisms in the viral population of some patients in the absence of any DAA selective pressure.1-4 Subsequent studies indicated that virological failure with certain DAA-containing regimens was associated with the detection of nonstructural (NS) 3 or NS5A RASs at baseline.5, 6 The potential impact of baseline RASs on treatment outcome is now well recognized, and current American Association for the Study of Liver Diseases (AASLD)/Infectious Diseases Society of America (IDSA) and European Association for the Study of the Liver (EASL) treatment guidelines include considerations for pretreatment resistance testing for multiple DAA-containing regimens.7, 8 Nevertheless, evolving HCV sequence analysis technologies and a lack of standardization of resistance analysis methods and reporting have contributed to uncertainty in the overall impact of baseline RASs across different treatment regimens. These factors have also led to varying perspectives among experts in the field on how drug resistance information should be considered in clinical decision making.9, 10
In this article, we describe comprehensive and independent FDA analyses of the impact of baseline HCV RASs on treatment efficacy for current FDA-approved, IFN-free HCV DAA treatment regimens for HCV GT1- or GT3-infected patients. These HCV GTs are the most common worldwide,11 and GT1a and GT3 are also generally the most challenging to treat with IFN-free DAA regimens. Our analyses focused on patients who were previously naïve to the DAA classes in their treatment regimen in order to characterize the efficacy impact of baseline RASs that are enriched in viral populations as natural or transmitted amino acid polymorphisms. We present a standardized approach for analysis of baseline NS5A RASs in NS5A inhibitor-naïve patients, whereby data submitted to the FDA were reanalyzed, as needed, to optimize comparability across different NS5A inhibitor-containing regimens and patient populations. Finally, we discuss how resistance data have been considered in the regulatory reviews of specific DAA-based treatments, focusing on the potential utility of pretreatment resistance testing to optimize treatment efficacy in clinical practice for certain regimens.
FDA Resistance Analysis Approaches
TERMINOLOGY AND GENERAL CONCEPTS
The FDA performs an independent assessment of resistance data for all antiviral drugs being considered for approval in the United States to verify the results presented in the new drug application (NDA) and to identify potentially novel resistance pathways. Analyses of HCV drug resistance consider both genotypic data (amino acid substitutions in DAA target genes detected by nucleotide sequence analysis) and phenotypic data (effect of specific amino acid substitutions on HCV DAA susceptibility in cell culture). To characterize the effect of baseline drug resistance characteristics on treatment outcome in clinical trials, these analyses typically censor patients who failed to achieve the primary efficacy endpoint (sustained virological response at post-treatment week 12; SVR12) for reasons unrelated to virological failure, such as missing data or early treatment discontinuation attributed to an adverse event; the analyses presented in this article also censored such cases.
Terminology used to describe HCV drug resistance has been evolving, and in recent months the field has moved to use the term “resistance-associated substitution” (or RAS) to describe HCV amino acid substitutions that are associated with treatment failure and/or reduced DAA susceptibility.7-9 FDA reviews and United States drug labels often use the term “polymorphism” when referring to RASs that are detected at baseline in patients who are naïve to the DAA class in question. Such RASs differ from the reference amino acid as a result of natural viral genetic variation (i.e., not as a result of DAA selection) or possibly are present in virus transmitted from a DAA-experienced patient. “Treatment-emergent” RASs are those that became detected or enriched in the viral population during or following treatment, presumably as a result of drug selective pressure. The term “resistance-associated” is preferred in FDA reviews over “drug resistant,” because the latter implies that the drug in question provides no efficacy benefit against the virus in any context, whereas in reality the presence of a DAA RAS by itself does not preclude the use of the DAA. As illustrated by multiple examples described in this article, the impact of baseline RASs on treatment outcome can be influenced profoundly by other factors that also impact treatment efficacy independently, such as patient disease characteristics (e.g., cirrhosis), treatment duration, and the activity and resistance barrier of all drugs in the regimen.
Historically, pharmaceutical sponsors used Sanger population nucleotide sequencing for genotypic resistance analyses, which can detect the predominant or consensus sequence as well as minor genetic variants that comprise at least 10%-25% of the viral population (variable sensitivity levels within this range have been reported). More recently, researchers and sponsors have moved toward the use of next-generation sequencing (NGS), a technology with many different platforms that perform high-throughput sequencing. Analyses of NGS data are not well standardized and have numerous possible analysis parameters with large amounts of data associated with a sequence run.12 In general, a completely independent resistance analysis of NGS data requires access to the raw sequence reads in fastq format and data tables that are populated at specific NGS sensitivity cutoffs. The FDA typically requests that sponsors submit both raw and analysis-ready NGS data to allow for an independent analysis of resistance results from clinical trials. Although varying NGS sensitivity cutoffs have been used in published HCV resistance analyses, for our regulatory reviews and the analyses described here a 15% NGS sensitivity cutoff was used to identify baseline amino acid substitutions of interest. This sensitivity cutoff for baseline analyses is used because it is comparable to the sensitivity of Sanger sequencing and thus allows for comparisons of data generated across both technologies. Furthermore, multiple studies have shown that baseline RASs detected in a smaller minority of viral populations generally have less of an impact on treatment outcome.13-16 Also, as the sensitivity of NGS is pushed to lower detection thresholds, reproducibility of results across different laboratories, NGS platforms, and analysis algorithms is likely to decline, which would be problematic if these analyses are used to guide resistance testing and treatment decisions in clinical practice.
Phenotypic analyses can play an important role in characterizing HCV DAA resistance mechanisms and are often used to complement genotypic resistance analyses. Table 1 provides examples of phenotypic analysis results for HCV replicons carrying specific NS5A or NS3 RASs. Although cell-culture–based phenotypic testing may help to identify or confirm key amino acid positions/substitutions associated with drug resistance, it is important to recognize that the lack of a clear or consistent resistance phenotype in cell culture does not preclude the clinical relevance of a particular baseline RAS. There are multiple instances in which detection of a baseline RAS is associated with treatment failure, yet the RAS itself does not confer a major decrease in HCV susceptibility to the DAA in cell culture, at least as a single amino acid change.6, 17-19 For example, the NS5A RASs, Q30H, Q30L, and L31M, were associated with reduced efficacy of elbasvir (EBR)/grazoprevir (GZR) in patients with HCV GT1a infection, despite these amino acid changes having a relatively modest effect (<1- to 10-fold, depending on assay method and substitution) on EBR activity in HCV GT1a replicon assays.19 Combinations of HCV RASs can confer greater levels of phenotypic resistance, whereas single-baseline HCV RASs that do not confer clear phenotypic resistance to clinically relevant drug concentrations may still contribute to virological failure by reducing the resistance barrier of the regimen. Underlying viral populations with complex combinations of RASs that confer enhanced resistance are more likely to be generated and selected by treatment when one or more RASs already predominates in the viral population, which can explain why additional, treatment-emergent RASs are often detected when patients with baseline RASs experience virological failure.
