PTEN Down-Regulation Promotes β-Oxidation to Fuel Hypertrophic Liver Growth After Hepatectomy in Mice
Potential conflict of interest: Nothing to report.
This study was funded by Synergia grants CRSII3_141798/1 and CRSII3_160717/1 from the Swiss National Foundation, and from the Clinical Research Priority Program (“Liver Tumors”) of the University of Zürich.
Abstract
In regenerating liver, hepatocytes accumulate lipids before the major wave of parenchymal growth. This transient, regeneration-associated steatosis (TRAS) is required for liver recovery, but its purpose is unclear. The tumor suppressor phosphatase and tensin homolog (PTEN) is a key inhibitor of the protein kinase B/mammalian target of rapamycin axis that regulates growth and metabolic adaptations after hepatectomy. In quiescent liver, PTEN causes pathological steatosis when lost, whereas its role in regenerating liver remains unknown. Here, we show that PTEN down-regulation promotes liver growth in a TRAS-dependent way. In wild-type mice, PTEN reduction occurred after TRAS formation, persisted during its disappearance, and correlated with up-regulated β-oxidation at the expense of lipogenesis. Pharmacological modulation revealed an association of PTEN with TRAS turnover and hypertrophic liver growth. In liver-specific Pten–/– mice shortly after induction of knockout, hypertrophic regeneration was accelerated and led to hepatomegaly. The resulting surplus liver mass was functional, as demonstrated by raised survival in a lethal model of resection-induced liver failure. Indirect calorimetry revealed lipid oxidation as the primary energy source early after hepatectomy. The shift from glucose to lipid usage was pronounced in Pten–/– mice and correlated with the disappearance of TRAS. Partial inhibition of β-oxidation led to persisting TRAS in Pten–/– mice and abrogated hypertrophic liver growth. PTEN down-regulation may promote β-oxidation through β-catenin, whereas hypertrophy was dependent on mammalian target of rapamycin complex 1. Conclusion: PTEN down-regulation after hepatectomy promotes the burning of TRAS-derived lipids to fuel hypertrophic liver regeneration. Therefore, the anabolic function of PTEN deficiency in resting liver is transformed into catabolic activities upon tissue loss. These findings portray PTEN as a node coordinating liver growth with its energy demands and emphasize the need of lipids for regeneration. (Hepatology 2017;66:908–921).
Abbreviations
-
- Acc
-
- acetyl-CoA carboxylase
-
- AKT
-
- protein kinase B
-
- AUC
-
- area under the curve
-
- bpV
-
- bisperoxovanadium
-
- Cpt1a
-
- carnitine palmitoyltransferase 1a
-
- CROT
-
- carnitine octanoyltransferase
-
- 4E-BP1
-
- 4E binding protein 1
-
- Fasn
-
- fatty acid synthase
-
- FGF21
-
- fibroblast growth factor 21
-
- GAPDH
-
- glyceraldehyde 3-phosphate dehydrogenase
-
- GSK3b
-
- glycogen synthase kinase 3 beta
-
- Hadha/b]
-
- hydroxyacyl-CoA dehydrogenase/3-ketoacyl-CoA thiolase/enoyl-CoA hydratase (trifunctional protein), alpha/beta subunit
-
- HDL
-
- high-density lipoprotein
-
- H&E
-
- hematoxylin and eosin
-
- Lipe
-
- hormone-sensitive lipase
-
- Lpl
-
- lipoprotein lipase
-
- LR
-
- liver regeneration
-
- Lw/Bw
-
- liver weight to body weight ratio
-
- mTOR
-
- mammalian target of rapamycin
-
- mTORC1
-
- mammalian target of rapamycin complex 1
-
- PH
-
- partial hepatectomy
-
- PI3K
-
- phosphoinositide 3-kinase
-
- Plin2
-
- perilipin 2
-
- PPARα
-
- peroxisome proliferator-activated receptor alpha
-
- PTEN
-
- phosphatase and tensin homolog
-
- PtenC
-
- Pten controls
-
- PtenKO
-
- Pten knockout
-
- RAS
-
- renin-angiotensin system
-
- RER
-
- respiratory exchange ratio
-
- S6K
-
- S6 kinase
-
- Scd1a
-
- stearoyl-CoA desaturase 1a
-
- SFSS
-
- small-for-size syndrome
-
- SREBP1
-
- sterol regulatory element-binding protein-1
-
- STAT3
-
- signal transducer and activator of transcription 3
-
- TAM
-
- Tyro3, Axl, and Mertk
-
- TGs
-
- triglycerides
-
- TRAS
-
- transient regeneration-associated steatosis
-
- wt
-
- wild type
Following tissue loss, liver first regenerates its functional units, the hepatocytes. Their repopulation occurs through both hypertrophic and hyperplastic mechanisms.1, 2 Overall, regeneration is highly efficient, with removal of 70% of the liver leading to complete regrowth within a week in mice.3 The enormous growth rate points to the need for suitable energy sources that fuel liver regeneration (LR).
