First international external quality assessment for hepatitis delta virus RNA quantification in plasma
Potential conflict of interest: Nothing to report.
We thank the ANRS (Agence nationale de recherches sur le SIDA et les hépatites virales) for their funding of the organization of this study.
Abstract
Infection by the hepatitis delta virus (HDV), a satellite of the hepatitis B virus (HBV), increases viral liver disease severity. Its diagnosis is thus vital for HBV-infected patients. HDV-RNA load (HDVL) should be assessed and monitored in plasma using real-time reverse-transcriptase polymerase chain reaction assays. Taking advantage of the recently-developed World Health Organization (WHO) HDV international standard (WHO-HDV-IS), the first international external quality control for HDVL quantification was performed. Two panels of samples were sent to 28 laboratories in 17 countries worldwide. Panel A comprised 20 clinical samples of various genotypes (1, 2, and 5-8) and viral loads, including two negative controls. Panel B, composed of dilutions of the WHO-HDV-IS, allowed the conversion of results from copies/mL into IU/mL for HDVL standardization and interlaboratory comparisons. Comprehensive analysis revealed a very high heterogeneity of assay characteristics, including their technical steps and technologies. Thirteen labs (46.3%) properly quantified all 18 positive samples; 16 (57.1%) failed to detect one to up to 10 samples, and several others underestimated (>3 log IU/mL) HDVL of African genotype strains (1 and 5-8). Discrepancies were mainly attributed to either primers or probe mismatches related to the high genetic variability of HDV and, possibly, to the complex secondary structure of the target genomic RNA. The labs were grouped in four clusters by the statistical analysis of their performances. The best clusters comprised the 17 labs that obtained the expected HDVL values, including five that otherwise failed to quantify one or two samples. Conclusion: The results of this international quality-control study underline the urgent need to improve methods used to monitor HDV viremia and will be instrumental in achieving that goal. (Hepatology 2016;64:1483-1494)
Abbreviations
-
- anti-HDV
-
- antibody to HDV
-
- CF
-
- conversion factor
-
- CV
-
- coefficient of variation
-
- FNRL-HDV
-
- French national reference laboratory for HDV
-
- HBsAg
-
- hepatitis B surface antigen
-
- HBV
-
- hepatitis B virus
-
- HDV
-
- hepatitis D virus
-
- HDV-1Eu/As
-
- HDV-1 of European or Asian origin
-
- HDVL
-
- HDV-RNA viral load
-
- HDVAb
-
- HDV antibody
-
- IQR
-
- interquartile range
-
- LLOQ
-
- lower level of quantification
-
- NA
-
- nucleic acid
-
- NC
-
- negative control
-
- PCA
-
- principal component analysis
-
- RT-PCR
-
- reverse-transcriptase polymerase chain reaction
-
- WHO
-
- World Health Organization
-
- WHO-HDV-IS
-
- WHO HDV international standard
The World Health Organization (WHO) estimates that approximately 240 million people are currently chronically infected with hepatitis B virus (HBV), and, among them, approximately 20 million are also infected with the satellite hepatitis delta virus (HDV).
HDV infection increases the probability of severe liver disease. Patients with HDV and HBV experience fulminant hepatitis at least 100 times more often than those with HBV alone, and, in cases of superinfection, they experience a chronicity rate reaching 70%-90%. Chronic hepatitis delta often evolves to cirrhosis (60%-70%) and hepatocellular carcinoma.1-9 The current treatment is disappointing; based on a 12- to 18-month regimen of pegylated interferon, it provides a sustained virological response in only 25%-40% of patients.10 Very recently, new hopes for treatment have emerged with specifically-targeted anti-HDV compounds, such as entry and farnesylation inhibitors or nucleic acid polymers, that are currently in phase 2 clinical trials.11-13 However, these new treatments are also sparking an urgent need for improved tools to quantify HDV viremia.
