B lymphocytes limit senescence-driven fibrosis resolution and favor hepatocarcinogenesis in mouse liver injury
Potential conflict of interest: Nothing to report.
Supported by grants MIUR-FIR RBFR12I3UB (to B.C.), MIUR-FIRB RBAP11H2R9-004 (to P.V.), AMANDA Project (Regione Lombardia/CNR) to P.V., and CNR-DSB Aging Project (to P.V) and Prot. No. 0059711 (to F.F.).
Abstract
Hepatocellular carcinoma (HCC) is a frequent neoplasia and a leading cause of inflammation-related cancer mortality. Despite that most HCCs arise from persistent inflammatory conditions, pathways linking chronic inflammation to cancer development are still incompletely elucidated. We dissected the role of adaptive immunity in the Mdr2 knockout (Mdr2–/–) mouse, a model of inflammation-associated cancer, in which ablation of adaptive immunity has been induced genetically (Rag2–/–Mdr2–/– and μMt-Mdr2–/– mice) or with in vivo treatments using lymphocyte-specific depleting antibodies (anti-CD20 or anti-CD4/CD8). We found that activated B and T lymphocytes, secreting fibrogenic tumor necrosis factor alpha (TNFα) and other proinflammatory cytokines, infiltrated liver of the Mdr2–/– mice during chronic fibrosing cholangitis. Lymphocyte ablation, in the Rag2–/–Mdr2–/– and μMt-Mdr2–/– mice, strongly suppressed hepatic stellate cell (HSC) activation and extracellular matrix deposition, enhancing HSC transition to cellular senescence. Moreover, lack of lymphocytes changed the intrahepatic metabolic/oxidative state, resulting in skewed macrophage polarization toward an anti-inflammatory M2 phenotype. Remarkably, hepatocarcinogenesis was significantly suppressed in the Rag2–/–Mdr2–/– mice, correlating with reduced TNFα/NF-κB (nuclear factor kappa B) pathway activation. Ablation of CD20+ B cells, but not of CD4+/CD8+ T cells, in Mdr2–/– mice, promoted senescence-mediated fibrosis resolution and inhibited the protumorigenic TNFα/NF-κB pathway. Interestingly, presence of infiltrating B cells correlated with increased tumor aggressiveness and reduced disease-free survival in human HCC. Conclusion: Adaptive immunity sustains liver fibrosis (LF) and favors HCC growth in chronic injury, by modulating innate components of inflammation and limiting the extent of HSC senescence. Therapies designed for B-cell targeting may be an effective strategy in LF. (Hepatology 2018;67:1970-1985).
Abbreviations
-
- ALT
-
- alanine aminotransferase
-
- αSMA
-
- α-smooth muscle actin
-
- β-gal
-
- β-galactosidase
-
- Ccl2
-
- C-C motif chemokine ligand 2
-
- CCN1
-
- cysteine-rich angiogenic inducer 61
-
- CI
-
- confidence interval
-
- Col1a
-
- collagen, type I, alpha
-
- ECM
-
- extracellular matrix
-
- FACS
-
- fluorescence-activated cell sorting
-
- FBS
-
- fetal bovine serum
-
- HCC
-
- hepatocellular carcinoma
-
- HCG
-
- hepatocarcinogenesis
-
- HCV
-
- hepatitis C virus
-
- HF
-
- hepatic fibrosis
-
- HO-1
-
- heme oxygenase-1
-
- HR
-
- hazard ratio
-
- HSCs
-
- hepatic stellate cells
-
- IgG
-
- immunoglobulin G
-
- IL
-
- interleukin
-
- iNOS
-
- inducible NO synthase
-
- LF
-
- liver fibrosis
-
- LPS
-
- lipopolysaccharide
-
- mAb
-
- monoclonal antibody
-
- MHCII
-
- major histocompatibility complex class II
-
- Mmp-13
-
- matrix metalloproteinase-13
-
- NOS
-
- NO synthase
-
- NF-κB
-
- nuclear factor kappa B
-
- NK
-
- natural killer
-
- NKT
-
- natural killer T
-
- NO
-
- nitric oxide
-
- PBS
-
- phosphate-buffered saline
-
- PDGFR-β
-
- Platelet-derived growth factor receptor-β
-
- SAA
-
- serum amyloid A protein
-
- SA-β-gal
-
- senescence-associated β-galactosidase
-
- SASP
-
- senescence-associated secretory phenotype
-
- Tgfβ1
-
- transforming growth factor beta 1
-
- Timp3
-
- tissue inhibitor of metalloproteinases-3
-
- TIS
-
- therapy-induced senescence
-
- TLR4
-
- Toll-like receptor 4
-
- TNFα
-
- tumor necrosis factor alpha
-
- TUNEL
-
- terminal deoxynucleotidyl transferase dUTP nick end labeling
-
- wt
-
- wild type
Hepatocellular carcinoma (HCC) is a frequent neoplasia and a leading cause of cancer-related death worldwide.1 It commonly develops in patients with chronic liver diseases, which may result from viral hepatitis or exposure to metabolic toxins.1 Chronic hepatic inflammation is a major risk factor for liver tumor development, given that more than 90% of HCCs are inflammation driven,2 thus providing a rationale for the continued development of an immune-based approach to anticancer therapy.3 Nonetheless, the identification of the cellular actors causing transition from liver cirrhosis to dysplasia and HCC is constantly evolving.
