Impact of surveillance for hepatocellular carcinoma on survival in patients with compensated cirrhosis
Potential conflict of interest: Dr. Piscitello consults for Exact Sciences. Dr. Mannalithara consults and owns stock in Conatus. She owns stock in Gilead.
This study was supported by National Institutes of Diabetes and Digestive and Kidney Diseases grants DK-34238 and DK-92336 (to W.R.K.) and T32 DK07198 (to J.D.Y.).
Abstract
Surveillance for hepatocellular carcinoma (HCC) has been recommended in patients with cirrhosis. In this study, we examined the extent to which the competing risk of hepatic decompensation influences the benefit of HCC surveillance by investigating the impact of availability of liver transplantation (LTx) and the rate of progression of hepatic decompensation on survival gain from HCC surveillance. A multistate Markov model was constructed simulating a cohort of 50-year-old patients with compensated cirrhosis. The primary outcome of interest was all-cause and HCC-specific mortality. The main input data included incidence of HCC, sensitivity of screening test, and mortality from hepatic decompensation. Treatment modalities modeled included LTx, resection, and radiofrequency ablation. In the base case scenario, LTx would be available to prevent death in a certain proportion of patients. In the absence of surveillance, 68.2% of the cohort members died within 15 years; of these decedents, 25.1% died from HCC and 43.6% died from hepatic decompensation. With surveillance, the median survival improved from 10.4 years to 11.2 years. The number of subjects under surveillance needed to reduce one all-cause and one HCC-specific death over 15 years was 28 and 18, respectively. In sensitivity analyses, incidence of HCC and progression of cirrhosis had the strongest effect on the benefit of surveillance, whereas LTx availability had a negligible effect. Conclusion: HCC surveillance decreases all-cause and tumor-specific mortality in patients with compensated cirrhosis regardless of LTx availability. In addition, incidence of HCC and sensitivity of surveillance test also had a substantial impact on the benefits of surveillance. (Hepatology 2018;68:78-88).
Abbreviations
-
- BCLC
-
- Barcelona Clinic Liver Cancer
-
- HCC
-
- hepatocellular carcinoma
-
- LTx
-
- liver transplantation
-
- PSA
-
- probabilistic sensitivity analysis
-
- RFA
-
- radiofrequency ablation
Despite recent advances in the treatment of hepatocellular carcinoma (HCC) that help patients with early stage HCC achieve better outcomes, an unacceptable proportion of patients present with an advanced, symptomatic stage of disease. To detect HCC at an early stage, surveillance for HCC has been recommended in patients at risk, principally in those with cirrhosis.1 Several studies have shown that HCC surveillance leads to early detection of HCC, affords the patient a chance to receive curative treatment, and decreases mortality.2, 3 Based on these results, the American Association for the Study of Liver Disease, European Association for the Study of the Liver, European Organization for Research and Treatment of Cancer, and Asian Pacific Association for the Study of the Liver practice guidelines have recommended HCC surveillance in selected, high risk patients.4-6
Surveillance for HCC, however, is not uniformly embraced. This may in part be related to lack of support from authorities such as the US National Cancer Institute, which believe that conclusive data for its benefits outweighing risks are lacking.7 A unique aspect of HCC is that the vast majority of affected patients have underlying chronic liver disease and cirrhosis. In addition to HCC, these patients are at risk of developing hepatic decompensation. Thus, death from hepatic decompensation, constituting a competing risk for HCC, diminishes the effectiveness of surveillance. Clearly, patients who succumb to hepatic decompensation derive no benefit.
In this study, we recognized that the impact of hepatic decompensation on the effectiveness of HCC surveillance may be mitigated by the availability of liver transplantation (LTx) or, as exemplified in the recent success in antiviral therapy against hepatitis B or C, by slowing the rate of progression to hepatic decompensation. Ideally, high-quality randomized studies with adequate statistical power would provide robust answers to these questions.8 In the absence of such empirical data, however, simulation modeling provides a means to evaluate the extent to which HCC surveillance may affect survival in patients with liver cirrhosis. The goals of this study were to (1) determine the magnitude of the long-term survival benefit of HCC surveillance in patients with compensated cirrhosis and (2) identify determinants of the benefits of surveillance, specifically the impact of LTx availability and progression of liver disease.