HDV is highly endemic in Mediterranean countries, Eastern Europe, the Middle East, northern South America, and Sub-Saharan and Central Africa. HDV is also highly prevalent in Turkey, Pakistan, Iran, and Mongolia. The HDV burden in Western and Southern Europe is currently low and mainly attributed to immigration from highly endemic regions.14, 15 Globally however, it is very likely that the prevalence of HDV is underestimated, given that there is still a considerable lack of information for several regions where HBV is highly prevalent, such as Asia, South America, and Africa.16 Using full-length genomic nucleotide sequences and phylogenetic analyses, eight HDV genotypes17, 18 and many subgenotypes have been defined based on divergences of >20% and >10%, respectively (E. Gordien and F. Le Gal, personal data and manuscript in preparation). Genotypes (and subgenotypes) have distinct and specific geographical distributions. Briefly, HDV-1 is the most prevalent genotype worldwide; HDV-2 is more common in the Far East, Japan, Taiwan, and parts of Russia (Yakutia); HDV-3 is exclusively found in the Amazon basin and in northern South America; HDV-4 is present in Japan and Taiwan; and, finally genotypes 5-8 are native to Africa.
Because HDV is a satellite of HBV, all patients positive for hepatitis B surface antigen (HBsAg) must also be screened for total anti-HDV antibodies. Plasma HDV-RNA viral load (HDVL) must be assessed in all anti-HDVAb (HDV antibody)-positive patients to identify those who are RNA positive (indicating active replication) and thus at risk of poor liver disease outcome. The French national reference laboratory for HDV (FNRL-HDV) developed the first consensus “in-house” assay. It is based on TaqMan real-time reverse-transcriptase polymerase chain reaction (RT-PCR) technology and capable of quantifying RNA load for most, if not all, known strains of HDV.19 Several other in-house and commercial assays have been developed in specialized laboratories using real-time RT-PCR technologies,19-29 but they performed poorly compared to the FNRL-HDV assay in recent studies.30, 31 The high genetic variability of HDV is a key factor in the discrepancies observed for these tests, which, furthermore, suffer from a lack of standardization. The recent development by the WHO of an HDV international standard (WHO-HDV-IS) will greatly contribute to improve this point (http://www.who.int/biologicals/expert_committee/BS_2227_HDV_RNA.pdf). Indeed, a standardized quantification tool is vital for monitoring treatment efficacy and improving knowledge on HDV-RNA kinetics during the natural history of the disease. For the present study, we performed an unprecedented comprehensive international quality-control study to evaluate almost all available commercial and in-house assays for plasma HDV-RNA quantification.
Materials and Methods
PARTICIPATING CENTERS
A total of 28 laboratories (L1-L28) from 17 globally distributed countries participated in this HDV assay international quality-control study (Table 1). Quantifications of samples were performed by each lab with their routine in-house (n = 22) or commercial (n = 6) assay.
PANELS OF SAMPLES
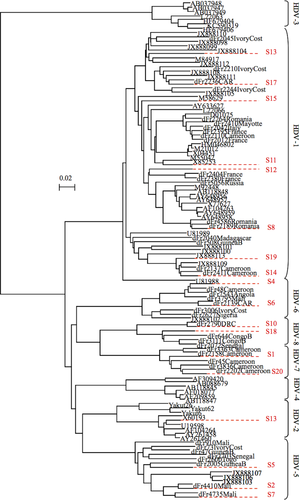
Panel A was composed of 20 plasma samples (S1 to S20), including two negative controls (HBsAg negative), selected from the FNRL-HDV biobank to representatively cover the large genetic diversity of HDV. Strains included in panel A were: four HDV-1 of African origin (HDV-1Af); four HDV-1 of European or Asian origin (HDV-1Eu/As); and one HDV-2, three HDV-5, two HDV-6, two HDV-7, and two HDV-8 (Fig. 1). Complete genome nucleotide sequences of these 18 HDV strains were determined as described17, 18 to evaluate the adequacy of each assay's primers and probes.
Panel B comprised eight samples composed of two negative controls and of two dilutions (1:10 and 1:100), each in triplicate of the HDV-WHO-IS (available as lyophilized plasma at a concentration of 575,000 IU/mL). The 1:100 dilutions were used to evaluate the capacity of assays to detect low levels of viremia. Panel B permitted the conversion of all results from copy/mL into IU/mL for subsequent comparative analyses. For each assay, a conversion factor (CF) was calculated: CF = 575,000 IU/mL/LxB (copy/mL). LxB is the mean value obtained by each lab (Lx) for the different triplicates of panel B (adjusted for the dilution) expressed in copy/mL. Thus, each value obtained for the samples of panel A, in copy/mL, was multiplied by this CF.