Systemic metabolic changes after partial hepatectomy (PH) are thought to provide regenerative triggers, but might also serve to satisfy energy demands. Liver is the major glucose provider, and hypoglycemia inevitably develops when liver mass is lost. Indeed, hypoglycemia is an essential regenerative signal.1 Moreover, hypoglycemia is thought to trigger a systemic response leading to a redistribution of lipids from the periphery into the regenerating liver.4 Unlike pathological steatosis (i.e., fatty liver disease), this transient regeneration-associated steatosis (TRAS) in hepatocytes is a physiological process observed in every regenerating liver.4 In mouse, TRAS peaks 16 hours post-PH and then gradually declines to lean values by 48-72 hours, thus around the major wave of parenchymal growth.3 TRAS is needed for regeneration, because its disruption impairs liver recovery.4, 5 Although first described more than 60 years ago,6 the function of TRAS remains unknown.
The tumor suppressor phosphatase and tensin homolog (PTEN) is a key inhibitor of the growth-promoting phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR) axis. Specifically, PTEN counteracts the phosphoinositide-dependent activation of AKT-mTOR through PI3K. The AKT-mTOR axis is considered a key regulator of cell-autonomous and systemic metabolism, orchestrating the metabolic and energetic needs with cellular growth.7 By opposing AKT-mTOR activation, PTEN holds a powerful position, and even subtle reductions in this “quasi-insufficient” tumor suppressor can have serious consequences.8
Loss of PTEN in resting hepatocytes promotes lipid import and lipogenesis, rapidly leading to pathological steatosis that can progress to cancer.9-11 After hepatectomy, PI3K-AKT-mTOR signaling is known to contribute to recovery. Intriguingly, PI3K seems to promote hyperplasia by phosphorylating signal transducer and activator of transcription 3 (STAT3), whereas the phosphoinositide-dependent AKT-mTOR axis stimulates hypertrophic growth.1, 12 Notably, AKT signaling regulates many of the metabolic adaptations associated with regeneration after tissue loss.13 No direct evidence supports a role for PTEN in liver regeneration to date. However, the proregenerative functions of several microRNAs have been associated with suppression of PTEN after resection.14-16
Down-regulation of PTEN after hepatectomy14-16 suggests an enhancement of regenerative AKT-mTOR activities. PTEN reductions might hence regulate not only parenchymal growth, but also adaptations to altered metabolic demands following tissue loss. Given the steatotic phenotype of resting Pten–/– liver,9, 10 TRAS might relate to PTEN down-regulation after hepatectomy. Furthermore, recent evidence indicates also catabolic roles for AKT-mTOR in hepatic lipid metabolism,17 perhaps implying that PTEN contributes to turnover of TRAS. Therefore, PTEN down-regulation after hepatectomy might serve to orchestrate tissue growth with the resulting energy demands. To this end, we explored PTEN and associated changes in regenerating liver. More specifically, we aimed at defining a role for PTEN in liver growth and TRAS, with the goal to shed light onto the function of TRAS after tissue loss.
Materials and Methods
Additional information can be found in the Supporting Information.
ANIMALS
Male wild-type (wt) mice (C57BL/6) were from Envigo (Horst, NL). Male hepatocyte-specific inducible Pten knockout (PtenKO) animals (AlbCre-ERT2Tg/+Ptenfl/fl) and corresponding controls (AlbCre-ERT2+/+Ptenfl/fl, PtenC) were kindly provided by M. Foti (University of Geneva, Geneva, Switzerland). All animal experiments were in accord with Swiss Federal Animal Regulations and approved by the Veterinary Office of Zürich (KEK-ZH-Nr. 2012-203).
ANIMAL SURGERY AND SUBSTANCES
PH (68%) and 91% hepatectomy (a model of lethal liver failure) were performed as reported.18 Substances (Supporting Table S1) were intraperitoneally injected in 100-μL volume at doses and times indicated in the results. See the Supporting Information for further details.
HISTOLOGICAL STAINING
Hematoxylin and eosin (H&E), periodic acid Schiff, and immunochemical stainings (see Supporting Table S2 for antibodies) were performed on 3-μm archived liver sections and Oil Red O on cryosections.