| NS5A Inhibitors (Fold-Change in EC50 Values) | ||||||
|---|---|---|---|---|---|---|
| NS5A RASa | Ledipasvir | Ombitasvir | Daclatasvir | Elbasvir | Velpatasvir | Pibrentasvir |
| GT1a-K24R | 4 | ≤1 | 2 | ≤1 | ≤1 | ≤1 |
| GT1a-M28T | 61 | 8,965 | 205 | 15 | 8 | 2 |
| GT1a-M28V | ≤1 | 58 | 1 | 1 | ≤1 | 2 |
| GT1a-Q30H | 183 | 3 | 435 | 6 | 2 | ≤1 |
| GT1a-Q30R | 632 | 800 | 365 | 16 | 2 | 2 |
| GT1a-L31M | 554 | 2 | 105 | 10 | 16 | ≤1 |
| GT1a-H58D | 1,127 | 243 | 367 | 6 | 7 | ≤1 |
| GT1a-H58P | ≤1 | ≤1 | ≤1 | ND | ≤1 | ≤1 |
| GT1a-Y93H | 1,677 | 41,383 | 1,600 | 220 | 609 | 7 |
| GT1a-M28T+Q30H | ND | ND | 76,833 | 2,286 | ND | ND |
| GT1a-Q30H+Y93H | 34,960 | ND | 98,167 | ND | 2,835 | 17 |
| GT1a-Q30R+Y93H | 33,691 | 354,981 | 52,667 | ND | 18,698 | 260 |
| GT1b-L31M | 3 | ≤1 | 3 | 1b | 2 | 2 |
| GT1b-Y93H | 1,807 | 77 | 12 | 17b | 3 | ≤1 |
| GT1b-L31M+Y93H | 20,270 | 142 | 16,000 | ND | 44 | ≤1 |
| GT3a-A30K | n/a | n/a | 117 | n/a | 50 | ≤1 |
| GT3a-Y93H | n/a | n/a | 3733 | n/a | 724 | 2 |
| References | 18, 32, 67 | 18, 37 | 18, 48, 49, 67 | 19, 51, 68 | 55, 67, 69 | 59, 70 |
| NS3/4A Protease Inhibitors (Fold-Change in EC50 Values) | ||||
|---|---|---|---|---|
| NS3 RAS | Simeprevir | Paritaprevir | Grazoprevir | Glecaprevir |
| GT1a-Q80K | 11 | 3 | 8 | ≤1 |
| GT1a-R155K | 86 | 36 | 9 | ≤1 |
| GT1a-Q80K+R155K | 1830 | 19 | 41 | ≤1 |
| References | 18 | 37 | 51 | 59 |
- a Selected examples are shown. Analyses of baseline RASs described throughout this article considered two sets of NS5A position lists, including a “Primary” list (any change from subtype reference at position 28, 30, 31, or 93) and an “Extended” list (any change from subtype reference at position 24, 28, 30, 31, 58, 92, or 93).
- b Data shown are from stable HCV replicon cell lines. Transiently transfected HCV replicon data are not available.
- Abbreviations: EC50, 50% effective concentration in HCV replicon assay; n/a, not applicable because the NS5A inhibitor is not included in a DAA regimen that is indicated in the United States for the treatment of HCV GT3 infection; ND, no data available.
Conversely, depending on the regimen and other factors noted above, a DAA can still be effective even against a viral population with RAS(s) that confer a major reduction in DAA susceptibility. For example, a single NS5A Y93N substitution confers >10,000-fold resistance to ledipasvir (LDV; NS5A inhibitor) in a GT1a replicon,18 and 2 treatment-naïve, GT1a-infected patients who received 12 weeks of ledipasvir/sofosbuvir (LDV/SOF) treatment in the ION-1 trial had virus at baseline with the Y93N RAS, yet both achieved SVR12. Ultimately, investigations into the mechanisms and clinical consequences of HCV drug resistance should take into account relevant patient characteristics, clinical drug exposures, sequence analysis findings, and the phenotypic impact of individual and combinations of multiple amino acid substitutions on viral drug resistance and replication capacity.
BASELINE HCV RASs/POLYMORPHISMS CONSIDERED
Although all available DAA target amino acid sequence data are considered in FDA reviews, baseline NS5A RASs and the GT1a NS3 Q80K RAS have been most frequently associated with DAA treatment failure. The capacity to detect an association between a particular HCV RAS and treatment outcome depends largely on the prevalence of that RAS in the study population. Because of the high prevalence of the NS3 Q80K polymorphism in HCV GT1a (approximately 35%-40% in clinical trials, generally higher in the United States relative to Europe), sample sizes are usually sufficient to determine its impact on treatment outcome. It is possible that other NS3 RASs at key resistance-associated positions (e.g., amino acids 155, 156, and 168) influence the efficacy of certain NS3/4A protease inhibitor-containing regimens, but RASs at these positions are infrequently detected in protease inhibitor-naïve patients.
Characterizing the effect of baseline NS5A RASs on treatment outcome with NS5A inhibitor-containing regimens in NS5A inhibitor-naïve populations has been particularly challenging. To assess whether NS5A RASs influence treatment outcome, a pooled list of known NS5A resistance-associated positions is initially considered because the low frequency of RASs in NS5A inhibitor-naïve patients at any given position often makes it difficult to determine their precise individual impact on treatment outcome. Furthermore, any amino acid changes at these positions are considered because sample sizes are usually inadequate to determine the relative impact of specific amino acids at the same NS5A position.