Innate immune cells indisputably play a key role in fibrosis initiation and progression, and the occurrence of tumor-associated macrophages has been linked to immune suppression, angiogenesis, and tumor progression.4 However, adaptive immunity also has a fundamental impact on the development and progression of HCC, eliciting both antitumor and protumor effects.5-8 In addition, the regulation of cellular senescence, a process that opposes tumor initiation and progression, is also influenced by T-cell function. In fact, CD4+ T lymphocytes can either induce growth arrest and senescence in cancer cells by secreting proinflammatory cytokines or trigger the clearance of senescent premalignant hepatocytes.9-11
Interestingly, a role for B cells in the pathogenesis of liver fibrosis (LF) has been also hypothesized.12 To unravel the role of adaptive immune responses in the pathogenesis of HCC, we utilized the Mdr2 knockout mice (Mdr2–/–) mouse model lacking the liver-specific P-glycoprotein, responsible for phosphatidylcholine transport across the canalicular membrane.13 The absence of phospholipids from bile leads to bile regurgitation into the portal tracts, causing important portal inflammation followed by slowly developing HCC, which closely mimics the human disease.14 Thus, this model is very suitable for investigating the role of adaptive immunity in chronic inflammation during premalignancy, senescence immune surveillance, and cancer.
Materials and Methods
MICE AND TREATMENTS
C57BL6/J-Abcb4tm1Bor (Mdr2–/–), B6.129S2-Ighmtm1Cgn/J and C57BL6/J wild-type (wt) mice were from The Jackson Laboratory (USA). B6.129S6-Rag2tm1FWA N12 mice were purchased from Taconic (USA). Mice were bred and housed under specific pathogen-free conditions at the Animal Facility of the Humanitas Clinical and Research Center in compliance with national and international law and policies. Food and water were provided ad libitum. To deplete CD20+ B lymphocytes, 5-month-old male mice were treated once every 2 weeks with intravenous injections of anti-mouse CD20 monoclonal antibody (mAb; clone 18B12, 7.5 μg/g of body weight; Biogen) for 8 weeks. To deplete CD4+ T and CD8+ T cells, mice were subjected to intravenous injections of Rat anti-mouse CD4 and anti-mouse CD8a mAbs (clones GK1.5 and 53-6.7; BioXcell, West Lebanon, USA). CD4- and CD8-depleting antibodies were administered every 7 days at doses of 7.5 μg/g of body weight, for 8 weeks. Control groups received the corresponding isotype controls (immunoglobulin G [IgG] 1, IgG2a, and IgG2b; BioXcell). Animals were sacrificed for analysis 1 week after the last injection. Experiments were performed according to protocols approved by the Istituto Clinico Humanitas and the Italian Institutional Animal Care and Use Committee.
LIVER LYMPHOCYTE ISOLATION
Mice were euthanized by CO2 inhalation. The hepatic portal vein was cannulated and perfused with 10 mL of ice-cold phosphate-buffered saline (PBS). After the removal of the gall bladder, the liver was rinsed with ice-cold PBS, chopped on ice into small ∼1-mm3 pieces, and then enzymatically digested in Iscove's modified Dulbecco's medium medium supplemented with 0.2 mg/mL of Liberase TL (Sigma-Aldrich, NJ, USA) and 0.01 mg/mL of DNase I (Sigma-Aldrich) for 30 minutes at 37°C. The enzymatic reaction was stopped using ethylenediaminetetraacetic acid (5 mM), and the liver suspension was meshed through a 70-μm cell strainer (Falcon, Tewksbury MA, USA). After centrifugation, the cell pellet was resuspended in 40% Percoll solution (92% Percoll, 7% PBS 10×, and 1% NaHCO3 7.2%; pH 7.2) in Hank's balanced salt solution supplemented with 100 UI/mL of heparin. After a centrifugation (1,800 rpm, 20 minutes, 4°C), the pellet was harvested and resuspended in RPMI 1640 medium with 5% fetal bovine serum (FBS).
IN VITRO CULTURE
Sorted liver CD19+ B220+ B and CD3+NK1.1_ T cells were resuspended at 2 × 105 cells/μL in RPMI 1640, 10% FBS, and 2 mM of l-glutamine and transferred into a flat-bottomed 96-well plate in 100 μL/well. Another 100 μL of RPMI was added that contained or not stimulating reagents at 2× final concentration. In case of in vitro liver T-cell stimulation, plates were previously coated with anti-mouse CD3 antibody (2 μg/mL, clone 145-2C11; eBioscience, MA, USA) for 3 hours at 37°C. Liver B cells were stimulated with lipopolysaccharide (LPS; 10 μg/mL; Sigma-Aldrich). Cocultures between liver B cells and splenic wt T cells were performed in the presence of soluble anti-mouse CD3 antibody (2 μg/mL; eBioscience). Supernatants were collected after 3 (T cells) or 5 days (B cells) of culture for cytokine secretion determination, and then cell proliferation was evaluated by 3H-thymidine incorporation. In vitro cocultures with hepatic stellate cells (HSCs) were established in normal or 5-μM pore-size Transwell inserts (Costar Corporation, Corning, NY, USA) 24-well plates, at a ratio 1:10 (HSC:T/B cells). Supernatants and HSCs for gene expression analysis were harvested after 5 days of culture.