Patients and Methods
MARKOV SIMULATION METHOD
A multistate Markov microsimulation model was constructed simulating a cohort of 50-year old patients with compensated cirrhosis. The cycle length of the model was 1 month and the Markov cycle was repeated for 180 cycles or 15 years. HCC surveillance would be performed every 6 months. Figure 1 demonstrates the Markov state transition model used in this study. In the model, the benefits of surveillance would result from HCCs diagnosed at an early stage, which may be treated with a potentially curative modality, namely LTx, surgical resection, or radiofrequency ablation (RFA). A cohort of 100,000 subjects was modeled under two scenarios; no surveillance (n = 50,000) and surveillance (n = 50,000)
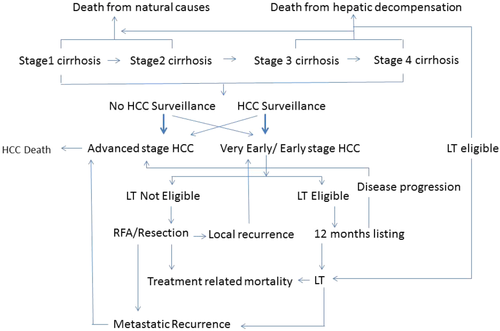
Subjects in all groups could, over time, experience hepatic decompensation and/or develop HCC. HCC would progress from early to advanced stages. Subjects under surveillance would be more likely to be diagnosed with an early stage HCC, whereas those not under surveillance would be more likely to be diagnosed at an early stage less frequently. The extent to which subjects under surveillance are diagnosed with early stage HCC would depend on the sensitivity of the surveillance test. Subjects with HCC may die because of treatment complications, progressive or recurrent HCC, decompensated liver disease, or unrelated causes.
The primary outcome of interest was mortality from all causes and from HCC. In addition to the number of deaths in each cohort, we calculated the number of subjects that needed to undergo surveillance to save one life (number needed to be in surveillance [NNS]) from all-cause and HCC-specific mortality. This was obtained following the standard method to compute the number needed to treat (inverse of the absolute risk reduction). In addition, relative risk reduction was calculated by the ratio of the difference between mortality without surveillance and mortality with surveillance divided by mortality without surveillance. TreeAge Pro (v20151120; Williamstown, MA) was used to generate the Markov model.
INPUT VARIABLES
The main input data included in this analysis were extracted from the published literature; however, because not all of the variables needed to execute the model could be populated in a robust, evidence-based fashion, an expert opinion was used to supplement the available data. Sensitivity analyses were undertaken (as described in detail below) to evaluate the degree to which the study conclusion would be affected by the uncertainty in the data.
Key transition probabilities in the model included annual incidence of HCC (3%; range, 1%-5%)9, 10 and median annual mortality of incurable HCC (50%; range, 45%-55%).11, 12 The mean interval of tumor progression between early, detectable lesions to advanced stage was set to be 1 year, based on the reported doubling time of HCC of approximately 6 months.13 Median time-to-event for annual HCC mortality and mean tumor progression were converted into transition probabilities using standard declining exponential functions (1 − e−ht, where h is ln(2)/Median Time or 1/Mean Time). We modeled cirrhosis progression stochastically as well. Transition probabilities for the four stages of cirrhosis were used based on data from a large meta-analysis, and the probability of death from hepatic decompensation was based on cirrhosis stage.14