HEPATOCYTE SIZE
Hepatocyte area was assessed on histology under exclusion of lipid droplets and vessels. Size was measured through forward scatter by flow cytometry.
WESTERN BLOTTING
The procedure was performed as reported.3, 18 Antibodies are listed in the Supporting Information.
QUANTITATIVE REAL-TIME POLYMERASE CHAIN REACTION
RNA extraction, complementary DNA synthesis and qPCR were performed as described.3, 18 Taqman gene expression assays (Applied Biosytems, Rotkreuz, Switzerland) are listed in the Supporting Information.
INDIRECT CALORIMETRY AND Echo-MAGNETIC RESONANCE IMAGING
Indirect calorimetry experiments were conducted at the Small Animal Metabolic Phenotyping core facility (University of Geneva) and approved by the Geneva Health Head Office. Energy expenditure and the respiratory exchange ratio (RER) were derived from O2 consumption and CO2 production; RER differences between PtenKO and PtenC were assessed through AUC (area under the curve) analysis.
LIPID AND GLYCOGEN MEASUREMENTS
Quantitation kits were used to measure triglycerides (TGs; ab65336; Abcam, Cambridge, UK). High-density lipoprotein (HDL; MAK045-1KT; Sigma-Aldrich, Buchs, Switzerland), and glycogen (MAK016-1KT; Sigma-Aldrich).
STATISTICAL ANALYSIS
Data are presented as mean ± SD unless stated otherwise. Differences between groups were assessed by a two-tailed t test assuming unequal variance. Five to 8 mice/group were analyzed. For survival after 91% hepatectomy, 10 animals/group were included. Differences were considered significant at P < 0.05 and indicated by an asterisk (*). Statistical analyses were performed using Prism software (version 6.0; GraphPad Software Inc., La Jolla, CA).
Results
PTEN IS ASSOCIATED WITH TRAS TURNOVER, WEIGHT GAIN, AND HYPERTROPHY IN REGENERATING LIVER
To explore the roles of PTEN and TRAS, we assessed lipid-associated parameters and PTEN levels following standard (68%) hepatectomy (PH) in wt mice. Histology confirmed the TRAS peak at 16 hours and its gradual disappearance around the times of major parenchymal growth.3, 6 The steatotic peak was preceded by the induction of perilipin 2 (Plin2), which promotes hepatocyte-adipocyte transdifferentiation and is required for lipid droplet formation (Fig. 1A).19 Around the steatotic peak, Cd36 (fatty acid translocase) was up-regulated, consistent with peripheral import of fat.4 Furthermore, β-oxidation genes (carnitine palmitoyltransferase 1a [Cpt1a], hydroxyacyl-CoA dehydrogenase/3-ketoacyl-CoA thiolase/enoyl-CoA hydratase (trifunctional protein), alpha/beta subunit [Hadha/b]) were elevated at the expense of lipogenic genes (stearoyl-CoA desaturase 1a [Scd1a], acetyl-CoA carboxylase (Acc), fatty acid synthase [Fasn]; Fig. 1B), suggesting that fat is being accumulated for energetic needs.
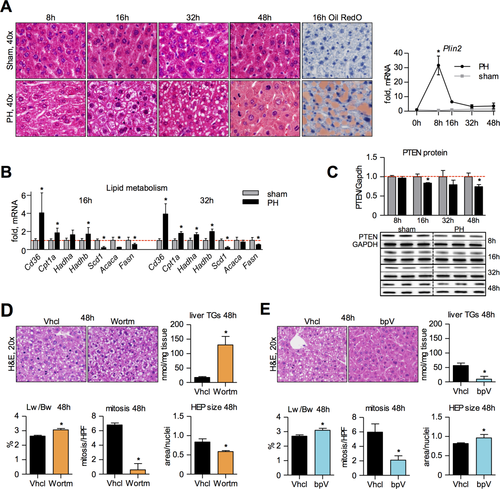
PTEN protein was down-regulated at the TRAS peak to levels (≤80%) known to induce carcinogenesis in mice.8 PTEN down-regulation occurred after Plin2 induction and persisted during lipid disappearance (Fig. 1C). Reduction in PTEN may hence be associated with TRAS turnover, but not with its accumulation.
To estimate PTEN's function during LR, we simulated elevated PTEN activity through inhibition of PI3K. Injection of wortmannin (0.75 mg/kg) at 13 hours post-PH led to reduced mitoses, a reduced hepatocyte size (however, only if lipid vesicles were excluded from hepatocyte area), but an increased liver-to-body-weight-ratio (Lw/Bw) at 48 hours. The latter was likely attributed to lipid accumulation, which was strongly elevated compared to vehicle controls (Fig. 1D).