In an effort to standardize baseline resistance analyses for currently available NS5A inhibitor-containing regimens, for this article we reanalyzed baseline sequence data considering two lists of NS5A amino acid positions: (1) a “primary” class list that includes key NS5A inhibitor resistance-associated positions 28, 30, 31, or 93, (where amino acid substitutions have been detected at baseline in NS5A inhibitor-naïve patients and were associated with NS5A inhibitor resistance both in cell culture and in clinical studies), and (2) an “extended” class list that includes these same four positions plus positions 24, 58, and 92, (where substitutions have also been associated with NS5A inhibitor resistance, either in clinical studies or in cell culture). Across various data sets from registrational trials in NS5A inhibitor-naïve patients, NS5A RASs based on the primary class list have been detected at baseline in 9%-14%, 17-23%, and 16% of patients with HCV GTs 1a, 1b, or 3, respectively, with up to ∼2-fold higher prevalence when considering the extended list.
Considerations for Resistance Testing in Clinical Practice
Another key challenge in the regulatory review of HCV DAA regimens has been determining how resistance results from clinical trials should be interpreted and considered in clinical practice, and whether pretreatment drug resistance testing should be recommended in drug labeling to guide treatment decisions for specific regimens or patient populations. Clearly, the need for pretreatment resistance testing depends on the impact of a particular RAS or set of RASs on treatment outcome with a particular regimen, but a number of other factors must also be considered, such as the frequency of baseline RAS detection in the target population, clinical availability of resistance testing, availability of alternative treatment options, resistance consequences of treatment failure, and clinical (i.e., disease progression) consequences of treatment failure. These considerations have been factored into the regulatory reviews of DAA-based regimens since the first FDA-approved DAAs. For example, there was evidence from the boceprevir program that certain baseline NS3 RASs were associated with reduced treatment efficacy among patients who had a poor response to the pegylated (Peg)-IFNα/RBV background therapy, but HCV resistance testing in clinical practice was not widely available in the United States at the time, and the proportion of patients with both a poor Peg-IFNα/RBV response and one or more of these baseline NS3 RASs was so small (∼1%) that it did not justify a broad recommendation for resistance testing to guide treatment decision making.20, 21 Even when a specific recommendation for pretreatment resistance testing is not justified or included in drug labeling (i.e., prescribing information), we have supported including available information in drug labels about the impact (or lack thereof) of baseline RASs on treatment outcome for consideration by clinicians.
Impact of Baseline HCV Polymorphisms for Specific DAA Regimens
SIMEPREVIR PLUS Peg-IFNα/RBV OR SOFOSBUVIR
The clinical development of simeprevir (SMV; NS3/4A protease inhibitor) with Peg-IFNα/RBV was the seminal case demonstrating an effect of a baseline HCV RAS on treatment efficacy, resulting in regulatory action.22 Based on pooled analyses of phase 3 trials of SMV plus Peg-IFNα/RBV, efficacy was significantly reduced among HCV GT1a-infected patients whose virus had the NS3 Q80K baseline RAS relative to those without Q80K, with an SVR12 rate that was only modestly higher than that achieved with placebo plus Peg-IFNα/RBV (Fig. 1A).
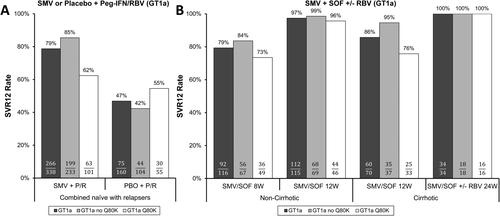
The Q80K baseline RAS is common in HCV GT1a, particularly in North America with an estimated frequency of 40%-50%, but is infrequent in HCV GT1b (≤1% in clinical trials). Among patients with HCV GT1a with Q80K who experienced virological failure with SMV plus Peg-IFNα/RBV, 83% (29 of 35) developed virus with a treatment-emergent NS3 R155K substitution, which conferred enhanced resistance to SMV as well as cross-resistance to other NS3/4A protease inhibitors available at that time. Given the high prevalence of Q80K in HCV GT1a, its impact on SMV plus Peg-IFNα/RBV efficacy, and the potential that treatment failure may compromise the efficacy of other NS3/4A protease inhibitors, the label recommended that patients infected with GT1a be screened for Q80K before treatment with SMV plus Peg-IFNα/RBV, and that alternative treatments be considered if the polymorphism is detected.23
Although SMV plus Peg-IFNα/RBV was the first example where pretreatment resistance testing was strongly justified to optimize treatment efficacy, SMV has primarily been used clinically in an IFN-free combination with SOF (uridine nucleotide analogue NS5B polymerase inhibitor), with or without RBV. In COSMOS, a small phase 2 trial that evaluated SMV plus SOF with or without RBV, SVR12 rates >90% were observed with no apparent reduction in overall efficacy attributable to the detection of Q80K at baseline.24 Based on these results, SMV plus SOF regimens were approved with only a consideration for Q80K screening for the treatment of HCV GT1a-infected patients.25
Two follow-up phase 3 trials were conducted to characterize more precisely the efficacy of SMV plus SOF. OPTIMIST-1 evaluated 8- and 12-week regimens in patients without cirrhosis,26 and OPTIMIST-2 evaluated a 12-week regimen in patients with cirrhosis.27 Considering data from all three trials of SMV plus SOF,28 the detection of Q80K at baseline was associated with an approximately 10% lower SVR12 rate for patients without cirrhosis treated for 8 weeks and a 19% lower SVR12 rate in patients with cirrhosis treated for 12 weeks (Fig. 1B). However, there was no apparent effect of Q80K for patients without cirrhosis who received SMV plus SOF for 12 weeks. Similarly, Q80K did not affect treatment outcomes for patients with cirrhosis who received SMV plus SOF with or without RBV for 24 weeks, although these results from COSMOS were less robust attributed to small sample sizes and pooling of data for regimens dosed with or without RBV. These results support the 12- and 24-week treatment durations recommended in labeling,23 and the results in particular from patients without cirrhosis provide a clear example where an intensified treatment regimen (i.e., longer duration) can reduce the impact of a baseline RAS. Of note, current AASLD/IDSA treatment guidelines no longer recommend the use of SMV plus SOF in HCV GT1-infected patients with cirrhosis,7 and EASL treatment guidelines do not recommend SMV plus SOF for HCV GT1-infected unless other IFN-free options are unavailable.8
LEDIPASVIR/SOFOSBUVIR
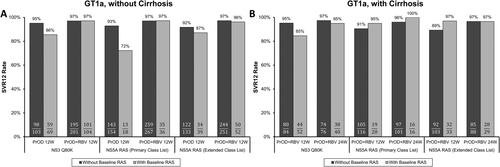
The effect of baseline NS5A RASs on SVR12 rates was analyzed in HCV GT1a- or GT1b-infected patients in the ION-1, ION-2, and ION-3 trials,29-32 which evaluated the efficacy of LDV/SOF, with or without RBV, for 8, 12, or 24 weeks. Among treatment-naïve, HCV GT1a-infected patients without cirrhosis who received LDV/SOF for 8 weeks, detection of NS5A RASs at baseline was associated with a 5% lower SVR12 rate (captured entirely by the primary class list, such that no additional virological failures were identified with the extended class list) compared to patients without baseline RASs (Fig. 2A). However, NS5A RASs did not reduce the efficacy of the LDV/SOF 12-week duration in treatment-naïve, HCV GT1a-infected patients with or without cirrhosis. No treatment-naïve, HCV GT1b-infected patients with NS5A RASs experienced virological failure with either treatment duration (data not shown). Based on overall higher efficacy, LDV/SOF treatment for 12 weeks is recommended in United States drug labeling for both treatment-naïve HCV GT1-infected patients with and without cirrhosis.33 The label also notes that an 8-week treatment duration can be considered for patients without cirrhosis with a pretreatment HCV RNA level <6 million IU/mL, based on a post-hoc efficacy analysis. Two of the 3 HCV GT1a-infected patients with baseline NS5A RASs who experienced virological failure with the 8-week duration had a baseline HCV RNA level ≥6 million IU/mL; thus, the SVR12 rate with LDV/SOF for 8 weeks for GT1a-infected patients with baseline NS5A RASs and pretreatment HCV RNA <6 million IU/mL was 93% (14 of 15; Fig. 2A).