In patients undergoing LTx, postoperative mortality was set to be 9% in the first 12 months and then 2% annually thereafter.15 Procedure-related mortality was assigned to be 1% and 5% in the first 6 months after RFA16, 17 and liver resection,18-20 respectively. There would be no procedure-related mortality for RFA or liver resection beyond 6 months after the treatment. We modeled that 70% (combining metastatic recurrence at 4.4% annually over 5 years and de novo HCC or intrahepatic “early” HCC recurrence at 17.8% annually) of patients receiving RFA or resection develop HCC recurrence over 5 years, whereas 13% of patients receiving LT develop metastatic recurrent disease over 5 years. 21-23 Progression of cirrhosis stage and the risk of death from hepatic decompensation would persist except in patients undergoing LTx. In all cohorts, age-specific mortality from unrelated causes was taken from the life expectancy data of the United States population.24
BASE CASE SCENARIO AND SENSITIVITY ANALYSES
We estimated that 30% in the no-surveillance cohort would be diagnosed at an early stage of HCC and would be eligible for curative treatment in the same fashion as early HCC patients of the surveillance cohort.25 We have modeled that 75% of individuals with early stage HCC would receive potentially curative treatment (LTx, RFA or resection).26, 27
In the base case scenario, LTx would be available for 17% of patients who would have died of hepatic decompensation.28 We estimated that 33% of early stage HCC patients would be eligible for LTx listing; the remaining two thirds would receive either RFA or resection, depending on their cirrhosis stage. Patients with stage 1 cirrhosis would receive resection, whereas RFA would be available for patients in all other stages. The waiting time before receiving LTx for HCC would be 12 months, at which time 80% would undergo LTx, whereas the remaining 20% of HCC patients listed for LTx would drop out due to tumor progression, death from cirrhosis, death from natural causes, or other reasons.29, 30
We assumed that 18% of patients with early stage HCC had very early stage HCC (single tumor <2 cm).31 Because patients with Barcelona Clinic Liver Cancer (BCLC) stage 0 HCC do not qualify for Model for End-Stage Liver Disease exception score in the current organ allocation system in the United States, we have modeled that they receive resection or RFA. Alternatively, they can wait for 6 months without any HCC treatment to simulate a situation in which patients wait until the tumor grows into United Network for Organ Sharing T2 stage to be listed for LTx. LTx would be considered as a treatment option in 33% of patients for BCLC stage 0 patients only after waiting for 6 months. We have assumed that there is no metastatic recurrence after curative treatment for very early stage HCC, but patients continue to have risk of developing de novo HCC after curative treatment.
One-way sensitivity analysis was undertaken to identify variables that have an important impact on the results of the analyses. These include (1) no progression of cirrhosis beyond stage 1, (2) annual incidence of HCC (range, 1%-5%), (3) sensitivity of HCC surveillance test (range, 50%-90%), (4) availability of LTx for hepatic decompensation (range, 0%-100%), (5) time to develop a late stage cancer from an early stage (range, 4-36 months), and (6) annual mortality due to late stage HCC (range, 40%-80%). Finally, we incorporated discounting for future years of life, recognizing the time preference in evaluation of life over time. Without making a value judgment about the effect of hepatic decompensation and HCC on the utility of the patient, we used the standard discount rate of 3% uniformly in the model and assessed its effect on the results.
Probabilistic sensitivity analysis (PSA) was performed to address uncertainty beyond limited one-way sensitivity analyses. Uncertainty regarding the base case value of each variable (i.e., second-order uncertainty) and the impact of such uncertainty on base case results was examined in PSA by sampling all variables over their ranges (Table 1), using triangular or beta distributions. The Markov analysis was performed for 100 iterations (100,000 subjects in each iteration, 50,000 per strategy), where each iteration represented a different sampling of variables from the assumed distributions.