Panel A and B samples were prepared at the FNRL-HDV by dilution in human plasma negative for all HBV markers. They were then coded and quantified three times, using the methodology of Le Gal et al.,19 by three different technicians blinded to sample composition. Samples were then stored at −80°C until they were packed in dry ice and sent to the 28 participating laboratories.
TECHNICAL CHARACTERISTICS OF THE DIFFERENT ASSAYS
All labs were requested to provide the main characteristics and the protocols of their routinely used assay, that is, the volume of sample analyzed, nucleic acid (NA) extraction method, quantification standard and internal control used, real-time RT-PCR reverse transcription and PCR profiles, technologies and devices, dynamic range of quantification, and sequences of the primers and probes.
STATISTICAL ANALYSIS
Viral loads, HDV genotypes, and technical parameters were considered for the statistical analyses. Negative (or undetectable) HDVL results obtained with panel A were considered as null. Similarly, “positive unquantifiable” (or detectable) results were replaced by the lower limit of quantification (LLOQ) value of the respective assay as reported by the participating center.
First, a principal component analysis (PCA) was done to transform possibly correlated variables into uncorrelated “principal components.” This enabled a distribution of lab data in potential clusters according to HDVL values. Second, a hierarchical classification of the principal components was performed to define groups of labs with different quantification capacities. A direct hierarchical cluster (bypassing the PCA) was also performed to check the robustness of the results. All these analyses were done with HDVL data obtained with panel A expressed in log copies/mL and in log IU/mL after conversion using the results of panel B.
Data from panel B were also used to evaluate the repeatability and sensitivity of the different assays.
All statistical tests were two-sided, with P values of <0.05 denoting statistical significance. Statistical analyses were performed on R software (version 2.14; http://www.R-project.org) with the FactoMineR package (http://factominer.free.fr).
Results
CHARACTERISTICS OF ASSAYS
The participating centers provided almost all of the main protocol parameters of their assays, which were highly heterogeneous, as concerns reagents, NA extraction, real-time RT-PCR technologies, and devices used (data not shown). Plasma sample and NA extraction elution volumes ranged, respectively, from 100 μL to 1 mL and from 20 to 500 μL, using either manual (n = 13) or automated (n = 15) extraction methods. Fifty-seven percent of the laboratories (16) used an internal RNA (14) or DNA (2) control. Additionally, RNA or DNA quantification standards were used by respectively 10 and 18 laboratories. Concerning real-time RT-PCR technologies, TaqMan probes were used most frequently (n = 17), followed by Fret probes (n = 4), Molecular Beacons (n = 2), and SYBR Green dye (n = 2). Finally, amplification protocols were performed with various real-time PCR devices, including ABI7500/7900 (Life Technology), LC480/640/2.0 (Roche), CFX96 (Biorad), Rotor-Gene (Qiagen), Mx3005P (Stratagène), DTlite (DNA-Technology), and SPS7700 (Seiko).
REPEATABILITY, PRECISION, SENSITIVITY, AND SPECIFICITY ACCORDING TO PANEL B
Quantification results obtained with the WHO-HDV-IS (panel B, according to dilution factor) ranged from 3.74 log to 7.98 log cp/mL (median, 5.97 log cp/mL). These results allowed us to calculate the conversion factor for each assay. Of note, repeatability for triplicates was rather good across the different labs (coefficient of variation [CV] ranging from 0.1% to 11.5% calculated from values expressed in log IU/mL). Also, higher mean CV values were found in two-step (2.65%), compared to one-step (0.9%), RT-PCR assays. However, a lack of sensitivity was observed for two labs that did not detect the 1:100 WHO-HDV-IS dilution. Furthermore, three labs identified either one (n = 2 labs) or the two (n = 1 lab) negative controls (NCs) as positive.
GLOBAL QUANTIFICATION RESULTS
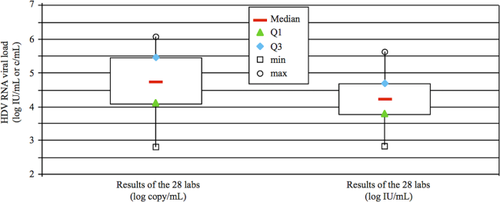
Quantification results obtained with panel A showed a very wide breadth of HDVL, expressed either in log copies/mL (interquartile range [IQR], 1.39) or in log IU/mL (IQR, 0.91; Table 2; Fig. 2). We note that the normalization of HDVL using the WHO-HDV-IS did lower the dispersion of the values for all labs, as expected. Therefore, we performed all subsequent analyses with values expressed in log IU/mL.
