Quantitative maternal hepatitis B surface antigen predicts maternally transmitted hepatitis B virus infection
Potential conflict of interest: Nothing to report.
This research was funded by grants from the Ministry of Health and Welfare (DOH-99-DC-1001 and DOH-100-DC-1019), Joint Research Grants from National Taiwan University and Cathay General Hospital (102-CGN07 and 103-CGN13), and a grant from Cardinal Tien Hospital (CTH-99-1-2A28).
Abstract
Despite immunoprophylaxis, hepatitis B virus (HBV) transmission in highly viremic mothers remains a global health issue. Using quantitative maternal surface antigen (HBsAg) to predict HBV infection in infants has not been investigated. We enrolled 526 mother-infant pairs with positive maternal HBsAg under current immunoprophylaxis. Maternal viral load and quantitative HBsAg were measured in the peripartum period. Infant HBsAg seropositivity for more than 6 months was defined as chronic infection. Rates of chronic infection in infants at various maternal HBsAg levels were estimated using a multivariate logistic regression model. Results showed that maternal HBsAg was positively correlated with maternal viral load (r = 0.69; P < 0.001) and accurately predicted maternal viral load above 6, 7, and 8 log10 IU/mL with an area under the receiver operating characteristic curve (AUC) of 0.97, 0.98, and 0.95. Nineteen infants were chronically infected. After adjustment for the other risk factor, maternal HBsAg level was significantly associated with risk of infection (adjusted odds ratio for each log10 IU/mL increase, 15.02; 95% confidence interval [CI], 3.89-57.94; P < 0.001). The AUC for predicting infection by quantitative maternal HBsAg was comparable to that by maternal viral load (0.89 vs. 0.87; P = 0.459). Estimated rates of infection at maternal HBsAg levels of 4, 4.5, and 5 log10 IU/mL were 2.4% (95% CI, 0.1-4.6; P = 0.04), 8.6% (95% CI, 4.5-12.7; P < 0.001), and 26.4% (95% CI, 12.6-40.2; P < 0.001). Conclusion: Quantitative maternal HBsAg predicts infection in infants as well as maternal viral load does. Antiviral therapy may be considered in pregnant women with an HBsAg level above 4-4.5 log10 IU/mL to interrupt mother-to-infant transmission. (Hepatology 2016;64:1451-1461)
Abbreviations
-
- ALT
-
- alanine aminotransferase
-
- AUC
-
- area under the curve
-
- CI
-
- confidence interval
-
- HBeAg
-
- hepatitis B e antigen
-
- HBIG
-
- hepatitis B immunoglobulin
-
- HBsAg
-
- hepatitis B surface antigen
-
- HBV
-
- hepatitis B virus
-
- OR
-
- odds ratio
-
- PCR
-
- polymerase chain reaction
-
- ROC
-
- receiver operating characteristic
Universal immunization beginning at birth and other vaccination strategies have resulted in a substantial reduction of hepatitis B virus (HBV) transmission in many countries, including Taiwan, with historically high endemicity.1-4 However, current immunoprophylaxis strategy does not completely eradicate HBV transmission. Maternal transmission is the main route of transmission in HBV-infected children despite immunoprophylaxis at birth.4-7 Hepatitis B e antigen (HBeAg)-positive mothers with high viral load are most likely to transmit the virus, even with active-passive immunoprophylaxis.5, 6, 8-11 Transmission from highly viremic mothers remains a major challenge in eradicating the HBV-related diseases.
In recent years, there have been gradually increasing reports on the efficacy of antiviral nucleos(t)ide analogues in highly viremic HBV-infected pregnant women to reduce maternal viral load and thus mother-to-infant transmission.12-17 Although these studies support both the safety and efficacy of antiviral therapy in mothers and infants for prevention of transmission, the standard screening and intervention protocol has not been established. The optimal cut-off level of maternal viral load for antiviral treatment is still under debate.18-21 In addition, because many highly endemic areas for HBV infection are resource-constrained countries, the cost and availability of HBV-DNA testing may limit the use of prenatal maternal HBV-DNA quantification in resource-limited areas.22
Quantitative serum hepatitis B surface antigen (HBsAg), as a sensitive and reliable commercial assay, has become a focus for translational and clinical researches recently.23, 24 Studies have demonstrated the value of quantitative HBsAg in predicting long-term outcomes of chronic HBV infection, including HBsAg clearance, HBV-DNA undetectability, and development of HCC.25-28 Similar to HBV DNA, HBsAg level is highest in the immune tolerance phase, starts to decline in the immune clearance phase, and decreases after HBeAg seroconversion.29-32 Because mother-to-infant HBV transmission occurs in highly viremic mothers, it is very likely that mothers with high viral load also have high HBsAg levels. We hypothesize that mothers with high HBsAg levels may have a higher risk to infect their children. The aim of this study was to investigate the relationship between quantitative maternal HBsAg and maternal viral load, and the relationship between quantitative maternal HBsAg and maternally transmitted HBV infection.