HUMAN SAMPLES
A tissue microarray composed of 116 samples of HCC was obtained from the Department of Hepatobiliary and General Surgery of Humanitas Clinical and Research Center with the approval of the institutional review board (Prot. No. CE Humanitas ex D.M. 8/2/2013-248/14) and after the patient's informed consent. All patients had hepatitis C virus (HCV)-related cirrhosis. The series under examination is enriched by complete clinical, pathological, and phenotypical data.15 Each case was represented by six tumoral spots, three taken from inner and three from outer HCC areas, and three extratumoral spots. CD20 expression was semiquantitatively evaluated in each spot and expressed as the mean percentage of immunoreactive cells in the tissue for each case. The histological tumor grading was evaluated in accord with Broder's classification.
IMMUNOHISTOCHEMISTRY
Livers were systematically set up following the practice guideline suggested by Fickert et al.16 Paraffin-embedded tissue sections were stained for terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL; Calbiochem) and with the following antibodies: ki67 (clone D3B5, 1:400; Cell Signaling Technology); α-smooth muscle actin (αSMA; RB 9010-P1, 1:100; Thermo Fisher Scientific); γH2A.X (clone 9718, 1:100; Cell Signaling Technology, MA, USA); CD3 (MCA1477, 1:1,000: Bio-Rad Laboratories); Ym1 (1:400, no. 01404; Stem Cell Technologies, Vancouver, Canada); major histocompatibility complex class II (MHCII; 1:150, ab25333; Abcam, Cambridge, UK); heme oxygenase 1 (HO-1; 4915-1050, 1:400; Bio-Rad Laboratories); CD45R (BMS14-0452-82, 1:400; eBioscience); and anti-CD20 L26 (760-2531, Ventana, AZ, USA). One whole section from each animal (at least eight for each group) was scanned with vs-120dot-slide. The images were quantified using Image Pro-premier software (http://rsb.info.nih.gov/ij/). The immune-reactive area was calculated relative to the total liver section area. Detection of β-galactosidase (β-gal) activity was performed with the Senescence Detection kit (BioVision, CA, USA) on snap-frozen livers, following the manufacturing instruction. Microscopic analysis was performed using an Olympus BX51 (Olympus Corporation). Five high-power fields were counted on two liver sections from each mouse (20× or 40×, >150 counted cells per field).
STATISTICAL ANALYSIS
All statistical analyses were performed using the Mann-Whitney U or Student t test. For histopathology, data extrapolated using Image Pro-premier software were analyzed with GraphPad Prism (version 7.0; GraphPad Software Inc., San Diego, CA). Statistical analyses of the results were performed using the Kruskal-Wallis test, followed by Dunn's test. P values <0.05 were considered statistically significant.
For human studies, data are expressed as means ± SD. Variables were compared using the chi-square test (or Fisher's exact test) for categorical data and Student t test (or the Mann-Whitney U test) for continuous variables. Kaplan-Meier curves were used to analyze differences of overall and disease-free survivals that were compared using the log-rank test. Proportional hazard/Cox regression analysis was used to identify independent prognostic factors. Hazard ratio (HR) with confidence interval (CI) was used as effect size. A P value <0.05 was considered significant. All tests were two-sided. All analyses were performed with the software Stata 13 (StataCorp LP).
Results
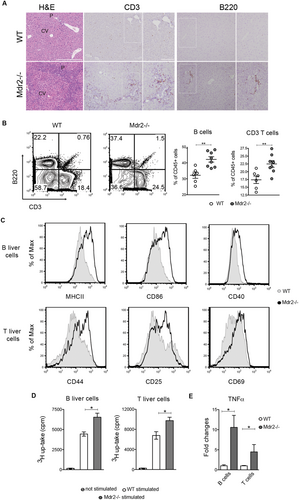
INTRAPORTAL ACCUMULATION OF LYMPHOCYTES IN INJURED LIVER OF Mdr2–/– MICE
To investigate the contribution of the adaptive immune cells in the inflammatory responses linked to HCC development, we evaluated the hepatic immune microenvironment of Mdr2–/– mice at the stage of chronic hepatitis. At the age of 7 months, mutant livers showed a prominent periportal ductular proliferation accompanied by leukocyte infiltration (Fig. 1A). Flow cytometry analysis on liver single-cell suspensions revealed markedly increased frequencies of CD3+ T cells and B220+ CD19+ B cells in the CD45+ fraction of mutant liver cells with respect to controls (Fig. 1B). No significant alterations were detected for natural killer T (NKT; CD3+ NK1.1+) and natural killer (NK; CD3– NK1.1+) subsets (Supporting Fig. S1A). Importantly, B- and T-cell infiltrates were localized predominantly at the periportal areas, where tissue injury arose (Fig. 1A), suggesting a primary involvement in the liver disease. To elaborate the role of lymphocytes in our model, we isolated, by fluorescence-activated cell sorting (FACS), intrahepatic CD19+ B- and CD3+ T-cell populations from perfused livers of Mdr2–/– and wt mice. Liver mutant B cells were in an increased activation state, characterized by enhanced expression of activation and costimulatory markers, such as MHCII, CD86, and CD40 (Fig. 1C and Supporting Fig. S1B). Consistent with their phenotype, Mdr2–/– B cells exhibited higher ability to prime and activate wt T cells in vitro (Supporting Fig. S1C,D). Likewise, T lymphocytes from mutants displayed greater levels of CD44, CD25, and CD69 markers compared to their normal counterpart (Fig. 1C and Supporting Fig. S1B). Next, we analyzed liver B- and T-cell functions following ex vivo stimulation with LPS and anti-CD3 mAb, respectively. Liver lymphocytes from Mdr2–/– mice displayed increased proliferative ability and produced greater amounts of the tumor necrosis factor alpha (TNFα) cytokine compared to controls (Fig. 1D,E). Thus, immune activity of B and T cells is enhanced during the pretumoral stage.