| Country | No. | Laboratories |
|---|---|---|
| Australia | 1 | WHO Reference Laboratory For Hepatitis B (Melbourne) |
| Belgium | 1 | University Ziekenhuis (Ghent) |
| Denmark | 1 | Aalborg University Hospital (Aalborg) |
| England | 1 | UCLH NHS Foundation (London) |
| France | 6 |
University hospital Pitié Salpêtrière (Paris) University hospital Lyon Sud (Lyon) CERBA laboratory (Saint-Ouen-L'Aumône) University hospital Dupuytren (Limoges) University hospital Hôtel Dieu (Nantes) University hospital Avicenne (Bobigny) |
| Germany | 5 |
Paul Erhlich Institute (Langen) Hannover Medical School (Hannover) MVZ Laboratory (Karlsruhe) Lipsdiag GmbH Company University Hospital Heidelberg (Heidelberg) |
| Greece | 2 |
University of Thessaly (Larissa) University of Athens (Athens) |
| Italy | 2 |
Azienda Ospedaliero-Univeritaria (Torino) Policlinico Universitario “G. Martino” (Messina) |
| Luxemburg | 1 | Fast Track Diagnostic Company |
| Mauritania | 1 | Biomédical-24 laboratory (Nouakchott) |
| Russia | 1 | Central Research Institute of Epidemiology (Moscow) |
| Scotland | 1 | West of Scotland Specialist Virology Center (Glasgow) |
| Spain | 1 | Hospital Universitario Valle Hebron (Barcelona) |
| Switzerland | 1 | Institute of Microbiology, CHUV (Lausanne) |
| Taiwan | 1 | Chang Gung Memorial Hospital (Taipei) |
| Turkey | 1 | Institute School of Medicine (Ankara) |
| USA | 1 | Laboratory Assay Development and Reference (Atlanta) |
| No. of Detected/Quantified Samples | Median HDV-RNA Load (Log10 Copy/mL)a | Median HDV-RNA Load (Log10 IU/mL)a, b | |
|---|---|---|---|
| Lab1 | 18 | 5.42 | 3.96 |
| Lab2 | 18 | 2.83 | 4.83 |
| Lab3 | 9 | 4.44 | 4.44 |
| Lab4 | 14 | 3.35 | 3.04 |
| Lab5 | 17 | 6.10 | 3.88 |
| Lab6 | 18 | 5.72 | 5.63 |
| Lab7 | 18 | 5.65 | 4.40 |
| Lab8 | 17 | 6.04 | 5.54 |
| Lab9 | 13 | 3.88 | 3.91 |
| Lab10 | 17 | 4.26 | 3.38 |
| Lab11 | 18 | 4.58 | 4.23 |
| Lab12 | 18 | 5.61 | 4.39 |
| Lab13 | 15 | 3.58 | 2.97 |
| Lab14 | 18 | 5.54 | 4.08 |
| Lab15 | 17 | 5.20 | 4.12 |
| Lab16 | 18 | 4.58 | 4.60 |
| Lab17 | 18 | 5.34 | 4.68 |
| Lab18 | 18 | 4.17 | 3.37 |
| Lab19 | 10 | 4.72 | 4.35 |
| Lab20 | 16 | 5.54 | 3.50 |
| Lab21 | 18 | 3.95 | 4.22 |
| Lab22 | 18 | 3.65 | 3.91 |
| Lab23 | 15 | 2.85 | 2.85 |
| Lab24 | 18 | 5.50 | 5.41 |
| Lab25 | 17 | 5.02 | 4.93 |
| Lab26 | 16 | 4.75 | 3.29 |
| Lab27 | 8 | 4.74 | 4.77 |
| Lab28 | 16 | 5.43 | 5.42 |
- a Calculated from values of quantified samples.
- b Values converted in IU/mL according to the results obtained with panel B (WHO HDV international standard).
The two NCs of panel A were correctly identified as negative by all but two labs, these latter finding, respectively, 1.15 and 2.1 log IU/mL for one of the NCs. All 18 positive samples of panel A were correctly identified as such by 13 labs (43%). The remaining 15 labs detected HDV-RNA in 17 samples (n = 5), 16 samples (n = 3), 15 samples (n = 2), 14 or 13 samples (n = 2) or <11 samples (n = 3; Table 2). Moreover, five labs identified one to seven samples as positive unquantifiable, close to their LLOQ (data not shown).