Subjects and Methods
SUBJECTS
From April 2007 to July 2013, 568 HBsAg-positive mothers with 594 deliveries from National Taiwan University Hospital, Cardinal Tien Hospital, Cathay General Hospital, Mackay Memorial Hospital, Taipei Tzu Chi Hospital, and En Chu Kong Hospital were enrolled. These women were routinely tested for qualitative HBsAg and HBeAg in the early third trimester according to the national screening program for pregnant women. Mothers positive for HBsAg were asked to join the study at the time of prenatal visits or at delivery. All mothers were negative for human immunodeficiency virus. Mothers receiving antiviral therapy during pregnancy were not included in this study.
PROTOCOL
After informed consents were obtained, mothers' serum specimens were collected in the third trimester of pregnancy or in the peripartum period.11 Maternal serum alanine aminotransferase (ALT), HBV genotype, viral load, and quantitative HBsAg were tested. In addition to HBeAg data obtained from routine prenatal visits, maternal HBeAg was rechecked at the same time when maternal viral load and quantitative HBsAg were tested. All infants received three doses of hepatitis B vaccine, either H-B-Vax II (5 μg/0.5 mL; Merck Sharp & Dohme, Rahway, NJ) or ENGERIX-B (20 μg/1 mL; GlaxoSmithKline Biologicals, Rixensart, Belgium) within the first week of birth, at 1 month, and at 6 months. For newborns of HBeAg-positive mothers, 0.5 mL (100 IU) of hepatitis B immunoglobulin (HBIG) was administered within 24 hours of birth. For newborns of HBsAg-positive but HBeAg-negative mothers, administration of self-paid HBIG was optional.4, 6 Preterm infants received the same immunization strategy as term infants except for delayed initiation of vaccination until these infants reached 2,000 g and had stable medical condition.33, 34 After 2013, an additional birth dose of vaccine was administered to those whose birth weight was <2,000 g, followed by three regular doses of vaccines. The on-schedule rates of vaccination were defined as the administration of the first, second, and third dose of HBV vaccines no longer than 7 days, 1.5 months, and 7 months after birth, respectively.
LABORATORY METHODS
Hepatitis B serological markers were tested using the AxSYM microparticle enzyme immunoassay (Abbott Laboratories, Abbott Park, IL). Maternal serum HBV DNA was quantified by a real-time polymerase chain reaction (PCR) assay (Abbott RealTime HBV assay; Abbott Molecular Inc., Des Plaines, IL). Lower and upper limits of quantification are 15 (51.2 copies/mL) and 109 IU/mL (3.4 × 109 copies/mL), respectively. HBV genotype was determined by real-time PCR with subsequent melting curve analysis (LightCycler hybridization probes assay system; Roche Diagnostics, Indianapolis, IN). This assay determines HBV genotype when viral load is not less than 1,000 copies/mL.35
Quantitative HBsAg was measured by a chemiluminescent microparticle immunoassay (Architect HBsAg; Abbott Laboratories). The detection range is from 0.05 to 250 IU/mL. All serum samples were diluted to 1:20 before HBsAg quantification. If the HBsAg level was found to be higher than 250 IU/mL, samples were diluted to 1:400 to 1:800 to obtain a reading within the calibration curve range.
DEFINITION OF HBV INFECTION IN INFANTS
Because the seropositivity of the HBV core antibody at age 0-24 months mainly results from passive transfer of the maternal antibody,36 we used seropositivity of HBsAg as the marker of infection. Infants were tested for HBsAg at 4-8 months and/or at 1-2 years of age.11 If an infant tested HBsAg positive, HBsAg testing was repeated 6 months later to confirm chronic infection. The institutional review board of the above-mentioned hospitals approved the protocol.