Data from treatment-experienced, HCV GT1a-infected patients without cirrhosis indicated a numerically lower SVR12 rate for those with baseline NS5A RASs who received LDV/SOF for 12 weeks (77% [10 of 13]); again, this signal was captured entirely by the primary class list (Fig. 2B). Similar findings of an impact of NS5A RASs on LDV/SOF efficacy in treatment-experienced patients were recently reported by Zeuzem et al.34 Data were available for only 3 treatment-experienced, HCV GT1b-infected patients without cirrhosis with primary NS5A RASs who received LDV/SOF for 12 weeks, 2 of whom achieved SVR12 (data not shown). Of note, all 4 treatment-experienced, HCV GT1-infected virological failure patients without cirrhosis with baseline NS5A RASs who received the 12-week LDV/SOF regimen in these trials had virus with two baseline NS5A RASs detected from the primary list, which may explain the reduced treatment efficacy observed in this group (discussed below). In GT1a- and 1b-infected, treatment-experienced, patients without cirrhosis, SVR12 rates were 100% with the LDV/SOF 24-week and LDV/SOF + RBV 12-week regimens, regardless of the presence of NS5A RASs (Fig. 2B).
Based on overall efficacy results from the ION-2 and SIRIUS trials,31, 32, 35, 36 LDV/SOF for 24 weeks is recommended in labeling for treatment-experienced, HCV GT1-infected patients with compensated cirrhosis, whereas LDV/SOF + RBV for 12 weeks can be considered for those who can take RBV. It was difficult to draw conclusions about the impact of baseline NS5A RASs on treatment outcome in this population because of the small numbers of patients in the analysis subgroups (Fig. 2C). In the LDV/SOF 24-week treatment group, 2 of 13 (15%) GT1-infected patients with baseline NS5A RASs experienced virological failure. Ultimately, the more intensive treatment regimens recommended in labeling are intended to maximize efficacy in treatment-experienced patients with cirrhosis, regardless of the presence or absence of baseline NS5A RASs.
Further analyses of patients who received LDV/SOF for 12 weeks in phase 3 trials indicated that 86% (76 of 88) and 82% (110 of 134) of patients with baseline NS5A RASs had only one NS5A RAS detected based on the primary and extended position lists, respectively, and among these subgroups SVR12 rates were high (99% and 98%, respectively; Fig. 2D). Reduced efficacy was observed only for the small subgroup of patients in whom two or more NS5A RASs were detected from the primary NS5A position list. This subgroup represented 2% (12 of 534) of pooled GT1-infected patients who received LDV/SOF for 12 weeks, of whom 67% (8 of 12) achieved SVR12; 2 (17%) of these 12 subjects had cirrhosis and both achieved SVR12.
Taken together, SVR12 rates with LDV/SOF in HCV GT1-infected patients were generally high regardless of the presence or absence of baseline NS5A RASs for regimens currently recommended in labeling; however, numerically lower SVR12 rates were observed for certain subgroups (with cirrhosis, or two or more NS5A RASs). At the time of approval, LDV/SOF represented the first highly effective, IFN-free, and RBV-free treatment option approved for HCV GT1-infected patients; there was no commercially available NS5A polymorphism screening assay, and there were promising, but limited, data on retreatment options for LDV/SOF virological failure patients. The regimens recommended in the United States label were intended to maximize treatment efficacy for all patients, particularly for treatment-experienced patients with cirrhosis.32, 33 Based on the low or inconsistent impact of baseline NS5A RASs on LDV/SOF ± RBV treatment efficacy, a pretreatment resistance testing recommendation is not included in the label. Nevertheless, data on the impact of baseline NS5A RASs are summarized in the label to be considered by clinicians on a case-by-case basis for treatment decision making, and both AASLD/IDSA and EASL treatment guidelines include baseline NS5A resistance testing considerations for HCV GT1a-infected, treatment-experienced patients.7, 8
OMBITASVIR/PARITAPREVIR/RITONAVIR PLUS DASABUVIR
The original FDA resistance analyses of ombitasvir/paritaprevir/ritonavir + dasabuvir (PrOD; NS5A inhibitor, ritonavir-boosted NS3/4A protease inhibitor, and non-nucleoside NS5B-palm polymerase inhibitor) were unusual because only a subset of patients in phase 3 trials had baseline samples analyzed, based partly on results from a large phase 2b trial that indicated baseline RASs did not significantly influence treatment outcome.37, 38 Nevertheless, pooled analyses of data from phase 3 trials indicated that baseline NS3 Q80K and NS5A RASs were enriched among HCV GT1a-infected patients who experienced virological failure relative to those who achieved SVR. Ultimately, the most efficacious regimen overall for GT1a-infected patients was recommended in the label (PrOD + RBV for 12 weeks for GT1a-infected patients without cirrhosis, PrOD + RBV for 24 weeks for GT1a-infected patients with compensated cirrhosis) to minimize the likelihood of virological failure and treatment-emergent drug resistance across multiple DAA classes.37, 39
Following the original NDA approval, the sponsor conducted retrospective analyses of a more complete set of samples from the phase 3 trials PEARL-IV,40 SAPPHIRE-II,41 and TURQUOISE-II,42 to characterize more precisely the impact of baseline RASs on treatment efficacy.43 Independent analyses of these results confirmed that the regimens recommended in labeling resulted in high SVR12 rates in clinical trials regardless of the presence of baseline RASs.