When PTEN was inhibited by bisperoxovanadium (bpV; 3.3 mg/kg) at 13 hours post-PH, Lw/Bw and hepatocyte size were increased, whereas TRAS was diminished (Fig. 1E). Notably, mitotic counts were also reduced through bpV, suggesting that PTEN inhibition during LR specifically affects the phosphoinositide-dependent AKT-mTOR axis, which promotes hypertrophy at the expense of hyperplasia.12 In contrast, PI3K inhibition additionally affects phosphoinositide-independent STAT3 activation, hence impacting on both hyperplasia and hypertrophy.12 Taken together, these findings indicate that PTEN down-regulation is associated with TRAS turnover, weight regain, and hypertrophy in regenerating liver.
HEPATOCYTE-SPECIFIC Pten DEFICIENCY ACCELERATES FUNCTIONAL LIVER RECOVERY THROUGH HYPERTROPHY
PTEN inhibition after hepatectomy promotes liver weight recovery, however bpV acts systemically and may exert unspecific effects. We therefore used inducible hepatocyte-specific Pten knockout mice to define the impact of PTEN deficiency on LR. Knockout was induced by Tyro3, Axl, and Mertk (TAM) in AlbCreERT2tg/+-Ptenfl/fl (PtenKO) 4 days before hepatectomy to avoid pre-existing fatty liver that may impair regenerative capacity.20 Pten expression was not significantly altered post-PH in Cre-lacking control mice (PtenC; Fig. 2A), suggesting that PTEN down-regulation (Fig. 1C) is regulated posttranscriptionally following resection. Regenerating livers in PtenKO mice remained Pten deficient, reflecting liver reconstitution from differentiated (albumin-positive) hepatocytes (Fig. 2A).
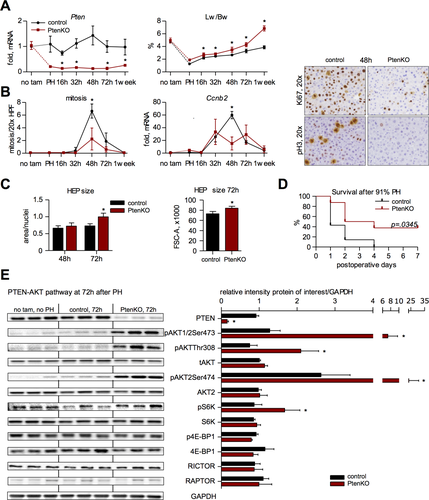
At PH, the starting weight of the liver remnant was slightly increased in PtenKO relative to PtenC. Post-PH, the difference in liver weight became more pronounced over time, leading to hepatomegaly in PtenKO after a week (Fig. 2A). Notably, accelerated weight gain was not associated with proliferation, but with enhanced hepatocellular hypertrophy typified through deficient S/M phase progression and p27 up-regulation (Fig. 2B,C and Supporting Figs. S1 and S2).
To determine whether accelerated weight gain leads to improved liver recovery, we performed 91% hepatectomy, which causes lethal liver failure in wt mice.18 The best measure to assess recovery of liver function is 7-day survival, the critical period after liver loss. Remarkably, survival was raised to 40% after 91% resection in PtenKO mice (Fig. 2D), indicating that the surplus hypertrophic liver mass generated by PTEN deficiency is functional.
Given the significant hypertrophy at 72 hours post-PH in PtenKO, we investigated AKT-mTOR-S6K (S6 kinase) signaling, known to promote a hyperplasia-to-hypertrophy switch.12, 21 Activating AKT phosphorylation was markedly increased by PTEN deficiency (Fig. 2E). Likewise, enhanced S6K phosphorylation indicated elevated mammalian target of rapamycin complex 1 (mTORC1) activity. Increased phosphorylation of AKT and S6K was also evident at 32 hours (Supporting Fig. S3), consistent with AKT-mTORC1-S6K signaling as a hypertrophic driver in regenerating liver from PtenKO.
Pten DEFICIENCY PROMOTES GLUCOSE STORAGE AND LIPID METABOLISM IN REGENERATING LIVER
Acceleration of LR in PtenKO is expected to rely on additional energy supply. We hence assessed hepatic energy stores, mainly consisting of TGs and glycogen.