| Variable | Input Data | |
|---|---|---|
| Base Case Scenario | Range | |
| Progression of cirrhosis stage per year14 | ||
| Stage 1 to 2 | 7% | 4%-10% |
| Stage 1 to 3 | 4% | 2%-6% |
| Stage 2 to 3 | 7% | 4%-10% |
| Stage 2 to 4 | 4% | 2%-6% |
| Stage 3 to 4 | 8% | 6%-10% |
| Annual hepatic decompensation mortality per cirrhosis stage14 | ||
| Stage 1 | 1% | 0.8%-1.3% |
| Stage 2 | 3% | 2.6%-4.3% |
| Stage 3 | 20% | 15%-25% |
| Stage 4 | 57% | 44%-70% |
| Annual incidence of HCC9, 10 | 3% | 1%-5% |
| Sensitivity of screening test1 | 70% | 60%-80% |
| Detection of early stage HCC without surveillance25 | 30% | 25%-35% |
| Probability of transition from early to advanced HCC13 | 63% | 40%-86% |
| Annual mortality of incurable HCC11, 12 | 50% | 45%-55% |
| Probability of receiving curative treatment for early stage HCC26, 27 | 75% | 70%-80% |
| Treatment-related mortality | ||
| LTx15 | 9% in the first 12 months and then 2% annually after the first year | 3%-15% in the first 12 months and then 1.5%-2.5% annually after the first year |
| Surgery19, 20 | 5% in the first 6 months and 0% thereafter | 2.5-7.5% in the first 6 months and 0% thereafter |
| RFA16, 17 | 1% in the first 6 months and 0% thereafter | 0.5-1.5% in the first 6 months and 0% thereafter |
| Probability of liver transplant listing28 |
Hepatic decompensation: 17% HCC: 33% |
Hepatic decompensation: 11-23% HCC: 29%-36% |
| Duration of liver transplant waitlist15 | 12 months | 6-18 months |
| Risk of recurrence after treatment within 5 years | ||
| LTx22 | 13% over 5 years | 10%-16% |
| Surgery23 | 70% over 5 years | 65%-75% |
| RFA21 | 70% over 5 years | 65%-75% |
Results
BASE CASE SCENARIO
Figure 2 summarizes the overall result indicating that HCC surveillance improves survival. The median survival in the surveillance cohort was 11.2 years compared with 10.4 years in the no-surveillance cohort. In Table 2, mortality from all causes at 15 years improved from 68.2% without surveillance to 64.7% with surveillance. Without surveillance, HCC was the cause of death in 25.1% of patients and hepatic decompensation in 43.6% of patients. In the surveillance cohort, the proportion of patients dying of HCC decreased to 17.8%, whereas that of hepatic decompensation increased to 47.1%.
| Mortality | NNS | Relative Risk Reduction | ||||
|---|---|---|---|---|---|---|
| Overall | HCC | Overall | HCC | Overall | HCC | |
| 3 years | 120 | 101 | 6.7% | 34.4% | ||
| No surveillance | 12.3% | 2.9% | ||||
| Surveillance | 11.5% | 1.9% | ||||
| 5 years | 54 | 46 | 7.9% | 35.1% | ||
| No surveillance | 23.5% | 6.2% | ||||
| Surveillance | 21.6% | 4.0% | ||||
| 10 years | 29 | 22 | 6.8% | 34.1% | ||
| No surveillance | 50.2% | 13.1% | ||||
| Surveillance | 46.8% | 8.7% | ||||
| 15 years | 28 | 18 | 5.2% | 32.8% | ||
| No surveillance | 68.2% | 17.1% | ||||
| Surveillance | 64.7% | 11.5% | ||||
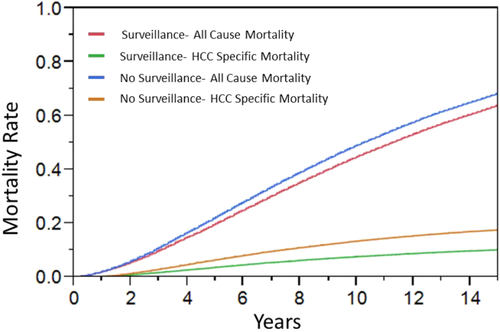
Table 2 also provides the NNS and relative risk reduction calculations. The NNS to reduce one death decreased progressively from 3 years to 15 years. At 15 years, the NNS to avoid one HCC death was 18 and one death from all cases was 28. Relative risk reduction was fairly constant regardless of the time frame of assessment. Surveillance was associated with an approximate 30%-35% relative reduction in HCC mortality throughout. Relative reduction in overall mortality was 5.2% with surveillance at 15 years.