ABSENCE OF ADAPTIVE IMMUNITY REDUCES COLLAGEN DEPOSITION AND HSC ACTIVATION
The functional role of adaptive immunity in HCC pathogenesis was investigated utilizing Rag2–/–Mdr2–/– mice, which lack mature T and B cells.17 Liver histological examination confirmed that 7-month-old Mdr2–/– mice were affected by overt hepatic fibrosis (HF), characterized by marked portal to bridging oval cell/biliary hyperplasia, extracellular collagen deposition, and inflammatory reactions (Fig. 2A). As quantified by laser scanning, we found a rate of 2.6 ± 1.4% of Picrosirius red–positive collagen fibers in Mdr2–/– mice, representing a 12-fold increase over controls (0.2 ± 0.19%). Furthermore, a dramatic expansion of activated HSCs, positive for the marker, αSMA, was also observed in the mutant compared to WT mice (6 ± 2.2% vs. 0.8 ± 0.5%; Fig. 2B). Remarkably, collagen deposition, oval cell/biliary hyperplasia, and inflammatory cell infiltration were visibly reduced in liver of age-matched Rag2–/–Mdr2–/– mice (now referred as Dko), as demonstrated by the lower relative organ weight and histological score (Fig. 2C,D). Smaller αSMA+ areas and fewer collagen fibrils reflected a 50% reduction in HSC activation and collagen deposition in Dko mice (3 ± 1.2%; Fig. 2A,B). Consistently, liver mRNA expression of fibrogenic markers, including Col1a1, collagen, type I, alpha 1 (Col1a)1, Col1a2, and transforming growth factor beta 1 (Tgfβ1), were significantly reduced (Fig. 2F). Decreased hepatic inflammation in Dko was also confirmed by reduced serum levels of serum amyloid A (SAA) protein (Fig. 2E). Toll-like receptor 4 (TLR4) is required for hepatic fibrogenesis, driving HSC activation and enhancing their sensitization to TGFβ signaling.18 In line with the down-regulation of Tgfβ1 expression, liver Tlr4 mRNA was significantly reduced in Dko, compared to Mdr2–/–, mice (Fig. 2F). Additionally, expression of C-C motif chemokine ligand 2 (Ccl2), a potent mediator of macrophage recruitment and linked to TLR4 activation,18 was also decreased (Fig. 2F). To corroborate the role of lymphocytes in HSC activation and fibrosis, we performed cocultures of ex vivo purified Mdr2–/– B and T cells with wt HSCs. We confirmed that Mdr2–/– lymphocytes produce a high amount of Tnfα as well as of interleukin (IL)6 and IL1β (Supporting Fig. S2A). Furthermore, we found that B cells isolated from Mdr2–/– mice are more potent stimulators of HSC activation, measured by IL1α, Ccl2, Lox, and Col1a2 mRNA levels, when they are in direct cell-to-cell contact rather than separated in transwell cultures (Supporting Fig. S2B).
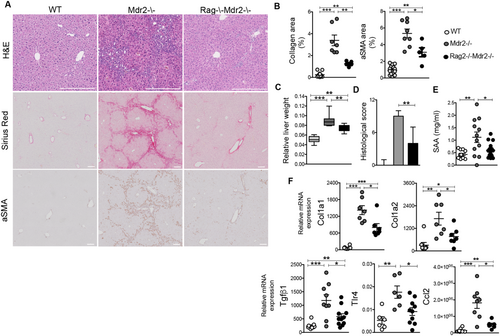
Overall, these data suggest that lymphocytes regulate the pathways sustaining HF following chronic liver injury.
ADAPTIVE IMMUNITY PROMOTES THE INFLAMMATORY PROTUMORIGENIC MICROENVIRONMENT OF FIBROTIC Mdr2–/– LIVERS
TNFα/NF-κB signaling promotes disease progression and HCC development in Mdr2–/– mice.6 Accordingly, cholangiohepatitis in 7-month-old Mdr2–/– mice was characterized by markedly increased liver mRNA and serum concentration of Tnfα (Fig. 3B), correlating with augmented hepatic activation of the NF-κB pathway, as demonstrated by the higher p65 phosphorylation in western blotting analysis (Fig. 3C). In contrast, the TNFα/NF-κB axis was suppressed in age-matched Dko mice (Fig. 3A-C), confirming that adaptive immune responses significantly contributed to the activation of this inflammatory signaling pathway.