Following hepatectomy, both PtenKO and PtenC displayed early drops in liver glycogen and serum glucose (Supporting Fig. S4A), a reported signal required for initiation of regeneration.4 Notably, glycogen stores recovered faster in PtenKO relative to PtenC, accompanied by down-regulation of gluconeogenic expression (peroxisome proliferative activated receptor gamma coactivator 1-alpha, phosphoenolpyruvate carboxykinase 1, glucose 6 phosphatase; Supporting Fig. S4A). Given the similar serum glucose levels, these findings indicate that PTEN deficiency counteracts glucose usage in regenerating liver.
Hepatic TG content was elevated in PtenKO at hepatectomy and peaked at 16 hours akin to PtenC (Fig. 3B). The subsequent decline in TG levels was delayed in PtenKO relative to PtenC. Therefore, loss of PTEN before hepatectomy leads to an expanded TRAS period. In contrast, inhibition of PTEN at the TRAS peak shortened this period (Fig. 1E), suggesting that PTEN deficiency does not directly enhance TRAS formation during LR. Indeed, PTEN loss did not affect Plin2 and Cd36 expression post-PH and even down-regulated fatty acid binding protein 4 (Supporting Fig. S4B), which is required for hepatocyte-adipocyte transdifferentiation and is enhanced in resting PtenKO liver.10
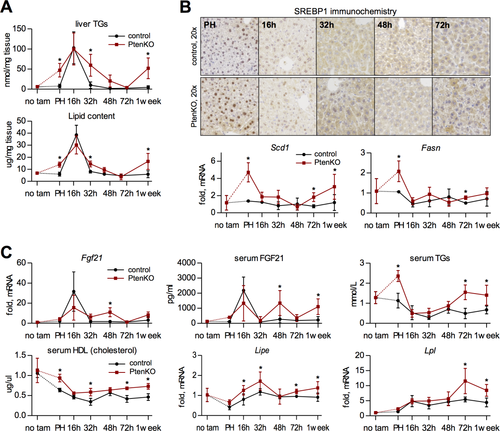
Resting PtenKO liver is known to amass fat partially through increased lipid synthesis.9, 11 Accordingly, lipogenic molecules (sterol regulatory element-binding protein-1 [SREBP1] and its transcriptional targets, Scd1 and Fasn) were up-regulated at hepatectomy in PtenKO liver remnants (Fig. 3B). After hepatectomy, however, the lipogenic program was suppressed (Fig. 3B), indicating little contribution of hepatic lipogenesis to TRAS. An expanded TRAS period may hence be secondary to the increased hepatoperipheral lipid shuttle pre-existing in PtenKO before PH.9 Indeed, the elevations in fibroblast growth factor 21 (FGF21), a hepatokine that promotes the import of peripheral lipids for their hepatic use,11, 22 were extended in PtenKO relative to PtenC and correlated with respective TRAS periods (Fig. 3C). In agreement, serum TGs were elevated in PtenKO at PH, but dropped during the TRAS peak similar to PtenC (Fig. 3C). Likewise, serum levels of HDL—transporting TGs into liver—were increased in PtenKO versus PtenC during LR (Fig. 3C), along with an elevated hepatic expression of hormone-sensitive lipase (Lipe) and lipoprotein lipase (Lpl; Fig. 3C)—lipases that free fatty acids from TGs.23, 24
Altogether, these results suggest that the expanded TRAS period in PtenKO results neither from increased lipogenesis nor from elevations in active lipid import. Rather, the systemic redistribution of lipids into liver after tissue loss4, 25 may be enhanced because of an elevated mobilization of peripheral fats pre-existing in PtenKO liver before PH.9, 10 Given that FGF21 can increase hepatic energy expenditure,22 the expanded TRAS period might further relate to a prolonged catabolic phase in regenerating PtenKO liver.
Pten DEFICIENCY PROMOTES LIPID OXIDATION AS AN ENERGY SOURCE AFTER HEPATECTOMY
To gain insight into concrete metabolic outputs during LR and the effects of PTEN loss thereupon, we measured RER (CO2/O2) post-PH by indirect calorimetry. The typical diurnal shift toward lipid oxidation was recorded for both PtenKO and PtenC (Fig. 4A; control data in Supporting Fig. S5). Upon resection, RER markedly dropped during the first 32 hours, identifying lipid oxidation as the favored energy source during the TRAS period of regenerating liver. Unlike PtenC, RER around the TRAS peak remained close to 0.7 in PtenKO, indicating increasing fat catabolism with lowering PTEN levels. From 32 hours onward, RER began to rise toward prehepatectomy levels. Another reduction in RER was recorded in mutant animals toward 72 hours, thus before TGs were disappearing in PtenKO liver. At 16 hours, peroxisome proliferator-activated receptor alpha (PPARα; a key transcription factor promoting β-oxidation) and its targets, CPT1A and carnitine octanoyltransferase (CROT), were up-regulated in PtenKO liver (Fig. 4B), along with increased expression of several β-oxidation genes (Supporting Fig. S6). Therefore, LR leads to a profound increase in lipid oxidation, which is enhanced through hepatic PTEN down-regulation and correlates with the disappearance of liver fat.