MODEL VALIDATION
Using the model, we calculated the expected survival of HCC patients according to the treatment received. Patients who received LTx had a post-LTx survival rate of 71% at 5 years, whereas those who underwent resection or RFA had an overall survival rate of 61% at 5 years, results that are consistent with a general consensus of survival results reported in the literature.21-23 Patients with BCLC stage 0 who were treated with RFA had a 5-year survival rate of 78%, which calibrates with the result seen in a recent meta-analysis.32 Next, we evaluated the overall survival of HCC patients from the onset and diagnosis of HCC (Supporting Figs. S1 and S2, respectively). The median survival of HCC patients was longer in the surveillance cohort than in the no-surveillance cohort (3.1 years versus 2.2 years from the time of HCC onset and 2.8 years versus 1.4 years from the time of HCC diagnosis). The 5-year overall survival from HCC diagnosis was 34% with surveillance and 15% without surveillance, both of which fall well within the range of survival rates reported in the literature.33
COMPETING RISKS ON THE EFFECTIVENESS OF HCC SURVEILLANCE
First, we tested a scenario in which cirrhosis did not progress and thus deaths from hepatic decompensation occurred at a lower frequency than the base case scenario. In Table 3, cirrhosis remained at stage 1 throughout the follow-up, which resulted in a reduction in deaths from hepatic decompensation and an increase in HCC mortality, with a net effect of overall reduction in all-cause mortality. The NNS to prevent one death from all causes at 15 years decreased by 37% (from 28 to 18) and that from HCC decreased by 24% (from 18 to 13).
| Mortality | NNS | Relative Risk Reduction | ||||
|---|---|---|---|---|---|---|
| Overall | HCC | Overall | HCC | Overall | HCC | |
| 3 years | 103 | 91 | 12.0% | 36.0% | ||
| No surveillance | 8.1% | 3.0% | ||||
| Surveillance | 7.1% | 2.0% | ||||
| 5 years | 47 | 42 | 14.6% | 35.9% | ||
| No surveillance | 14.6% | 6.7% | ||||
| Surveillance | 12.5% | 4.3% | ||||
| 10 years | 22 | 19 | 14.4% | 33.9% | ||
| No surveillance | 30.9% | 15.8% | ||||
| Surveillance | 26.5% | 10.4% | ||||
| 15 years | 18 | 13 | 12.9% | 32.6% | ||
| No surveillance | 43.9% | 22.7% | ||||
| Surveillance | 38.3% | 15.3% | ||||
In addition, we considered a scenario in which liver disease remained stable with concomitant reduction in the incidence of HCC, as would be expected in patients who have cirrhosis with viral hepatitis who have received effective antiviral therapy (Supporting Table S1). As expected, all-cause and HCC-specific mortality decreased compared with the base case scenario. The NNS for all-cause and HCC mortality increased; the NNS over 15 years for overall mortality increased by 57% (from 28 to 44) and that for HCC mortality increased by 90% (from 18 to 34).
OTHER SENSITIVITY ANALYSES
As expected, the annual incidence of HCC affected the effectiveness of HCC surveillance: higher annual incidence rates of HCC were associated with lower NNS to prevent one all-cause or HCC-specific mortality and larger relative risk reduction for all-cause mortality (Figs. 3A and 4A, Supporting Table S2, and Supporting Figs. S3A and S4A). Similarly, a higher sensitivity of HCC surveillance test was associated with a larger effect of surveillance, although its magnitude was smaller than that from variability in HCC incidence (Figs. 3B and 4B, Supporting Table S3, and Supporting Figs. S3B and S4B). It is noteworthy that the effect of surveillance test sensitivity was very small when assessed for the long term (e.g., 15 years) (Figs. 3B and 4B).