Among HCV GT1a-infected patients without cirrhosis treated with the nonrecommended PrOD (no RBV) regimen, the detection of NS3 Q80K or NS5A RASs (captured entirely by the primary class list) at baseline was associated with 10% and 21% lower SVR12 rates, respectively, relative to patients without these RASs (Fig. 3A). A Q80K substitution by itself in a GT1a replicon conferred only a modest ∼3-fold reduction in paritaprevir susceptibility (Table 1), but may contribute toward resistance when present in combination with certain other NS3 RASs that emerge with virological failure. Interestingly, all 6 patients with baseline NS5A RASs who experienced virological failure with the PrOD regimen (including 1 additional post-SVR12 relapser) also had virus with NS3 Q80K, indicating that either Q80K alone or the combination of baseline RASs in both targets may be most critical in contributing to virological failure with this suboptimal regimen. Importantly, SVR12 rates with the recommended PrOD + RBV regimen trended higher across all of the subgroups with or without baseline NS3 Q80K or NS5A RASs (Fig. 3A), supporting the use of this regimen for HCV GT1a-infected patients without cirrhosis without any consideration for baseline resistance testing.
Among HCV GT1a-infected patients with cirrhosis, the detection of baseline NS3 Q80K was associated with a 10% lower SVR12 rate for those who received the nonrecommended 12-week duration of the PrOD + RBV regimen, but there was no indication that baseline NS5A RASs affected efficacy with either treatment duration (Fig. 3B). Again, the recommended regimen (24-week duration) was associated with a high SVR12 rate across all subgroups analyzed.
The detection of dasabuvir baseline RASs in NS5B was uncommon and was not consistently associated with treatment outcome in these analyses or in the original NDA analyses.37 Furthermore, baseline RASs in any drug target did not influence efficacy for HCV GT1b-infected patients, because the SVR12 rate was nearly 100% across all phase 3 trials of the PrOD with or without the RBV regimen.
DACLATASVIR PLUS SOFOSBUVIR
The combination of daclatasvir (DCV; NS5A inhibitor) plus SOF was approved for the treatment of patients with HCV GT1 or GT3 infection based on results from the ALLY-1/2/3 trials.44-46 According to DCV labeling, the recommended treatment duration is 12 weeks, and dosing with RBV is recommended for HCV GT1-infected patients with decompensated cirrhosis or who have received a liver transplant, and for HCV GT3-infected patients with cirrhosis and posttransplant patients.47
For analyses of GT1-infected patients (ALLY-1/2 trials), resistance data were pooled across treatment groups because of the small sizes of treatment arms and key patient subpopulations.48 These analyses showed that the detection of baseline NS5A RASs (again captured entirely by the primary class list) was associated with a 22% lower SVR12 rate among HCV GT1a-infected patients (Fig. 4A), but not for GT1b-infected patients (data not shown). Four of 17 (24%) HCV GT1a-infected patients with baseline NS5A RASs experienced virological failure; all 4 subjects had Child-Pugh A/B cirrhosis (n = 2 each), and 3 of these 4 patients received DCV plus SOF with RBV. At the time of treatment failure, 3 of the 4 patients had additional treatment-emergent RASs in NS5A and/or NS5B, raising concerns about possible drug resistance consequences of failure. None of the 11 HCV GT1a-infected patients without cirrhosis with baseline NS5A RASs (primary class list) experienced virological failure with DCV/SOF ± RBV.
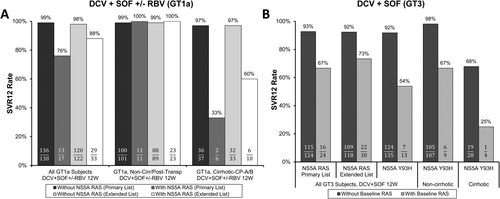
Although it was challenging to draw major conclusions based on such a small number of GT1a-infected patients with NS5A RASs, these results were not entirely unexpected, given that other trials had demonstrated an impact of NS5A RASs on DCV-based treatment efficacy.6, 46 It is also plausible that cirrhosis influenced the impact of baseline RASs, as was shown for SMV plus SOF. Therefore, based on the totality of information, DCV labeling recommends providers consider screening for the presence of baseline NS5A RASs (based on the primary class list) for HCV GT1a-infected patients with cirrhosis.47 The relatively flexible language is based on the limited data, favorable efficacy overall in patients with decompensated cirrhosis (particularly Child-Pugh B), and lack of a known alternative DCV + SOF–based regimen with improved efficacy for HCV GT1a-infected patients with cirrhosis and NS5A RASs.48 Of note, AASLD/IDSA HCV treatment guidelines7 no longer recommend DCV + SOF for HCV GT1-infected patients with compensated cirrhosis, whereas EASL treatment guidelines8 include baseline NS5A resistance testing considerations for treatment-experienced GT1a-infected patients regardless of cirrhosis status.
In HCV GT3-infected patients, there was also evidence that NS5A RASs reduced treatment efficacy in the ALLY-3 registrational trial, with SVR12 rates of 67% and 93% for those with and without NS5A RASs, respectively (primary list; Fig. 4B).49 This association was observed in both patients with and without cirrhosis and was driven primarily by NS5A Y93H, which accounted for 6 of the 8 virological failure patients with baseline NS5A RASs. Clearly, NS5A Y93H was the key DCV resistance pathway in this trial, given that baseline or treatment-emergent Y93H was detected in 15 of the 17 treatment failure patients. Interestingly, among patients with baseline Y93H who experienced virological failure, no additional treatment-emergent NS5A RASs were observed, indicating that in GT3, the >3,000-fold reduction in HCV susceptibility caused by Y93H alone was likely sufficient to confer HCV resistance to clinically relevant DCV levels.