STATISTICAL ANALYSES
Statistical analyses were performed using Stata software (version 11.2; StataCorp LP, College Station, TX). Two-sided P ≤ 0.05 was considered statistically significant. HBV DNA and quantitative HBsAg levels were log10-transformed. For statistical comparisons, we assigned a value of 15 IU/mL, the lower detection limit of the HBV-DNA quantification assay, to samples with detectable HBV-DNA levels less than 15 IU/mL.
Differences between infants with and without HBV infection were analyzed with the use of the Wilcoxon rank-sum test for continuous variables and Fisher's exact or chi-square tests for categorical variables. The factors examined included maternal age, maternal HBeAg status, maternal viral load, maternal HBsAg levels, maternal ALT levels, maternal HBV genotype, factors related to maternal-fetal hemorrhage (threatened abortion, threatened preterm labor, chorionic villus sampling, amniocentesis, forceps/vacuum delivery, and emergent cesarean section after any period of labor),37 type of birth,38 gestational age, infant sex, birth weight, timeliness of HBV vaccination, and feeding practice.39, 40 Multivariate logistic regression analyses were performed to obtain the odds ratio (OR) and 95% confidence interval (CI). Predictor variables in the multivariate logistic regression models were selected from the characteristics that were different between infants with and without HBV infection. Goodness of fit of the multivariate logistic regression model was determined using Hosmer-Lemeshow statistics. Using the multivariate logistic regression models, rates of infection were estimated at designated maternal viral load and maternal HBsAg levels.
The correlation between maternal viral load and quantitative maternal HBsAg was evaluated by the Spearman correlation coefficient. Receiver operating characteristic (ROC) analyses were used to evaluate the performance of quantitative maternal HBsAg to predict high maternal viral loads (≥6, 7, and 8 log10 IU/mL) and the performance of quantitative maternal HBsAg to predict infant's infection. The Youden's index was used to select the optimal cut-off point.41 The ROC contrast test was used to compare ROC curves.
Results
FOLLOW-UP AND OUTCOMES OF INFANTS
Enrollment and follow-up of participants are shown in Fig. 1. We enrolled 594 deliveries of 568 mothers, and 88.6% (526 of 594) of infants have returned for HBsAg testing. Infants tested for HBsAg and infants lost to follow-up had comparable maternal and birth characteristics (Supporting Table S1). The main reasons for infants not returning for testing were moving away and parental unwillingness to let their children receive painful blood sampling. All 162 infants with HBeAg-positive mothers and 90.9% (331 of 364) infants with HBeAg-negative mothers received HBIG administration. The HBV vaccine completion rate (three doses) was 100%. On-schedule rates for the first, second, and third doses of vaccine were 95.4%, 93.9%, and 92.4%, respectively.
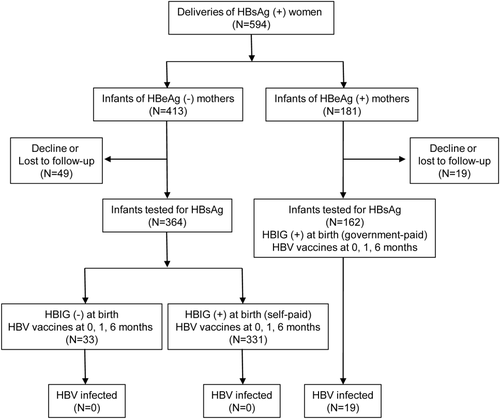
Among the 526 children tested for HBsAg, 63 (12%) were checked only at 1-2 years of age. For the children tested only at 1-2 years of age without testing before, both perinatal and horizontal transmissions were possible. Their HBsAg-positive mothers were the most probable sources of infection, either perinatal or postnatal horizontal transmission.42
Chronic HBV infection was confirmed in 19 infants; all were born to HBeAg-positive mothers. The infection rate was 3.6% (19 of 526) in infants born to HBsAg-positive mothers and 11.7% (19 of 162) in infants born to HBeAg-positive mothers.
CORRELATION BETWEEN QUANTITATIVE MATERNAL HBsAg AND VIRAL LOAD
Median time of maternal blood sampling for quantitative HBsAg and HBV DNA was 2 days postpartum, and the latest blood sampling time was 2 months postpartum. All the 162 HBeAg-positive mothers and 99.5% (362 of 364) of HBeAg-negative mothers had concordant HBeAg results with prenatal HBeAg screening. The 2 mothers with HBeAg seroconversion had quantitative HBsAg and HBV DNA tested on the day of delivery.