PCA was performed using only HDVL results. HDV genotypes and technical characteristics were used as additional variables. We kept the two first axes of the PCA, which quantified the size and dispersion of the measures, respectively.
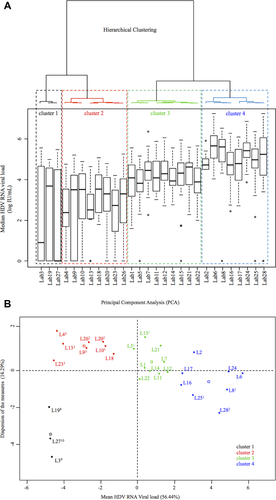
From these parameters, and according to the criterion of the similarity of lab results, we were able to classify the 28 labs into four clusters (Fig. 3A,B). Cluster 1 (in black) comprised labs 3, 19, and 27, which found HDVL results very far from both the expected values and the values of the others labs, that is, they did not detect 8-10 positive samples and dramatically underestimated the remaining ones. Cluster 2 (in red), with a median HDVL of 3.33 log IU/mL (2.85-3.91), regrouped eight labs that underestimated HDVL in all samples and failed to quantify at least one sample, except for L18. Cluster 3 (in green), with a median HDVL of 4.12 log IU/mL (3.88-4.39), comprised nine labs that detected all 18 positive samples of panel A (except L5 and L15, which did not detect S19). Moreover, in cluster 3, L1 identified S1 as positive unquantifiable and L5 identified S7 and S14 as positive unquantifiable. Cluster 4 (in blue), with the highest median HDVL values of 5.17 log IU/mL (4.60-5.63), comprised the remaining eight labs. In this last cluster, five labs detected the 18 positive samples and the remaining three labs reported negative results in ≤2 samples. L8 and L25 failed to detect S15 (HDV-1Eu/As) and L28 identified S4 (HDV-6) and S20 (HDV-7) as negative (Figs. 4 and 5).
QUANTIFICATION RESULTS ACCORDING TO TECHNICAL CHARACTERISTICS
Systematic analysis of the technical characteristics of the assays, as provided by the different labs, did not identify any significant differences for the performances of the labs, whatever the cluster considered. Similarly, no significant differences were observed between in-house and commercial kits (data not shown).
QUANTIFICATION RESULTS ACCORDING TO HDV (SUB)GENOTYPE
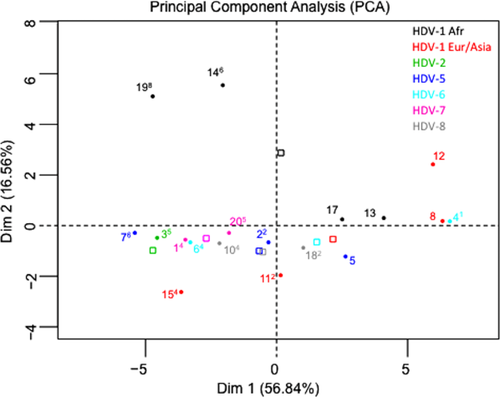
Performances of the different labs depended strongly on HDV genotype (Figs. 4 and 5). Thus, all samples with an African genotype (including HDV-1Afr) and HDVL values ranging from 3.9 to 6.7 log IU/mL were either not detected or dramatically underestimated by several laboratories. For example, S14 and S19, an African group of genotype 1 (Fig. 1), were not detected by six and eight laboratories, respectively, and were underestimated by several others (Figs. 4 and 5). A similar pattern was found with other African strains of HDV-5 (S7), HDV-6 (S6), HDV-7 (S1 and S20), and HDV-8 (S10 and S18). Of note, the Asian HDV-2 sample (S3) was also dramatically underestimated or missed by several laboratories (labs 3, 9, 19, 22, 23, 26, and 27; Fig. 5). Very surprisingly, four laboratories (labs 3, 8, 25, and 27) failed to detect S15, which is a ubiquitous strain in Europe and Asia (Figs. 4 and 5). Taken together, these results strongly suggested a major role for HDV genetic variability in quantification failure.