As noted above, dosing of DCV and SOF with RBV is recommended in labeling for HCV GT3-infected patients with cirrhosis.47 Pooling data from ALLY-148 and the ALLY-3+ trial50 (a published follow-up study to ALLY-3) in which patients received DCV plus SOF and RBV for 12 weeks, SVR12 was achieved in 18 of 19 (95%) GT3-infected patients with Child-Pugh A or B cirrhosis who had HCV without NS5A Y93H. Unfortunately, the efficacy benefit of adding RBV for HCV GT3-infected patients with the Y93H RAS, regardless of cirrhosis status, could not be determined because of insufficient data.
Including a GT3 NS5A Y93H screening recommendation in the DCV label was considered; however, a specific screening recommendation ultimately was not included in the label because (1) DCV plus SOF provided a 12-week, IFN- and RBV-free treatment option for HCV GT3, and available alternative regimens at the time of approval (including Peg-IFNα and/or RBV) were not ideal, (2) a commercial assay to detect Y93H in GT3 was not available at the time, and (3) there are no clear DAA resistance consequences of treatment failure for patients who already had the Y93H polymorphism detected at baseline.48, 49 Nevertheless, data on the impact of Y93H are described in the DCV label to be considered by clinicians on a case-by-case basis,47 and considerations for Y93H testing for patients with HCV GT3 infection are now included in both AASLD/IDSA and EASL guidelines.7, 8
ELBASVIR/GRAZOPREVIR
The combination of EBR/GZR (NS5A inhibitor and NS3/4A protease inhibitor) is also impacted by the presence of baseline NS5A RASs in HCV GT1a-infected patients. Pooling data across registrational trials of DAA-naïve patients, in GT1a-infected patients the detection of baseline NS5A RASs was associated with a nearly 30% lower SVR12 rate with the EBR/GZR 12-week regimen, and similar to other DAA regimens this signal was captured entirely by the primary class list of NS5A positions with no additional failures identified based on the extended class list (Fig. 5A).19, 51 The impact of NS5A RASs on treatment efficacy was observed regardless of past Peg-IFNα/RBV treatment history or cirrhosis status. Baseline HCV-RNA level by itself was not associated with treatment outcome; however, the presence of a low baseline HCV-RNA level (using a relatively low cutoff of <800,000 IU/mL) may reduce the impact of baseline NS5A RASs for a small subset of GT1a-infected patients. Of the 56 GT1a-infected patients with baseline NS5A RASs (primary list) who received EBR/GZR for 12 weeks, 14% (8 of 56) had baseline HCV RNA <800,000 IU/mL, and all 8 achieved SVR12, whereas 65% (31 of 48) of patients with baseline NS5A RASs and HCV RNA ≥800,000 IU/mL achieved SVR12. The majority of GT1a-infected patients with NS5A RASs had only a single polymorphism detected, and in contrast to the results with LDV/SOF, with EBR/GZR for 12 weeks the detection of only single baseline NS5A RASs was still associated with a 26% lower SVR12 rate (based on primary NS5A position list) relative to those patients without NS5A RASs (Fig. 5B). Of particular concern, HCV populations emerged with additional or evolving NS5A and/or NS3 RASs substitutions in nearly all HCV GT1a-infected patients with baseline NS5A RASs who failed treatment.
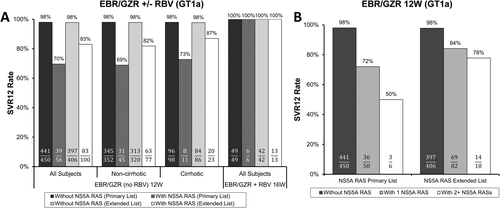
Further analyses indicated that an intensified regimen of EBR/GZR plus RBV for 16 weeks may reduce the impact of baseline NS5A RASs in HCV GT1a-infected patients.19, 52 This conclusion relies on only 6 patients who had an NS5A RAS at a key resistance-associated position, all of whom achieved SVR12 with this regimen (Fig. 5A). An 18-week duration of EBR/GZR plus RBV was also evaluated, but this treatment group did not include any GT1a-infected patients with a baseline NS5A RAS from the primary list. Despite the limited data for GT1a-infected patients with baseline NS5A RASs who received EBR/GZR plus RBV for 16 weeks, these patients represented a challenging group given that all 6 were Peg-IFNα/RBV treatment-experienced, 2 had cirrhosis, and various patterns of NS5A polymorphisms were represented. Based on these results and the desire to minimize treatment failure, EBR/GZR labeling,53 AASLD/IDSA treatment guidelines,7 and EASL treatment guidelines8 all include pretreatment NS5A resistance testing considerations to determine the optimal EBR/GZR regimen for patients with HCV GT1a infection.
As in other trials, the detection of baseline NS3 Q80K was common among HCV GT1a-infected patients in clinical trials of EBR/GZR, but there was no evidence that it affected treatment outcome. Among HCV GT1b-infected patients, the 12-week EBR/GZR regimen resulted in SVR12 rates of 94% (48 of 51) and 99.6% (247 of 248) for those with and without baseline NS5A RASs, respectively (primary list). A more detailed summary of FDA analyses of EBR/GZR resistance in clinical trials is described elsewhere.19, 51
SOFOSBUVIR/VELPATASVIR
The presence of NS5A RASs at baseline did not affect treatment outcomes for HCV GT1-infected patients who received 12 weeks of SOF and velpatasvir (VEL; NS5A inhibitor [SOF/VEL]) in the ASTRAL-1 trial (patients without cirrhosis or with compensated cirrhosis).54, 55 Only 2 HCV GT1-infected patients overall experienced virological failure in this trial; 1 had virus at baseline with NS5A RASs detected, but was subsequently found to have been infected with an unusual GT1 subtype (1c/h). Among HCV GT1a- and 1b-infected patients, SVR12 rates were 100% (19 of 19) and 100% (26 of 26), respectively, for those with baseline NS5A RASs (primary list).