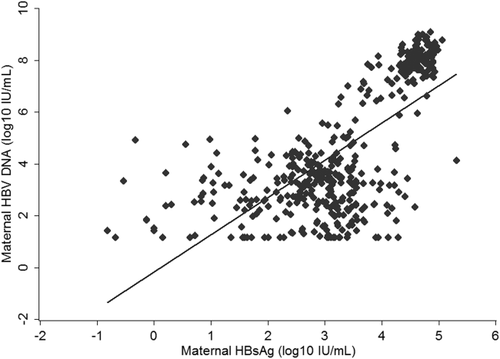
Table 1 shows maternal characteristics by HBeAg status. HBeAg-positive mothers were younger (33.4 ± 4.2 vs. 34.3 ± 4.0 years; P < 0.001) and had higher viral load (7.3 ± 1.6 vs. 2.9 ± 1.1 log10 IU/mL; P < 0.001) and higher HBsAg levels (4.3 ± 0.8 vs. 2.5 ± 1.1 log10 IU/mL; P < 0.001) than HBeAg-negative mothers.
| HBeAg(+) Mothers (N = 162) | HBeAg(−) Mothers (N = 364) | P Value | |
|---|---|---|---|
| Age at delivery, years | |||
| Mean ± SD | 33.4 ± 4.2 | 34.3 ± 4.0 | <0.001 |
| Maternal viral loada | |||
| Mean ± SD (log10 IU/mL) | 7.3 ± 1.6 | 2.9 ± 1.1 | <0.001 |
| Maternal HBsAg | |||
| Mean ± SD (log10 IU/mL) | 4.3 ± 0.8 | 2.5 ± 1.1 | <0.001 |
- a This category excludes 92 mothers with undetectable maternal HBV-DNA levels.
Figure 2 depicts the correlation between quantitative maternal HBsAg and maternal viral load. Quantitative maternal HBsAg had a strong positive correlation with maternal viral load (r = 0.69; P < 0.001) in all HBsAg-positive mothers. In HBeAg-positive mothers, HBV-DNA levels were positively correlated with HBsAg levels (r = 0.65; P < 0.001). In HBeAg-negative mothers, HBV-DNA levels had a suboptimal correlation with HBsAg levels (r = 0.12; P = 0.046).
Performances of quantitative maternal HBsAg to predict high maternal viral load are shown in Supporting Fig. S1. The predictive accuracy was very high, with an area under the ROC curve (AUC) of 0.97 (95% CI, 0.96-0.99), 0.98 (95% CI, 0.97-0.99), and 0.95 (95% CI, 0.93-0.97) to predict maternal viral load higher than 6, 7, and 8 log10 IU/mL, respectively. The optimal cutoff of maternal HBsAg level was 3.63, 4.30, and 4.32 log10 IU/mL, with a sensitivity of 97.1%, 91.1%, and 98.6% and a specificity of 88.9%, 97.4%, and 86.0% to predict maternal viral load higher than 6, 7, and 8 log10 IU/mL, respectively.
FACTORS RELATED TO MATERNALLY TRANSMITTED HBV INFECTION
Table 2 lists the risk factors included in the univariate analyses. High maternal viral load (OR for each log10 IU/mL increase, 2.38; 95% CI, 1.53-3.72; P < 0.001), high maternal HBsAg (OR for each log10 IU/mL increase, 15.58; 95% CI, 4.10-59.19; P < 0.001), and preterm delivery (OR, 3.96; 95% CI, 1.24-12.62; P = 0.020) were associated with a higher risk of infection in infants. Compared to infants with a gestational age ≥37 weeks, a higher percentage of preterm infants had a delay in the first (3.1% vs. 25%; P < 0.001) and second (4.5% vs. 27.8%, P < 0.001) dose of hepatitis B vaccine. Maternal viral load, quantitative maternal HBsAg, and preterm delivery were used as predictor variables in the multivariate analyses (Table 3). Maternal HBeAg was not used as a predictor variable in logistic regression analyses because of separation, a condition in which the independent variables perfectly predicted the outcome variable.