To address this hypothesis, we aligned the complete genomic sequences of the 18 positive samples of panel A and the sequences of primers and probes provided by 21 of the participating laboratories (data for six labs using commercial assays and one using an in-house assay were not available). Consistent with genetic variability within the target PCR regions, we found several potential mismatch positions within these sequences (Table 3). This concerned primarily forward primers and probes, which showed up to six and five mismatches, respectively. Inversely, with no more than one mismatch, reverse primers appeared relatively well adapted to their targets. Five laboratories used primers and probes designed within the hepatitis delta antigen coding region, but, unfortunately, only L7 provided data for them. The underestimation of the HDVLs in S5 and S8 (3 and 1.5 log IU/mL, respectively) by L7 was very likely linked to the presence of one and three mismatches to the target, respectively, in the reverse primer and probe sequences. Most cluster 3 and 4 laboratories, which were able to detect almost all samples, used primers and probes designed for the very well-conserved ribozyme regions of the viral genome without mismatches, except L28 (one), L2 (three), L21 (three), and L5 (four; Table 3). In contrast, laboratories within cluster 2, which reported low HDVL values for most panel A samples, used forward primers and probes exhibiting respectively two to six mismatches and one to four mismatches to their respective targets. According to Fisher's exact test for counting data, the number of mismatches was significantly higher in cluster 1 and 2 versus cluster 3 and 4 (P = 0.034). Very interestingly, L27, which failed to quantify 10 of the 18 positive samples, used a forward primer exhibiting only one mismatch, and, inversely, lab 2, which detected all samples correctly, used forward primer exhibiting three mismatches. Similarly, S19 (HDV-1Afr), which was not detected by eight laboratories (labs 4, 5, 9, 13, 15, 20, 26, and 27; Figs. 4 and 5), presented one to six mismatches within the corresponding forward primer and probe sequences. In all these latter cases of detection/quantification failures, the primer and probe sequences were also designed within the well-conserved ribozyme region of the viral genome. However, labs 21 (although using a forward primer with three mismatches) and 24 properly quantified S19. Taken together, one can speculate that, in addition to the genetic variability at the primary nucleotide sequence, the secondary structure of the HDV RNA, which is predicted to fit in a pseudo-knotted conformation in its ribozyme region,32 may be involved in quantification outcomes.
| Laboratories | Forward Primera | Reverse Primera | Probea | Amplification Region |
|---|---|---|---|---|
| Lab64 | 0 | 0 | 0 | Antigenomic and genomic ribozymes |
| Lab84 | 0 | 0 | 0 | Antigenomic and genomic ribozymes |
| Lab123 | 0 | 0 | 0 | Antigenomic and genomic ribozymes |
| Lab244 | 0 | 0 | 0 | Antigenomic and genomic ribozymes |
| Lab254 | 0 | 0 | 0 | Antigenomic and genomic ribozymes |
| Lab174 | 0 | 0 | 1 | Antigenomic and genomic ribozymes |
| Lab113 | 0 | 1 | SYBR Green | End LHDAg and antigenomic ribozyme |
| Lab143 | 0 | 0 | 1 | Antigenomic and genomic ribozymes |
| Lab271 | 1 | 0 | 1 | Antigenomic and genomic ribozymes |
| Lab284 | 1 | 1 | SYBR Green | End LHDAg and genomic ribozyme |
| Lab73 | 0 | 1b | 3(5) | HD antigen |
| Lab132 | 2(2) | 0 | 0 | Antigenomic ribozyme |
| Lab102 | 2(3) | 0 | 0 | Antigenomic ribozyme |
| Lab153 | 2(3) | 0 | 1 | Antigenomic ribozyme |
| Lab262 | 2(3) | 0 | 1 | Antigenomic ribozyme |
| Lab24 | 3(3) | 0 | 0 | Antigenomic ribozyme |
| Lab213 | 3(3) | 0 | 0 | Antigenomic ribozyme |
| Lab92 | 3(3) | 0 | 0 | Antigenomic ribozyme |
| Lab53 | 4(4) | 1 | 2(3) | Antigenomic ribozyme |
| Lab202 | 4(4) | 1 | 3(4) | Between the two ribozymes |
| Lab42 | 6(6b) | 0 | 4(4) | Antigenomic ribozyme and antigenomic and genomic ribozymes |
| Lab13 | NA | NA | NA | HD antigen |
| Lab31 | NA | NA | NA | HD antigen |
| Lab164 | NA | NA | NA | HD antigen |
| Lab182 | NA | NA | NA | NA |
| Lab191 | NA | NA | NA | HD antigen |
| Lab223 | NA | NA | NA | NA |
| Lab232 | NA | NA | NA | NA |
- The numbers in superscript correspond to the cluster (1, 2, 3, or 4) of classification of the laboratory (see Fig. 3A,B).