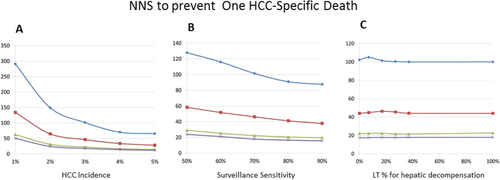
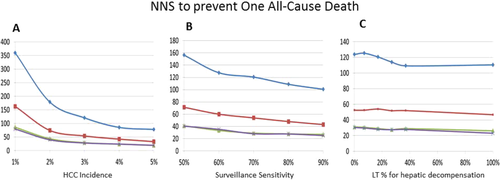
Other variables tested had a much smaller effect on the survival benefits of HCC surveillance. For example, availability of LTx had little impact on the effectiveness of surveillance, as gauged both by NNS and relative risk reduction (Supporting Table S4). Similarly, varying access to LTx for hepatic decompensation had little impact on the benefits of HCC surveillance (Figs. 3C and 4C, Supporting Table S5, and Supporting Figs. S3C and S4C). Finally, other variables subjected to sensitivity analyses—namely, time to develop late stage cancer and mortality of late stage HCC—had negligible impact on the overall results (data not shown).
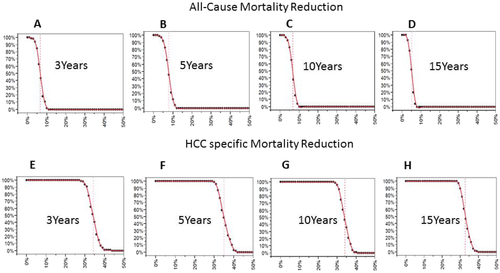
PSA was performed to address the second order uncertainty (uncertainty around the parameters used in the base case analysis) along with first order uncertainty (patient level) (Fig. 5 and Supporting Fig. S5). All-cause and HCC-specific mortality reduction were above 0%, indicating that there are no instances in which there would be no risk reduction; for example, the median 5-year risk reduction in all-cause mortality was 7.8%. The 2.5th and 97.5th percentiles of the PSA were 3.3% and 11.4%, respectively. The median 5-year risk reduction in HCC-specific mortality 34.8%. The 2.5th and 97.5th percentiles of the PSA were 30.8% and 40.7%, respectively.
Finally, a discounted life year analysis was performed with reducing the value of life by 3% per year. HCC surveillance offered, in the base case scenario, an increase of 0.24 life years for a subject relative to no surveillance (discounted life years: 8.52 versus 8.27). In general, it reduced the apparent benefits of surveillance in other scenarios without fundamentally altering the direction of effects (data not shown).
Discussion
In this study, we designed a multistate Markov model to investigate the survival benefit of HCC surveillance in patients with compensated cirrhosis and identify factors associated with the effectiveness of HCC surveillance in reducing mortality. In our simulated cohorts of 50-year-old patients, 68% of the cohort members died after 15 years of follow-up without HCC surveillance, which decreased to 65% with surveillance. These resulted in NNS to reduce one death over 15 years of 28 for all causes and 18 for HCC. While the survival benefit in patients with cirrhosis under surveillance may not be directly comparable to that in other cancer surveillance programs in otherwise healthy individuals, the NNS to avoid one cancer death has been reported to be 550 for prostate-specific antigen over 12 years of follow-up and between 233 and 746 for mammography over lifetime, depending on the age of the subject.34, 35
The results of our sensitivity analyses strongly indicate that HCC surveillance remains highly effective irrespective of the factors that were taken into consideration. We did find that HCC surveillance is most effective when the risk of death from hepatic decompensation decreases, even when the incidence of HCC is also decreased. In addition, the incidence of HCC and the sensitivity of surveillance test also had a substantial impact on the benefits of surveillance. These considerations are particularly relevant today when there are increasing number of older patients with cirrhosis (e.g., HCV) in whom current and future therapies may halt or slow the progression of cirrhosis.