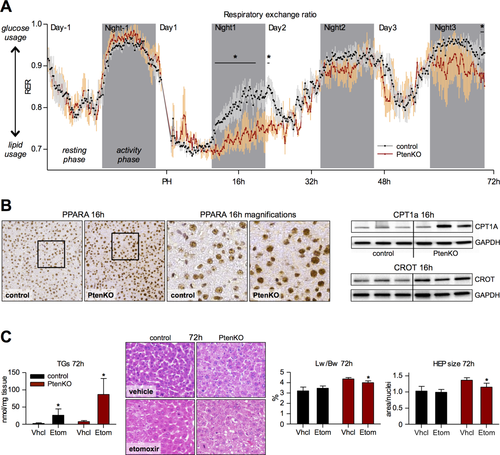
Pten DEFICIENCY FUELS HYPERTROPHIC LR THROUGH β-OXIDATION OF TRAS-DERIVED LIPIDS
The increases in lipid oxidation observed in PtenKO animals after hepatectomy may originate from the metabolism of TRAS lipids to fuel the regenerative process. If TRAS lipids are oxidized to provide energy for liver growth, inhibition of β-oxidation should result in persisting steatosis and a diminished liver weight after hepatectomy. We targeted the rate-limiting β-oxidation enzyme, CPT1A, using etomoxir. Low doses (10 mg/kg) were applied every 12 hours starting 16 hours post-PH, so as to inhibit lipid oxidation without compromising survival. At 72 hours post-PH—when liver is lean and PtenKO display hypertrophy with elevated liver weight—etomoxir did not significantly alter Lw/Bw or hepatocyte size in PtenC relative to vehicle controls, despite a mild increase in hepatic lipids (Fig. 4C). The lack of distinct effects may relate to the low dose, but also to the inefficacy of etomoxir in inhibiting the oxidation of medium and short-chain fatty acids. In PtenKO, however, etomoxir markedly increased hepatic TG content while reducing liver weight. Moreover, hepatocyte size of PtenKO was diminished by etomoxir and no more different from that of PtenC hepatocytes (Fig. 4C). Therefore, mild inhibition of β-oxidation does counteract hypertrophic parenchymal growth driven by PTEN loss; however, it appears insufficient to impact on the general regeneration process. We conclude that TRAS provides lipids for β-oxidation to fuel hypertrophic LR, a process promoted by PTEN down-regulation.
POTENTIAL CATABOLIC AND HYPERTROPHIC MEDIATORS DOWNSTREAM OF AKT
Overactive AKT signaling (Fig. 2E and Supporting Fig. S3) is likely responsible for the metabolic and hypertrophic changes in regenerating PtenKO liver. AKT can induce β-catenin26 (directly or through glycogen synthase kinase 3 beta [GSK3b] inhibition), which is an important promoter of lipid catabolism in the liver.27 On immunofluorescence, we observed increased nuclear expression of β-catenin at 16 hours post PH in PtenKO, along with elevated inhibitory GSK3b phosphorylation (Fig. 5A). In support of its catabolic role, hepatic targets of β-catenin (acyl-CoA dehydrogenases27) were up-regulated in PtenKO at 16 hours (Supporting Fig. S6). However, chronic overactivity of the hypertrophic driver, mTORC1,4 may likewise promote β-oxidation.17 We treated mice with moderate doses of the mTORC1 inhibitor rapamycin (1 mg/kg at 13 hours post-PH and subsequently every 24 hours). In PtenC at 72 hours postresection, rapamycin had little effect on liver weight, hepatocyte size, and TG content (Fig. 6B). In PtenKO, rapamycin reduced liver weight and hepatocyte size, but did not significantly impact on TG content. These findings portray mTOR as a major promoter of hypertrophy in Pten loss-driven regeneration, whereas associated β-oxidation may preferably be regulated by other AKT-dependent molecules such as β-catenin (Fig. 6C).