| Variable | OR (95% CI) | P Value |
|---|---|---|
| Maternal age (per 1-year increase) | 0.97 (0.87-1.09) | 0.647 |
| Maternal HBeAga | — | — |
| Maternal HBV DNA (per log10 IU/mL increase)b | 2.38 (1.53-3.72) | <0.001 |
| Maternal HBsAg (per log10 IU/mL increase) | 15.58 (4.10-59.19) | <0.001 |
| Maternal ALT > ULN (40 U/L) vs. ≤ULNc | 2.09 (0.58-7.47) | 0.257 |
| Maternal HBV genotype C vs. genotype Bd | 0.99 (0.34-2.85) | 0.987 |
| Cesarean vs. vaginal birth | 0.51 (0.18-1.44) | 0.202 |
| Presence of any factor related to maternal-fetal hemorrhagee | 0.86 (0.34-2.16) | 0.750 |
| Preterm (gestational age <37 weeks) vs. term | 3.96 (1.24-12.62) | 0.020 |
| Female vs. male infant | 0.83 (0.33-2.09) | 0.691 |
| Birth weight (per 1-kg increase) | 0.65 (0.22-1.89) | 0.430 |
| Delayed vaccination | 1.19 (0.34-4.19) | 0.786 |
| Breast vs. formula feedingf | 0.65 (0.21-2.00) | 0.450 |
- a The OR was not available because of separation, a condition in which the independent variables perfectly predicted the outcome variable.
- b This category excludes 92 mothers whose HBV DNA was undetectable.
- c This category excludes 9 mothers with insufficient samples.
- d This category excludes 235 mothers whose sample was insufficient for HBV genotyping or whose viral load was below 1,000 copies/mL and the genotype could not be reliably determined. This category also excludes 4 mothers coinfected with genotypes B and C.
- e This category excludes 4 mother-infant pairs with missing data.
- f The breastfeeding group consists of infants fed breast milk exclusively and infants fed both breast milk and formula in the first 6 months. This category excludes 4 mother-infant pairs with missing data.
- Abbreviation: ULN, upper limit of normal.
| Variable | Model 1 | Model 2 | ||
|---|---|---|---|---|
|
Adjusted OR(95% CI) |
P Value |
Adjusted OR(95% CI) |
P Value | |
|
Maternal HBV DNA (per log10 IU/mL increase) |
2.36 (1.51-3.69) | <0.001 | — | — |
|
Maternal HBsAg (per log10 IU/mL increase) |
— | — | 15.02 (3.89-57.94) | <0.001 |
|
Preterm (gestational age <37 weeks) |
3.02 (0.83-11.03) | 0.095 | 2.44 (0.68-8.71) | 0.169 |
Results of the multivariate logistic regression analyses are listed in Table 3. Maternal viral load and quantitative maternal HBsAg were analyzed in model 1 and model 2 separately because multicollinearity would occur if these two highly correlated variables were used in the same model. According to the Hosmer-Lemeshow test, the data were well fitted by model 1 (P = 1.000) and by model 2 (P = 1.000). After adjustment for the other risk factor, infants with higher maternal viral load (adjusted OR for each log10 IU/mL increase, 2.36; 95% CI, 1.51-3.69; P < 0.001) and infants with higher maternal HBsAg level (adjusted OR for each log10 IU/mL increase, 15.02; 95% CI, 3.89-57.94; P < 0.001) had a significantly higher risk of infection.
ESTIMATED INFECTION RATES AT VARIOUS MATERNAL VIRAL LOAD AND HBsAg LEVELS
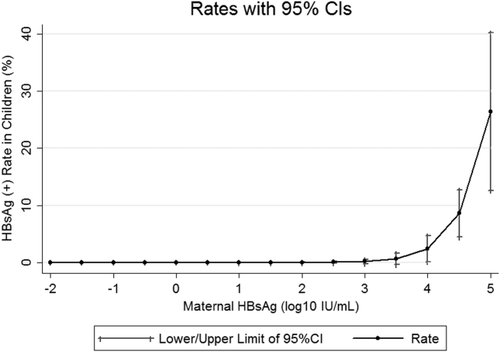
For better application in clinical settings, we used the multivariate logistic regression models listed in Table 3 to estimate infection rates at designated maternal viral load and HBsAg levels. At maternal viral load levels of 6, 7, 8, and 9 log10 IU/mL, rates of infection were 2.5% (95% CI, 0.1-4.9; P = 0.041), 5.7% (95% CI, 2.3-9.1; P = 0.001), 12.4% (95% CI, 7.1-17.7; P < 0.001), and 24.7% (95% CI, 12.0-37.3; P < 0.001), respectively. At maternal HBsAg levels of 3.5, 4, 4.5, and 5 log10 IU/mL, rates of infection were 0.6% (95% CI, -0.4 to 1.6; P = 0.22), 2.4% (95% CI, 0.1-4.6; P = 0.04), 8.6% (95% CI, 4.5-12.7; P < 0.001), and 26.4% (95% CI, 12.6-40.2; P < 0.001), respectively (Fig. 3). Infants with a maternal viral load above 6-7 log10 IU/mL or infants with a maternal HBsAg level above 4.0-4.5 log10 IU/mL have a substantial risk of infection.