To assess the impact of HCC surveillance in patients with cirrhosis, outcome should be measured from patients for whom surveillance is implemented, not just in a subset of patients with cirrhosis who had already developed HCC. There were numerous observational studies comparing survival post-HCC diagnosis, but no observational or randomized controlled trial study specifically evaluated the impact of HCC surveillance on overall survival among patients with cirrhosis from the beginning of HCC surveillance.25 A previous modeling study attempted to evaluate the impact of HCC surveillance in cirrhotic patients, but outcome was measured only in patients who had developed HCC from the time of HCC diagnosis, but not from the beginning of HCC surveillance.26 Cadier et al.36 recently performed a cost-effectiveness analysis of HCC surveillance, but they did not investigate the major determinant for effectiveness of HCC surveillance and failed to incorporate competing risk of death from hepatic decompensation. The contribution of our study is deeper insight of the medical effectiveness of HCC surveillance and its determinants, which would be a prerequisite for cost-effectiveness. Similarly, we have not delved into the discussion of harms and costs associated with false-positive results. Data to date indicate that the harms associated with HCC surveillance is minimal, attributable to radiocontrast toxicity or biopsy complications.37
The main purpose of HCC surveillance is to reduce mortality. Although a number of previous observational studies reported that HCC surveillance is associated with earlier diagnosis and increased survival, it is essentially impossible to exclude lead time (earlier detection of HCC without affecting the natural history of disease) or length (preferential detection of slowly growing HCC) biases from the data.1 Thus far, only two randomized controlled trials have evaluated the effect of HCC surveillance on mortality. These studies were performed in China in patients with HBV infection and showed inconsistent results. One study included 18,816 patients and showed a reduction of HCC mortality by more than a third.3 The other study, conducted in 5581 men, failed to show statistically significant mortality reduction despite detection of HCC in an earlier stage.38
Death from hepatic decompensation is the leading cause of death and a major competing risk of HCC in patients with cirrhosis. Our data suggest that if the underlying disease process can be controlled, surveillance becomes more beneficial, even in the absence of LTx. It is now well established that successful therapy of viral hepatitis in patients with cirrhosis reduces the development of hepatic decompensation.39 Furthermore, an increasing body of literature has shown that effective therapy of underlying liver disease may reverse cirrhosis in the long run.40 Thus, our data suggest that HCC surveillance would be beneficial in those patients, whereas it remains unknown whether HCC surveillance should continue indefinitely in those patients. However, further data are needed to inform whether and when the risk of HCC in those patients is sufficiently lowered to warrant discontinuation of surveillance.
In our validation attempt, we were pleased to see that our multistate model seemed to replicate data reported in the literature. In a study of 1155 consecutive patients with cirrhosis, the 6-year survival rates of patients with compensated and decompensated cirrhosis without LTx were 54% and 21%, respectively.41 Most of the deaths were attributed to complications of advanced liver disease (hepatic failure in 49%, HCC in 22%, and bleeding complications in 13%). A systematic review summarizing 118 studies on the natural history of cirrhosis showed similar results: the 10-year survival of patients with compensated cirrhosis was 61%.14 In our previous cohort study, the 15-year survival of patients with Child-Pugh class A cirrhosis was approximately 30%.42 Taken together, these data indicate that our estimates calibrate well with real-life data. Finally, previous studies consistently showed that 25%-30% of deaths in patients with cirrhosis were attributed to HCC, again similar to the estimates of our model.43
As with any studies based on simulation models, our data are an approximation of real-life observations only to the degree that the structure and input data of the model are reflective of the best knowledge available. For example, the heterogeneity in the biological characteristics and tumor behavior of HCC are difficult to model precisely. At the same time, an important advantage of simulation analyses is that they allow sensitivity analyses assessing the impacts of various components of the model. Our results clearly show that the risk of hepatic decompensation is a major driver for the effectiveness of surveillance, whereas tumor-specific variables may be much less so, suggesting that more elaborate models that take into account individual tumor biology are unlikely to change the main results. Furthermore, simulation modeling makes it possible to analyze the long-term outcomes associated with surveillance, whereas conducting a randomized trial of 15 years' duration is prohibitively costly and, to many investigators, unethical.
In conclusion, although routine implementation of HCC surveillance in patients with cirrhosis remains controversial in the United States, our study offers additional insight about the mortality benefits of surveillance of patients who have cirrhosis. HCC surveillance is most effective in preventing mortality in the setting where death from hepatic decompensation is lower, the incidence of HCC is higher, and sensitivity of HCC surveillance test increases, whereas availability of LTx had minimal impact. In all of the scenarios considered in this analysis, HCC surveillance decreases all-cause and tumor-specific mortality, which lead us to advocate that surveillance for HCC be conducted in patients with compensated cirrhosis, even if LTx is not available.