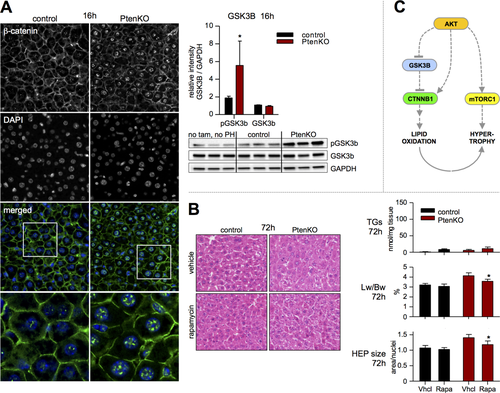
Discussion
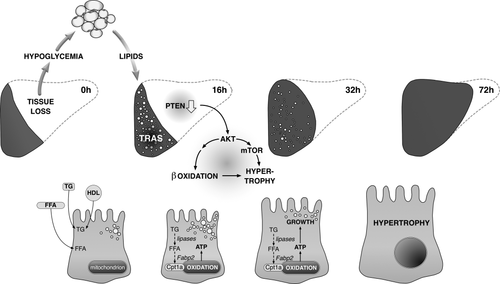
Transient steatosis occurs in every regenerating liver and meanwhile is recognized as an essential component of successful recovery after tissue loss. The precise function of TRAS has remained unknown thus far; however, the delivery of energy for the regenerative process provides a conceivable explanation. Here, we show that the tumor suppressor PTEN participates in LR, with its down-regulation promoting liver growth fueled by TRAS. More specifically, we demonstrate that (1) TRAS turnover is associated with down-regulation of PTEN post-PH; (2) PTEN deficiency promotes AKT-mTORC1 signaling and hypertrophic liver growth; and (3) β-oxidation of TRAS-derived lipids is required for hypertrophy driven by PTEN deficiency. Moreover, our data indicate a general shift to lipid oxidation during LR, consistent with the view that the burning of fat is a main energy source for the regenerative process.
Following PH, PTEN is down-regulated at a time when lipid accumulation peaks in regenerating liver. Our initial experiments using wortmannin and bpV suggested that PTEN regulates hypertrophic liver growth in association with TRAS turnover. To better appreciate the function of PTEN down-regulation, we explored regeneration in liver with hepatocyte-specific PTEN loss.
In quiescent liver, loss of PTEN causes pathological steatosis developing from elevated lipogenesis and an increased import of peripheral lipids, likely related to exaggerated insulin-PI3K-AKT signaling.9-11 In regenerating liver, Pten loss had a different outcome. Although Pten loss was associated with an expanded TRAS period, it had no impact on molecules that are up-regulated in resting PtenKO liver to promote lipid accumulation (i.e., import, vesicle formation, and lipogenesis)—in keeping with PTEN's role in normal regenerating liver, where TRAS formation occurred before PTEN down-regulation. These observations are remarkable inasmuch as they illustrate how, after tissue loss, the regenerative program dominates over other processes, adapting the function of PTEN (and likely other molecules) for its own purposes.
Whereas PTEN deficiency in resting liver mainly promotes energy storage, our studies on regenerating liver reveal a catabolic function for PTEN down-regulation. Calorimetric measurements demonstrated a clear shift toward lipid consumption during the TRAS period in PtenC. This shift was accentuated in PtenKO and preceded the disappearance of hepatic TGs post-PH. Lipid oxidation was accompanied by an elevation in β-oxidation molecules and a reduction in glucose utilization in regenerating PtenKO liver, emphasizing fat as a preferred energy source. Therefore, PTEN down-regulation after hepatectomy appears to foster a unique phenotype in that it promotes lipid oxidation while enhancing glucose storage.
Despite little effect of low-dose etomoxir on liver growth in wt mice, the marked calorimetric shift toward lipid oxidation post-PH in PtenC—together with the up-regulation of β-oxidation genes in wt mice—strongly suggests that TRAS is a general source of regenerative energy. The provision of energy conceivably explains why TRAS is required for LR4, 5, 19, 28, 29 and why animal models of deficient β-oxidation present with regenerative defects and persisting TRAS.4, 30 Notably, earlier experiments with the β-oxidation inhibitor octanoylcarnitine suggested that lipids are the dominant energy source early post-PH.31
Importantly, in both models of PTEN deficiency, liver weight and hepatocyte size were increased at times when TG content was close to nil (i.e., bpV 48 hours, PtenKO 72 hours), linking PTEN deficiency, TRAS turnover, and hypertrophic liver growth. When β-oxidation was mildly inhibited from the TRAS peak onward, hepatic TGs remained elevated at 72 hours in PtenKO, ongoing with a reduction in liver weight and hepatocyte size. Overall, these findings identify TRAS as a source of lipids that are being oxidized to fuel hypertrophic liver growth in a PTEN-dependent manner (Fig. 6).