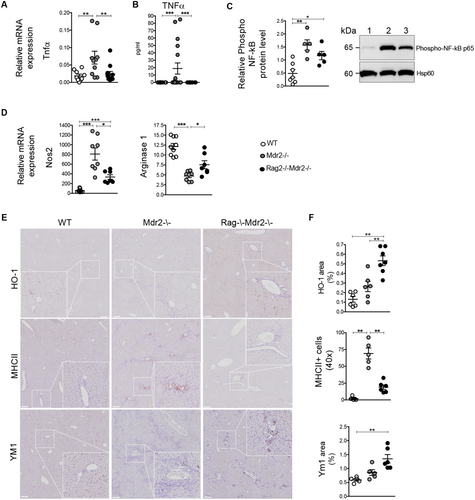
During the inflammatory phase of chronic wounds, TNFα, in combination with other factors, induces the synthesis of nitric oxide (NO), which contributes to liver fibrogenesis.19 In agreement, a marked increase in the inducible NO synthase (iNOS) Nos2 transcript level was detected in hepatic tissue of Mdr2–/– mice (Fig. 3D). In Dko mice, inos expression was significantly inhibited, resulting in higher Arginase I activity (Fig. 3D). Increased Arginase I in Dko mice likely reflected reduced liver damage, compared to Mdr2–/– mice. It was also noteworthy that blunted TNFα-induced up-regulation of inos in Dko mice correlated with significantly increased antioxidant HO-1 immunoreactivity (Fig. 3E,F, upper panel).
Arginine metabolism by NO synthase (NOS) or Arginase has profound consequences on macrophage polarization.20 We examined the macrophage phenotype in Mdr2–/– and Dko mice. Inflammatory M1-type macrophages are abundantly present in liver of Mdr2–/–, compared to wt mice, as demonstrated with MHCII immunostaining (Fig. 3E,F, middle panel). In contrast, M1 macrophage polarization was significantly suppressed in Dko mice in favor of a skewing toward anti-inflammatory M2-differentiated macrophages, as confirmed by the higher liver immunoreactivity for the Ym1 marker (Fig. 3E,F, bottom panel). Thus, adaptive immunity is critical to maintain the inflamed hepatic environment and to dictate macrophage polarization driving fibrosis progression.
CYSTEINE-RICH ANGIOGENIC INDUCER 61–DRIVEN SENESCENCE PROGRAM IS ENHANCED IN Rag–/–Mdr2–/– MICE
Cellular senescence plays a key role in restoring tissue homeostasis after liver injury, by limiting the proliferation of activated HSCs and hence the extent of extracellular matrix (ECM).21
Therefore we asked whether dampen of fibrosis in the Dko mice could be ascribed to cellular senescence activation. To identify senescent cells in situ, we stained liver sections for senescence-associated markers, Ki-67 and SA-β-gal. Immunohistochemistry for Ki-67 revealed that Mdr2–/– livers contained nearly 2-fold more proliferating cells compared with control and Dko mice (Fig. 4A,B). In addition, senescence-associated β-galactosidase (SA-β-gal)-positive cells accumulated at a relatively low frequency in fibrotic Mdr2–/– livers, almost comparable to that of wt controls (Fig. 4A,B). On the contrary, a marked accumulation of SA-β-gal+ cells was observed in the portal and periportal areas of Dko liver (Fig. 4A). The reduced expression of platelet-derived growth factor receptor-β (Pdgfrβ), a feature of myofibroblast activation, in livers of Dko mice (Fig. 4C) would suggest that SA-β-gal+ senescent cells are most likely derived from activated HSCs, which reverted or stalled to a less-reactive phenotype. Accordingly, in serial sections of Dko livers, most of the β-gal-positive cells also express αSMA marker (Fig. 4D). To further confirm this finding, HSCs were isolated from livers and cultured for 6 days. Purity of HSC cultures was assessed by endogenous retinoid fluorescence and oil red staining, marking cytoplasmic lipid droplets typically of HSCs (Supporting Fig. S3A,B). Accordingly, SA-β-gal+ senescent Dko HSCs secreted large amounts of inflammatory senescence-associated secretory phenotype (SASP) factors, including IL6, Ccl2, IL1α, and granulocyte macrophage/colony-stimulating factor (Supporting Fig. S3E). Interestingly, no production of TNFα was detected (data not shown).