- a Number of mismatches observed according to alignment of the sequences of the 18 positive strains of panel A. In parenthesis, total possible number of mismatches.
- b Mismatches located in the first five nucleotides of the 3′ end of the primer.
- Abbreviations: HD, hepatitis delta; LHDAg, large hepatitis delta antigen; NA, not available.
Discussion
The quantification of HDV RNA has become a major diagnostic issue, for both the management of patients and the evaluation of new specific anti-HDV therapies, which are currently in progress. Recent studies have shown that in comparison to the FNRL-HDV consensus assay,19 many in-house and commercial assays dramatically underestimate HDVL and even fail to detect HDV-RNA.30, 31 These discrepancies among assays were shown to be mainly related to the high genetic diversity of HDV and to primers and probes poorly adapted. This highlights the need for a standardized approach to the quantification of HDV RNA by real-time RT-PCR. Here, we report the results of the first quality-control study on HDV-RNA load assessment to be carried out at the international level. Our work involved 28 different laboratories, distributed worldwide, and took advantage of the recently developed WHO-HDV-IS to standardize results into IU/mL. In this manner, we were able to fully compare the relative performances of most in-house and commercial assays that have been developed worldwide over more than 10 years.
Most important, our study confirmed that most laboratories underestimate and/or fail to detect several African genotypes (i.e., HDV-5 to -8 and HDV-1Afr). Our alignment of primer/probe sequences against the 18 whole-genomic sequences of the samples of panel A revealed that several primer/probe mismatches were associated with discordant results among laboratories. We note, however, that two laboratories using nonmismatched primers grossly underestimated the viral load of sample 19. Furthermore, we observed discrepancies for two different HDV-1 African subgenotype strains that we recently characterized (E. Gordien and F. Le Gal, personal data and manuscript in preparation), despite only subtle sequence differences. These observations suggest that efficient quantification of viral RNA may be affected by structural features of the HDV genome, which is predicted to adopt a rod-like hairpin where 70% of the nucleotides form base pairs, interspersed with stems and loops.33 Therefore, not only the primary PCR target sequence, but also these structural properties must be taken into account for primer/probe design. Studies are now ongoing to identify (sub)genotype-independent target priming sites to improve the robustness of HDV-RNA quantification by real-time RT-PCR.
Because of its high degree of conservation, the ribozyme region is a preferred PCR target; indeed, it was the choice of at least 19 laboratories (information not available for three laboratories) in our study. Nonetheless, among those laboratories, we observed a high degree of discrepancy in viral load estimates, possibly related to the differences in (sub)genotypic nucleotide sequences or RNA structures discussed above. Unfortunately, we could not assess the value of PCR targeting the antigen-coding region because primers and probes for it were provided by only one laboratory using an in-house assay. Interestingly, however, this latter was able to detect all samples, although some were slightly underestimated, possibly attributed to mismatches observed within the probe in particular.
Technical features, including RNA extraction methods and real-time RT-PCR technologies and devices, seemed to have no significant influence on the final quantitative results.
However–and crucially–12 labs had no internal control for monitoring the different steps of their assays. Moreover, 2 of 28 assays used a DNA as internal control, meaning that NA extraction and RT steps were not evaluated. Another main point was the use of DNA, rather than RNA, as the standard of quantification. This point had been raised by Homs et al., who demonstrated that a full-length genomic RNA and a strong thermal-shock denaturation step were indicated for optimal HDVL quantification.34 A strong denaturation step has also been a key point for optimal results in our own experience.19 It could be argued that RNA should be more appropriate, like in human immunodeficiency virus or hepatitis C virus commercial assays, to properly quantify an RNA target.