In ASTRAL-3,56 HCV GT3-infected patients without cirrhosis or with compensated cirrhosis received SOF/VEL for 12 weeks or SOF plus RBV for 24 weeks. In the SOF/VEL arm of this trial, high SVR12 rates were observed among HCV GT3-infected patients without cirrhosis with or without baseline NS5A RASs (Fig. 6A). Among GT3-infected patients with cirrhosis who received SOF/VEL, 6 had baseline NS5A RASs (primary list), of whom 3 (50%) achieved SVR12. Two of these patients with cirrhosis had baseline Y93H detected, and both experienced virological failure, although baseline Y93H was associated with only a modestly lower SVR12 rate for GT3-infected patients without cirrhosis (SVR12 = 92% [12 of 13]; 9 of 10 treatment-naïve, 3 of 3 treatment-experienced). Overall efficacy of SOF/VEL for GT3-infected patients was lower for treatment-experienced patients relative to treatment-naïve patients, with SVR12 rates of 90% (64 of 71) and 98% (200 of 204), respectively. Again, limited data on the impact of baseline NS5A RASs were available when restricting the analyses to the treatment-experienced subgroup, with SVR12 rates of 83% (5 of 6) and 90% (59 of 65) for those with and without baseline NS5A RASs, respectively. Given the numerically lower SVR12 rate in ASTRAL-3 for HCV GT3-infected patients with cirrhosis or treatment-experience and the potential impact of baseline NS5A RASs, as well as indirect evidence from ASTRAL-4 that addition of RBV may improve treatment efficacy (summarized below), the addition of RBV to the 12-week SOF/VEL regimen, possibly in conjunction with pretreatment NS5A resistance testing, was considered for HCV GT3-infected patients with compensated cirrhosis.55 However, the data were considered insufficient at the time to draw conclusions about the potential risk versus benefit of adding RBV. A clinical trial evaluating SOF/VEL dosed with RBV in the GT3-compensated patients with cirrhosis population is ongoing, which may help clarify the optimal treatment regimen. Current AASLD/IDSA and EASL treatment guidelines include resistance testing recommendations (focused on Y93H) for GT3-infected patients according to their past treatment history and cirrhosis status,7, 8 which is a reasonable approach for clinicians to consider on a case-by-case basis to maximize treatment efficacy, pending additional clarifying data.

ASTRAL-457 evaluated SOF/VEL for 12 weeks, SOF/VEL plus RBV for 12 weeks, and SOF/VEL for 24 weeks, in patients with Child-Pugh B decompensated cirrhosis. Among HCV GT1-infected patients with decompensated cirrhosis, overall SVR12 rates were numerically highest in the 12-week SOF/VEL plus RBV arm (98%; 65 of 66), with no evidence that baseline NS5A RASs affected treatment outcome in this treatment group (Fig. 6B). Numerically lower SVR12 rates were observed in the RBV-free treatment arms for HCV GT1-infected patients with baseline NS5A RASs, although these differences are based on a small number of patients. Consistent with the results in HCV GT1-infected patients, the highest SVR12 rate overall for HCV GT3-infected patients with decompensated cirrhosis was observed in the 12-week SOF/VEL plus RBV arm (85%; 11 of 13 compared to 54% [7 of 13] in the 12-week SOF/VEL arm and 55% [6 of 11] in the 24-week SOF/VEL arm); however, no GT3-infected patients in the SOF/VEL plus RBV arm had baseline NS5A RASs, so we were unable to determine their impact on treatment outcome in this patient population. The 12-week SOF/VEL plus RBV regimen is recommended in labeling for HCV GT1- or GT3-infected patients with decompensated cirrhosis based on overall SVR12 rates in ASTRAL-4 and the desire to maximize treatment efficacy in this population with advanced disease.55, 58 Attributed to the complex nature of this population, additional considerations and treatment recommendations are described in AASLD/IDSA and EASL treatment guidelines, but without regard to baseline NS5A resistance characteristics.7, 8
GLECAPREVIR/PIBRENTASVIR
Based on pooled analyses of NGS resistance data from registrational trials of glecaprevir (GLE; NS3/4A protease inhibitor) and pibrentasvir (PIB; NS5A inhibitor [GLE/PIB]) conducted in patients naïve to NS3/4A protease inhibitors and NS5A inhibitors, baseline HCV RASs did not affect treatment outcome for patients with HCV GT1 infection. Among HCV GT1-infected patients who received GLE/PIB regimens recommended in United States labeling (8 weeks for patients without cirrhosis, 12 weeks for patients with cirrhosis), only 2 of 488 (<1%) patients overall experienced virological failure.59 Both virological failure patients had a GT1a infection, 1 of whom had an NS5A Y93N RAS detected at baseline; overall, Y93N was detected at a ≥15% level in 3 of 380 (<1%) GT1a baseline NS5A sequences. Considering the GLE/PIB regimens recommended in labeling, SVR12 rates were 97% (31 of 32) and 100% (42 of 42) for GT1a- and GT1b-infected patients, respectively, with baseline NS5A RASs (primary list). The NS3 Q80K baseline RAS was detected in 35% (135 of 384) of GT1a-infected subjects, 100% of whom achieved SVR12 with GLE/PIB for 8 or 12 weeks.
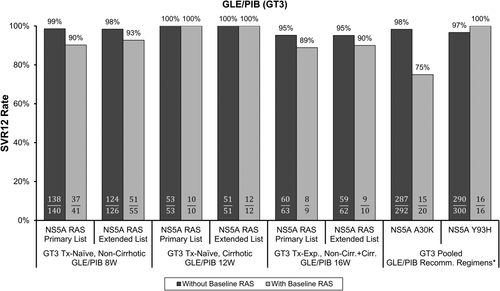
There was a signal that baseline NS5A RASs, particularly A30K, may cause a modest reduction in GLE/PIB efficacy for HCV GT3-infected patients. Overall, for regimens recommended in labeling, SVR12 rates ranged from 89% to 100% for GT3-infected patients with baseline NS5A RASs from the primary list (Fig. 7). Any differences in SVR12 rates for HCV GT3-infected patients with and without baseline NS5A RASs could be attributed to the detection of NS5A A30K. Across GLE/PIB clinical trials, A30K was detected in approximately 6% of HCV GT3a sequences; note that GT3a was the predominant GT3 subtype (98% of GT3 patients) in GLE/PIB clinical trials, and the reference sequence at this position varies according to GT3 subtype.59 Pooling data for label-recommended regimens, SVR12 rates were 75% (15 of 20) and 98% (287 of 292) for HCV GT3a-infected patients with and without baseline A30K, respectively. Most of the patients with baseline A30K were treatment-naïve and without cirrhosis, of whom 78% (14 of 18) achieved SVR12 with GLE/PIB for 8 weeks. A numerically higher SVR12 rate (93% [13 of 14]; failure had A30K + Y93H) was observed for treatment-naïve patients without cirrhosis with baseline A30K who received GLE/PIB for 12 weeks,59 indicating that a longer treatment duration may reduce the impact of A30K. However, this difference is based on only 4 and 1 virological failure patients in the 8- and 12-week treatment groups, respectively, and one of the failures in the 8-week group occurred on-treatment and thus would not have been prevented with a longer treatment duration. Data are not available to determine whether the addition of RBV or a third DAA may improve treatment efficacy in GT3-infected patients with baseline A30K. Furthermore, insufficient data were available to assess the impact of baseline A30K in GT3-infected patients with cirrhosis (n = 1, treatment-naïve, SVR12) or past treatment experience (n = 1, without cirrhosis, virological failure). Of note, the NS5A Y93H baseline RAS by itself did not affect treatment outcome, because SVR12 was achieved in 100% (16 of 16) of GT3-infected patients with baseline Y93H who received label-recommended regimens. Interestingly, among GT3 infected patients who experienced virological failure, Y93H was the most common treatment-emergent NS5A RAS (83% [15 of 18] of virological failure patients), and was usually detected in combination with A30K or other NS5A RASs, consistent with the presence of multiple NS5A RASs conferring enhanced HCV phenotypic resistance to PIB. The presence of A30K + Y93H in a GT3a replicon resulted in a 69-fold reduction in PIB susceptibility,59 whereas neither RAS alone significantly reduced PIB susceptibility (Table 1). No baseline resistance testing recommendations are included in United States labeling for GLE/PIB, although available data on the impact of the GT3 NS5A A30K baseline RAS are described for consideration by clinicians on a case-by-case basis.