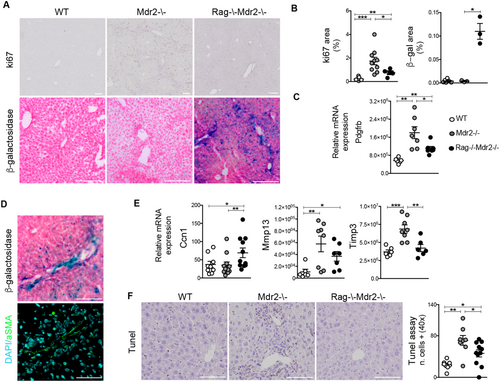
In liver injury, the hepatocyte-derived matricellular protein, CCN1, is critical for induction of HSC senescence, through a RAC/NADPH oxidase 1–dependent pathway.22 A significant increase in Ccn1 tissue expression was observed in Dko, compared to Mdr2–/–,mice (Fig. 4E). Importantly, enhanced Ccn1 expression correlated with increased Rac1 transcript level in purified HSCs from Dko, with respect to Mdr2–/–, mice (Supporting Fig. S3C). Up-regulation of Ccn1 has been linked to apoptosis.23 However, the presence of fewer scattered apoptotic hepatic cells, identified by TUNEL staining in Dko mice with respect to Mdr2–/–, confirmed that cellular senescence is the principal mechanism of restrained LF (Fig. 4F).
Finally, we examined livers for markers of extracellular proteolysis, related to wound resolution. Expression of collagenase 3 (matrix metalloproteinase-13; Mmp-13) diminished in Dko livers compared to the Mdr2–/– mouse (Fig. 4E), indicative of an overall protective effect against fibrosis.24 Furthermore, mRNA levels of tissue inhibitor of metalloproteinases-3 (Timp3) profibrotic gene, encoding for metalloproteinases inhibitor, were also down-regulated in the Dko mouse (Fig. 4E). These results suggest a major role for adaptive immunity in the perpetuation, rather than resolution, of cholangiohepatitis.
B LYMPHOCYTES ARE SUFFICIENT FOR THE ESTABLISHMENT OF HF
To dissect the relative contribution of B and T cells in hepatitis, we treated Mdr2–/– mice with lineage-specific depleting antibodies (anti-CD20 or anti-CD4/CD8, now referred to as B-Mdr2–/– and T-Mdr2–/–) intravenously for 2 months, starting from 5 months of age. Administration of anti-CD20 mAb efficiently produced B-cell depletion in peripheral blood and liver of mutant mice (Supporting Fig. S4A,B). Similarly to Dko mice, collagen deposition was markedly decreased in B-Mdr2–/– livers, as shown by reduced Sirius Red+ fibrills and mRNA expression of fibrogenic genes (Col1a1, Col1a2; Fig. 5A,B). In agreement, αSMA immune-reactivity and liver Pdgfrβ mRNA expression were reduced (Fig. 5C). This also correlated with a significant decrease of relative liver mass in B-Mdr2–/– mice (Fig. 5D).
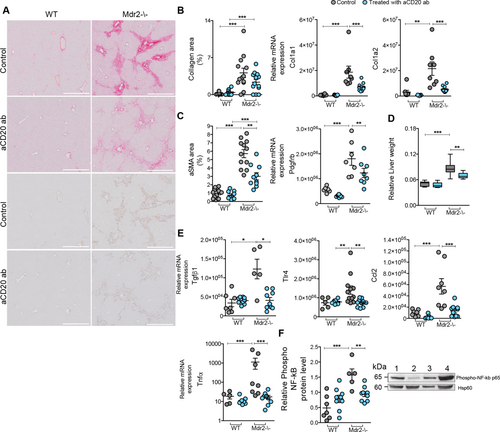
Transcript levels of liver Tgfb, Tlr4, and Ccl2, mediators of hepatic fibrogenesis, were also markedly decreased in B-Mdr2–/– mice compared to controls (Fig. 5E). Furthermore, we observed that in B-Mdr2–/– mice, the TNFα/NF-κB axis was significantly attenuated (Fig. 5E,F), demonstrating the mechanistic contributions of B cells in the inflammation of Mdr2–/– mice. Consistent with their decreased activation, the secretory phenotype of HSCs isolated from B-Mdr2–/– mice was similar to that observed for senescent Dko cell cultures, with high production of IL1α, Ccl2, and IL6 cytokines (Supporting Fig. S3D,E). To confirm the role of B cells in LF, we crossed the B-cell-deficient μMT mouse with the Mdr2–/– mouse and analyzed the progenies. Their analysis at 7 months of age revealed significant amelioration of LF and inflammation (Supporting Fig. S5), further suggesting that B cells play a critical role in amplification of the fibrotic process of Mdr2–/– mice, which also involved activation/regulation of the senescent program.
Interestingly, as reported for experimental models of LF induced in T-cell-deficient mice,12 LF was not significantly affected by the CD4/CD8 T-cell depletion (Supporting Fig. S6A,B). Similar to Mdr2–/– immune-competent mice, T-Mdr2–/– mice showed nearly intact collagen deposition, unaltered αSMA-expressing HSCs, and an up-regulated proinflammatory/fibrogenic tissue phenotype (Supporting Fig. S6C-G).