Nevertheless, the results of our study suggest that these variables did not significantly interfere with the quantitative performances of the different assays and instead strongly highlight the crucial nature of primer and probe design for robust HDVL estimates. Thus, according to their relative quantification performances, we classified the labs into four clusters. Laboratories grouped in clusters 1 and 2 gave poor results compared to those in clusters 3 and 4, which were able to detect and quantify HDV RNA from almost all samples. We note that samples could not be retested, and thus the possibility of punctual handling errors could not be eliminated when labs failed to quantify one sample. Thus, with the exception of laboratory 28 (two samples undetected), we can consider that all cluster 3 and 4 laboratories, representing half of the participating laboratories and including two that used commercial assays, found target values of the panel A samples (Fig. 3B). In contrast, the possible underestimation of some samples by cluster 3 laboratories, or their overestimation by cluster 4 labs, will need to be further addressed. In this respect, the normalization of the values was done with the HDV-WHO-IS prepared from a specific strain of the ubiquitous European/Asian genotype 1, which raises the issue of the use of an international standard consisting of a blend of different (sub)genotypes.
In summary, there is an urgent need to standardize HDV-RNA quantification assays. We note particularly that although TaqMan was most frequently used in the different assays, our results show that the only technical parameter to play a role in the performance of the different assays was primer and probe design.
In conclusion, this study allows us to imagine a standardized assay, available as a purchasable kit externally validated for performance and ease of use in the clinic. The first key point would be the use of an obligatory internal control at the RNA extraction step of the assay. Ideally, this internal control should be an armored or encapsulated RNA to enable the monitoring of all subsequent steps, including RNA extraction and complementary DNA synthesis. Additionally, a one-step RT-PCR would be suitable for full automation. The primers and probes for such an assay should be designed preferentially for the highly conserved ribozyme of the genome, while taking into account potential issues with the RNA secondary structure. Alternatively, another region could be chosen, as long as the overall genetic variability of HDV is considered. Furthermore, an RNA, rather than a DNA plasmid, should be used as the standard of quantification, and the HDV-WHO-IS should be used to standardize and express the results in IU/mL.34 As discussed above, one issue remains unresolved, that is, whether or not international standards for the different genotypes should be provided. Finally, all of the various steps should ideally be automated so as to provide an efficient easy-to-use kit providing high specificity and sensitivity, as well as the capacity to quantify the HDV RNA of all infectious strains whatever the genotype. Such a tool would greatly improve patient management and forward the development of new, specifically targeted anti-HDV therapies.
Acknowledgments
We thank all those who participated in the study: Paul Erhlich Institute, Langen (Germany); Hannover Medical School, Hannover (Germany); MVZ Laboratory, Karlsruhe (Germany); LIPSDIAG GmbH, Leipzig (Germany); University Hospital Heidelberg, Heidelberg (Germany); University Ziekenhuis, Ghent (Belgium); Aalborg University Hospital, Aalborg (Denmark); Hospital Universitario Valle Hebron Barcelona (Spain); CHU Pitié Salpétrière, Paris (France); CHU Lyon Sud, Lyon (France); Laboratoire CERBA, Saint-Ouen-l'Aumône (France); CHU Dupuytren, Limoges (France); CHU Hôtel Dieu, Nantes (France); CHU Avicenne, Bobigny (France); University of Thessaly, Larissa (Greece); University of Athens, Athens (Greece); Azienda Ospedaliero-Universitaria, Torino (Italy); Policlinico Universitario “G. Martino”, Messina (Italy); Institut de Microbiologie, Centre Hospitalier Universitaire Vaudois, Université de Lausanne, Lausanne (Switzerland); Institute School of Medicine, Ankara (Turkey); UCLH NHS Foundation, London (UK); Laboratory Assay Development and Reference, Atlanta, GA (USA); Central Research Institute Of Epidemiology, Moscow (Russia); Chang Gung Memorial Hospital, Taipei (Taiwan); Laboratoire Biomédical-24, Nouakchott (Mauritania); WHO Regional Reference Laboratory For Hepatitis B, Melbourne (Australia); West of Scotland Specialist Virology Center, Glasgow (UK); and Fast Track Diagnostic (Luxembourg).
We also thank Dr. Michael Chudy from the Paul Ehrlich Institute (PEI) for providing the WHO HDV international standard and Dr. Syria Laperche of the French National Institute for Blood Transfusion (INTS) for providing HBsAg-negative plasma.
We thank Fernando Neri Pinto, Elhame Anouhal, and Jessica Techer, the laboratory technicians who performed genotyping, viral load quantification, dilutions of the samples, and preparation of the panels.