PTEN down-regulation as a promoter of hypertrophic LR fits the role of the protein in various hypertrophic pathologies, where cell-type–specific Pten loss may be etiological (e.g., cerebellar hypertrophy,32 macrocephaly,33 or hypertrophic cardiomyopathy34). Notably, hypertrophy in these pathologies can be corrected by inhibition of mTORC1.32-34 Similarly, moderate-dose rapamycin treatment of PtenKO during the TRAS peak inhibited both hepatocellular hypertrophy and excess liver weight gain, consistent with the PDK1-AKT-mTOR axis as a promoter of hypertrophic regeneration12 and mTOR as a central regulator of cell size.35 Why hypertrophy causes proliferative suppression remains unclear; however, an analogous switch from hyperplastic to hypertrophic liver regeneration has been reported in mice treated with bpV.21 Given that hypertrophy is thought to be the default mode of liver regeneration,1, 36 AKT-mTORC1 overactivity may not acutely suppress, but simply diminish the need for proliferation.
Recent findings indicate that mTORC1-S6K can promote lipid oxidation in resting liver.17 However, rapamycin did not increase TG content in regenerating liver, suggesting either incomplete inhibition or promotion of TRAS catabolism by other PTEN-regulated molecules. β-catenin is a strong candidate, given its regulation though AKT and its requirement for efficient hepatic lipid oxidation.26, 27 Although AKT's role in fostering hepatic lipid accumulation is established,37 AKT can promote lipid catabolism in situations associated with energy deprivation such as exercise or fasting.38, 39 Intriguingly, acute energy deprivation provokes hypoglycemia, mobilization of peripheral fats, and a switch to lipid usage,40 thus processes that we also observe in regenerating liver. Furthermore, hepatocyte-specific deletion of sirtuin 1, a key energy sensor induced by fasting, causes deficient LR accompanied by persisting TRAS and a failure to up-regulate genes needed for lipid oxidation.30 More so, insulin levels drop upon fasting, whereas in regenerating liver insulin responsiveness appears to be dampened.41 In terms of energy changes, the instant events occurring after hepatic tissue loss may hence be comparable to the early response toward acute fasting, albeit coupled to the specific demands of a growing tissue. The reduced insulin responsiveness further suggests that AKT activation post-PH occurs independent of insulin, perhaps explaining the divergent outcomes (i.e., fat storage vs. usage) of AKT-mTOR signaling in resting (with overactive insulin signaling) versus regenerating PtenKO liver.
The contribution of hypertrophy to LR is being increasingly appreciated.36, 42 Indeed, the promotion of hypertrophic liver growth through PTEN down-regulation might be of clinical relevance. The most frequent cause of death attributed to liver surgery is small-for-size syndrome (SFSS). This clinical entity can develop following too extended resection, leaving behind marginal remnants that fail to recover because of deficient regeneration.3, 18 Intriguingly, a consistent feature of SFSS livers is persisting hypersteatosis, illustrating the intimate relationship between TRAS and successful recovery. No treatment currently exists for this entity; however, experimental approaches that prevent the SFSS also normalize TRAS.18 PTEN knockout was able to rescue 40% of mice after 91% hepatectomy, a lethal mouse model of SFSS.18 Therefore, transient inhibition of PTEN—such as through bpV—may offer new avenues to prevent or treat SFSS. Moreover, promotion of lipid oxidation may aid such measures, as suggested by the impact of lipid-plus-carnitine infusion on regeneration in rats.43
In summary, our study highlights the function of PTEN in compensatory liver hypertrophy and emphasizes the importance of TRAS for the regenerative process. Following PH, reductions in PTEN have two major consequences, that is, promotion of cellular hypertrophy through mTOR along with the enhancement of β-oxidation—a process that may be fostered through β-catenin and that provides the fuel for the hypertrophic expansion of functional parenchyme. The catabolic properties of PTEN down-regulation enhance the turnover of TRAS, which forms as a part of the metabolic adaptations imposed by the loss of liver tissue. More broadly, our findings affirm TRAS as an obligate component of successful LR, with fat as the prime regenerative fuel during the periods of low hepatic capacity. The appreciation of lipid catabolism in LR may point to novel options for management of regenerative deficiencies in the clinic, such as experienced in surgery-induced liver failure.
Acknowledgments
We thank Eleonora Maurizio, Pia Fuchs, and Dr. Ursula Süss for their excellent technical support.
REFERENCES
Author names in bold designate shared co-first authorship.