TUMOR DEVELOPMENT IS MARKEDLY REDUCED IN Rag2–/–Mdr2–/– MICE
Both accumulation of senescent cells and sustained inflammation could contribute to malignant transformation.6, 8, 10, 11 Therefore, we analyzed tumorigenesis in Dko mice, in which inflammation-driven fibrosis is restrained by cellular senescence but senescence-surveillance is partially impaired. At 16 months of age, 80% of Mdr2–/– mice developed visible nodules classified as HCC, whereas no age-matched wt mice presented with HCC (Fig. 6A,B). This finding in the Mdr2–/– mice coincided with strongly increased relative liver weight attributed to tumor burden (mean 0.09 ± 0.01 SD in Mdr2–/– mice vs. 0.07 ± 0.01 in Dko mice vs. 0.05 ± 0.01 in wt; Fig. 6C). Of note, HCC development was considerably attenuated in Dko mice, with a 2-fold reduction in overall tumor frequency and a reduced number of macroscopically detected tumors for the single mouse (Fig. 6A-D). In addition, tumor-bearing Dko mice displayed a lower number of total proliferative lesions, including pretumoral foci of cellular alteration and tumors (Fig. 6D). Furthermore, Dko animals displayed a significantly reduced serum aminotransferase (alanine aminotransferase; ALT) level, indicative of milder liver damage (Fig. 6E).Importantly, analogous results were obtained for μMT-Mdr2–/– mice at a similar age (Fig. 6 A-E).

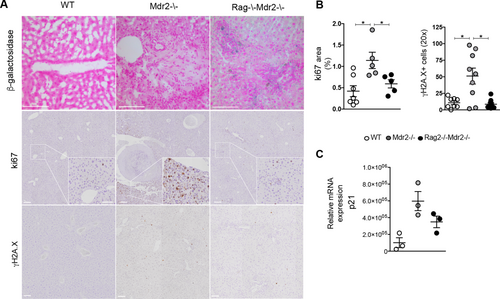
Next, we evaluated whether cellular senescence could be a potential contributor to HCC arrest in Dko mice. At 16 months, SA-β-gal+ cells persisted in liver of Dko mice, whereas Mdr2–/– hepatic tissues were almost negative (Fig. 7A). Hepatocyte proliferation with damaged DNA is a key factor in the formation of HCC.25 As expected, hepatocyte proliferation was evident in Mdr2–/– livers and associated with increased DNA double-strand breaks, as assessed by Ki-67 (1.14 ± 0.4% in Mdr2–/– mice vs. 0.4 ± 0.3% in wt controls) and γH2A.X staining (51 ± 36 positive cells in Mdr2–/– mice vs. 10 ± 7 in wt controls), respectively (Fig. 7A,B). On the contrary, in Dko mice, we observed reduced hepatocyte proliferation (0.5 ± 0.2%) and protection from DNA damage (8.6 ± 6.2 positive cells; Fig. 7A,B). In the Mdr2–/– mouse, p21 promotes both liver regeneration and hepatocarcinogenesis (HCG).26 In agreement, higher p21 mRNA was detected in Mdr2–/– hepatocytes compared to controls, whereas its expression was down-regulated in Dko hepatocytes (Fig. 7C), confirming lymphocytes as a critical component linking inflammation and HCG, by sustaining DNA damage, cell proliferation, and neoplastic transformation.
B CELLS ACCUMULATE IN POORLY DIFFERENTIATED HUMAN HCC
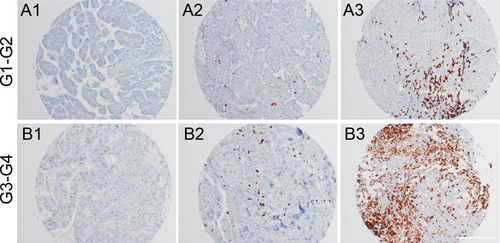
Next, we sought to investigate whether the presence of B lymphocytes mirrors, in human HCC, the protumorigenic role shown in our mouse models. Thus, we analyzed a monoinstitutional cohort of 116 surgically resected HCC patients with HCV etiology. The amount of B lymphocytes was analyzed in tumoral and extratumoral tissues. B cells were classified as absent, 1%-10% or ≥11% in, respectively, 21%, 86%, and 3% of HCC samples and in 2%, 86%, and 12% of extralesional liver parenchyma. Next, we explored a possible correlation between tumoral/extratumoral B-cell infiltrate and clinical (overall and disease-free survival), pathological (histotype, grade, stage, and microscopic or macroscopic angioinvasion), and phenotypical features (p53, B-catenin, stem cell markers, and Ki-67 expression) of the series. Interestingly, a higher amount of B lymphocytes within HCC showed a significant correlation with poor differentiation (G1-G2 vs. G3-G4: 2.3 ± 2.0 vs. 4.1 ± 4.0; P = 0.004) and with lower disease-free survival (HR = 1.07; 95% CI = 1.00-1.14; P = 0.05; Fig. 8). By contrast, at this late HCC stage, the presence of B lymphocytes in extratumoral tissue did not show any statistical correlation.
Discussion
In this study, we addressed the impact of the adaptive immune system on the fibrogenic response that accompanies the development of HCC in Mdr2–/– mice. We show that activated liver lymphocytes amplify the fibrogenic response by secreting proinflammatory cytokines, including TNFα. Ablation of adaptive immunity strongly suppressed HSC activation, promoting their transition to cellular senescence. Additionally, absence of liver lymphocytes led to a switch in the liver metabolic/oxidative state, resulting in skewed macrophage polarization toward an anti-inflammatory restorative M2 phenotype. Notably, lack of lymphocytes markedly halted HCG, hindering TNFα/NF-κB pathway activation. Mechanistically, we found that TNFα-producing CD20+ B cells, but not T cells, limit senescence-mediated LF resolution, favoring HCC progression. These results indicate that B lymphocytes have a decisive role in cancer development following chronic liver injury.