Conclusions
In this article, we presented multiple examples where HCV RASs detected at baseline can reduce the efficacy of DAA-based regimens in patients with chronic HCV GT1 or GT3 infection. Importantly, we also presented examples where intensified or alternative DAA-based regimens appeared to overcome the impact of baseline RASs. Therefore, highly effective treatment options are available for patients with or without HCV RASs, and in some cases regimens can be optimized based on RAS patterns detected at baseline.
There are some limitations with these analyses that should be considered. We utilized a consistent NGS sensitivity cutoff (15%) as well as standardized lists of resistance-associated positions for optimal comparisons of efficacy results across different DAA regimens. Nevertheless, cross-trial comparisons should always be interpreted cautiously, and in the presented trials, randomization to treatment arms was not stratified with consideration of pretreatment HCV resistance characteristics. In addition, within our standardized analysis lists, there was an uneven distribution of specific baseline NS5A RASs across treatment arms and trials, which was unavoidable because of small sample sizes. Therefore, it was often not possible to assess the precise impact of specific individual NS5A RASs or specific combinations of RASs on treatment outcome. Finally, these analyses focused on HCV GT1 and GT3, and although highly effective regimens are now available for all major HCV GTs, the role of HCV genetic variability for less common HCV GTs, such as GT5 and the many subtypes of GT6, has not been fully elucidated because of relatively limited clinical data.
Our analyses of baseline NS5A RASs for six different NS5A inhibitor-containing regimens indicate that any signal of an impact on efficacy in NS5A inhibitor-naïve patients with HCV GT1 or GT3 infection is captured by RASs from the four-position “primary” list (NS5A amino acid positions 28, 30, 31, or 93), if not specific individual positions within that list, and broadening the list to include other NS5A positions provides little added value and dilutes the signal of any impact on treatment efficacy. This does not rule out an impact of specific NS5A RASs outside of amino acid positions 28, 30, 31, or 93 on NS5A inhibitor-based treatment efficacy; however, such RASs are either rarely or never detected at baseline in NS5A inhibitor-naïve patients (e.g., H58D [GT1a], P32-Deletion), or appear to have less of an impact on treatment efficacy when detected in the absence of additional RASs from the four-position “primary” list.
Although the results in this article may provide insight into potential retreatment strategies for DAA-experienced patients with RASs following treatment failure, it is important to consider that drug resistance patterns and other clinical characteristics in DAA-experienced patients may differ from those in DAA-naïve patients, and analyses and discussion of DAA retreatment outcomes (i.e., retreatment with the same DAA class) are beyond the scope of this article. Nevertheless, studies of certain treatment intensification strategies similar to those that have been shown to reduce the impact of baseline RASs in DAA-naïve patients, such as a longer duration or adding DAAs or RBV, have demonstrated favorable efficacy results in DAA-experienced patients.60-63 More recently, the fixed-dose combination DAA regimens sofosbuvir/velpatasvir/voxilaprevir (SOF/VEL/VOX; VOX is an NS3/4A protease inhibitor) and GLE/PIB have been approved in the United States and Europe. Both regimens were studied in clinical trials that included DAA-experienced patients, including those with experience with NS3/4A protease inhibitors and/or NS5A inhibitors. In HCV GT1-infected patients, the three-DAA SOF/VEL/VOX regimen was highly effective in NS5A inhibitor-experienced patients, including those who also previously received an NS3/4A protease inhibitor.64-66 The two-DAA GLE/PIB regimen also had favorable efficacy in patients with prior NS5A inhibitor experience, whereas reduced efficacy was observed for patients with experience to both NS3/4A protease inhibitors and NS5A inhibitors, which was associated with the detection of baseline RASs in both drug targets.59 We speculate that an intensified GLE/PIB regimen including at least one additional fully active drug from a different class is necessary for optimal GLE/PIB efficacy in this challenging patient population, considering both the impressive efficacy of the 3-DAA SOF/VEL/VOX regimen as well as the findings described in this article.
The analysis of HCV drug resistance mechanisms in clinical trials has played a critical role in the regulatory review of HCV DAAs. The results and uniform analysis methods presented in this article should bring more clarity regarding the efficacy impact and regulatory perspectives on baseline HCV RASs for currently available DAA therapies, and also help guide future studies. Building on the tremendous successes and knowledge gained in HCV drug development in recent years, we expect that HCV genetic variability and drug resistance considerations will continue to evolve as novel treatment strategies are developed and introduced into clinical practice.
Acknowledgments
Most data analyzed for this report were submitted in Original or Supplemental New Drug Applications for the HCV DAAs described. The retrospective resistance analysis data for ombitasvir/paritaprevir/ritonavir plus dasabuvir are shown with permission from AbbVie, Inc. We acknowledge the study sponsors, as well as the numerous investigators and study volunteers, as the source of these data. We also thank the FDA DAA review team members for helpful discussions and perspectives on all of the regimens described in this article and, specifically, Dr. Jeffrey Murray, Dr. Debra Birnkrant, Dr. John Farley, and Dr. Edward Cox for helpful discussions and editorial suggestions.
REFERENCES
Author names in bold designate shared co-first authorship.