QUANTITATIVE MATERNAL HBsAg TO PREDICT INFECTION
Figure 4 shows the performances of maternal viral load and quantitative maternal HBsAg to predict infant infection. The AUC for maternal HBV DNA and quantitative maternal HBsAg to predict infection was 0.87 (95% CI, 0.82-0.92) and 0.89 (95% CI, 0.84-0.93), respectively. The AUC for quantitative maternal HBsAg to predict infection was comparable to that for maternal viral load to predict infection (P = 0.46). The optimal cutoff of maternal viral load level to predict infection was 5.95 log10 IU/mL, with a sensitivity of 100% and a specificity of 71.3%. The optimal cutoff of maternal HBsAg level to predict infection was 4.10 log10 IU/mL, with a sensitivity of 100% and a specificity of 71.3%.

Discussion
Timely postnatal immunoprophylaxis and completion of the three-dose vaccine series in infants born to HBsAg-positive mothers reduces up to 90%-95% of perinatal infections.6, 43, 44 Identifying and treating highly viremic mothers, whose infants are still at risk of infection despite immunoprophylaxis, is the crucial step to complete the last mile of hepatitis B eradication. Our study shows that quantitative maternal HBsAg is highly correlated with maternal viral load, and infants born to mothers with high HBsAg levels are at substantial risk of infection. To our knowledge, the current study is the first to demonstrate the significant relationship between quantitative maternal HBsAg and maternally transmitted HBV infection.
Multiple factors, such as factors related to maternal-fetal hemorrhage,37 type of birth,38 feeding practice,39, 40 and timeliness of vaccination, may affect mother-to-infant HBV transmission, but maternal viral load is the primary and most important cause.8-11 Our study included these aforementioned risk factors and had high follow-up rate and high vaccine on-schedule/completeness rate. Our study not only confirms that both maternal HBsAg and maternal viral load are significantly related to infant infection, but also shows that quantitative maternal HBsAg predicts infant infection as well as maternal viral load does.
Serum HBV DNA and quantitative HBsAg levels reflect similar, but not identical, conditions virologically. Quantification of serum HBV DNA represents merely viral replication. However, serum HBsAg is produced not only from translated messenger RNAs of transcriptionally active covalently closed circular DNA, but also from integrated HBV-DNA sequences.23 Although studies have reported a positive correlation between HBV DNA and quantitative HBsAg levels,29, 30, 45 the role of quantitative maternal HBsAg in predicting infant infection has not been investigated. Compared to HBV-DNA measurement, HBsAg quantification is inexpensive, with a cost of approximately 10%-20% of HBV-DNA quantification, and uses high-throughput platforms. Therefore, the novel results of our study are promising and have important clinical implications.
The limitation of this study is that the correlation between HBsAg and HBV DNA in HBeAg-negative patients is not as good as that in HBeAg-positive patients.45 Risk of transmission in HBeAg-negative mothers is very low but still present, possibly from those mothers with higher viral load. Our large-scale study reported an HBsAg-positive rate of 0.23% (4 in 1,773) in infants with HBeAg-negative mothers under current immunoprophylaxis.6 On the other hand, maternal HBeAg is not routinely tested in prenatal screening in many countries. Our aim of testing quantitative HBsAg is to identify mothers at high risk for transmission, which occurs in highly viremic mothers who are mostly positive for HBeAg. Although the correlation between quantitative HBsAg and HBV DNA is less satisfying in HBeAg-negative mothers, this does not compromise the value of quantitative HBsAg for our purpose.