Elements of both innate and adaptive immunity are pivotal in regulating inflammation-driven LF.2 However, their role in its evolution to cancer is still to be unraveled. Mdr2−/− mice recapitulate key features of human liver tumorigenesis, including inflammatory environment, genomic instability, and fibrosis.6, 27, 28 It might therefore allow elucidating pathogenic components linking inflammation and HCG. In Mdr2–/– mice, chronic hepatitis preceding the neoplastic stage was accompanied by a substantial periportal B- and T-lymphocytic infiltration. Similarly, in chronic inflammatory human liver diseases,29, 30 tertiary lymphoid follicles of T and B cells have a recognized implication for the disease pathogenesis.31 In line with this, LF and signs of liver injury were markedly reduced in the immunocompromised Rag2–/–Mdr2–/– mice.
The distinctive role of B and T cells in hepatic fibrogenesis has been dissected by administering lineage-specific depleting antibodies to Mdr2–/– mice, at the preneoplastic stage. Unlike T-Mdr2–/– mice, B-Mdr2–/– mice showed inhibition of fibrosis and accumulation of inactivated senescent HSCs similar to Dko animals, suggestive of a B-cell autonomous function. Anti-CD20 mAb treatment might halt several pathways through which B lymphocytes sustain HSC activation and ECM deposition. Our data indicate that hepatic B cells from fibrotic mice stimulate collagen production by direct secretion of profibrotic cytokines, such as TNFα and IL6. Moreover, B cells act to amplify proinflammatory circuits in effector immune cells, especially T cells. Additionally, B cells might influence macrophage polarization through an Fcγ receptor-dependent signaling,32 directly stimulate fibroblasts, or inhibit metalloproteinase activity by antibody secretion.33 Overall, B-cell depletion primarily affects fibrosis by causing activated HSCs to convert into a senescent state.
Suppression of the immune system is commonly regarded as a risk factor for the development of de novo malignancies in patients, likely related to reduced immune surveillance and increased susceptibility to infections.34, 35 Remarkably, tumor development was profoundly suppressed in Dko mice. Although the role of TNFα in HCG remains controversial,36 in Mdr2–/– mice, activation of the TNFα/NF-κB pathway is responsible for tumor development, whereas neutralizing TNFα antibody inhibits NF-κB activation and HCC development.6 Thus, the observed inhibition of the TNFα/NF-κB axis is most likely responsible for the reduced carcinogenesis in the absence of B cells. Interestingly, serum ALT levels were significantly reduced in immunodeficient mice, emphasizing the link between liver inflammation and HCG. Likewise, in patients with hepatitis-associated cirrhosis, a direct correlation has been described between serum ALT, HCC recurrence rate, and kinetics.37
One limitation of our study is that we have only utilized one liver cancer mouse model. In this regard, it is noteworthy that a potential role for B cells in fibrosis has been previously proposed in mice treated with CCl4.12 However, although it would be interesting to recapitulate our findings in other inflammation-driven models of HCC,7, 38 different etiological settings might determine different immune responses, influencing the progression to HCC in a disease-specific context.
Strikingly, in Dko mice, HCC development was considerably attenuated, despite the important accumulation of senescent cells. The senescence response is widely recognized as a potent tumor-suppressive mechanism, but also a promoter of cancer initiation, by virtue of SASP-derived factors, a source of growth factors for tumor cells.39 In accord, clearance of senescent cells is an important mechanism of tumor surveillance in the liver cancer model and adaptive immune cells are involved in this process.10, 40 Based on our results, it can be speculated that the senescence-driven inflammation have per se a dispensable role in neoplastic transformation, whereas HSC inactivation significantly limits the evolution of fibrosis. Moreover, lymphocyte-mediated inflammation is required for accumulation of DNA double-strand breaks in preneoplastic hepatocytes of Mdr2–/– mice, suggesting that B cells act by orchestrating the inflammatory and metabolic/oxidative environment that is a prerequisite for cancer development. Increasing evidence supports the role of B lymphocytes in tumor progression.41-43 Our data in human HCC highlight infiltrating B cells as a prognostic tool to classify distinct clinical behaviors, identifying also B-cell responses as a possible target of immunotherapeutic strategies. In this regard, induction of senescence in tumor cells by chemotherapy or radiation is now becoming an emergent antitumoral approach.44 In our system, cellular senescence accumulates in the preneoplastic stage of Dko mice, limiting LF without promoting neoplastic transformation at later stage. It is tempting to speculate that in chronic disease, therapy-induced senescence (TIS) may be crucial to dampen fibrosis, extending the latency of HCC. This aspect is even more relevant for patients affected by cirrhosis for which liver transplantation is the only effective therapy.45 To translate our findings in clinical medicine, B-cell targeting may be an effective therapeutic strategy of TIS.
Acknowledgments
We thank Dr. Elena Fontana and Dr. Chiara Novello for technical assistance and the Clinical Chemistry & Hematology Murine Laboratory at IRCCS S. Raffaele Scientific Institute for ALT analysis.
REFERENCES
Author names in bold designate shared co-first authorship.