There have been emerging data on the efficacy and safety of antiviral treatment in highly viremic HBsAg-positive pregnant women to prevent mother-to-infant transmission.12-17 However, there is still no consensus on the optimal cut-off value of maternal viral load for treatment.18-21 Various maternal viral load levels (6-9 log10 copies/mL, approximately 5.3-8.3 log10 IU/mL) have been used as the cutoff for antiviral treatment.12-16 The majority of HBV-infected children with immunoprophylactic failure had a maternal viral load above 7 log10 copies/mL (approximately 6.3 log10 IU/mL).8-11 But infected cases with a maternal viral load at 6 log10 copies/mL (approximately 5.3 log10 IU/mL) have been reported.9-11 Transmission at a lower maternal viral load level may be related to other coexisting risk factors.11 Our study included multiple risk factors and shows that a maternal viral load above 6-7 log10 IU/mL (approximately 6.7-7.7 log10 copies/mL) is associated with a significant risk of infection. The result provides evidence for determination of the criteria for eligibility to receive intervention in HBV-infected pregnant women to prevent mother-to-infant transmission.
Our study also shows that a maternal HBsAg level above 4.0-4.5 log10 IU/mL is associated with a significant risk of infection. According to the ROC analyses, the “optimal” cutoff of maternal viral load and maternal HBsAg to predict infection was 5.95 and 4.10 log10 IU/mL, respectively. Maternal viral load and quantitative HBsAg had the same sensitivity of 100% and specificity of 71% in predicting infection at the optimal cutoffs. It indicates that if we use the aforementioned optimal cutoffs, all mothers at high risk for transmission will be identified and treated, whereas 29% of mothers whose infants are not infected will take medications.
Statistically, the optimal cut-off value is determined according to the maximum of the Youden index (sensitivity + specificity - 1).41 But, in reality, to get the optimal balance between sensitivity and specificity depends on the purpose of the test. Because acquisition of HBV infection in infancy leads to increased lifetime risk for cirrhosis and hepatocellular carcinoma and current evidences have suggested a favorable safety profile for nucleos(t)ide use in both mothers and infants,12-17 a highly sensitive test with a low false-negative rate is preferable to a highly specific test with a low false-positive rate.
Selecting a cutoff for defining high-risk mothers for transmission not only determines the eligibility for receiving treatment, but also affects the cost-effectiveness of intervention. In countries with limited resources, a higher cutoff may be used to reduce the number of the mothers eligible for treatment and thus the cost of the intervention. Inevitably, a higher cutoff would result in lower health benefits because a small number of infected infants with a relatively lower maternal viral load or a relatively lower maternal HBsAg level would be missed in such a screening/intervention strategy. Our ROC analyses demonstrate that a maternal viral load cutoff at approximately 6 log10 IU/mL (approximately 6.7 log10 copies/mL) or a maternal HBsAg cutoff at 4.0-4.5 log10 IU/mL predicts infant infection with an excellent sensitivity and an acceptable specificity. Our multivariate analyses (Table 3) also show that a maternal viral load above 6-7 log10 IU/mL (approximately 6.7-7.7 log10 copies/mL) or a maternal HBsAg above 4.0-4.5 log10 IU/mL is associated with a significant risk of infection. We therefore suggest that the above two cutoffs are both appropriate for determining eligibility for receiving antiviral intervention in HBsAg-positive pregnant women to eliminate perinatal transmission. A proposed algorithm of different screening options for management of HBV-infected mothers and their children is provided (Fig. 5).
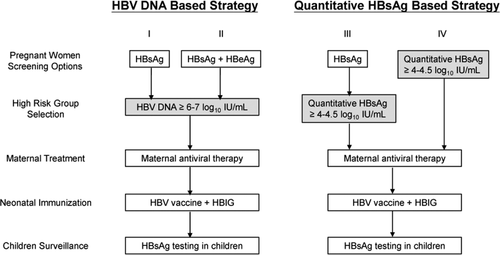
Currently, commercial quantitative HBsAg assays are becoming readily available and have the potential to replace qualitative HBsAg assays for diagnosing HBV infection and monitoring the status of infected individuals.23, 24 The present study shows that in addition to maternal viral load, quantitative maternal HBsAg can serve as an alternative marker to identify high-risk mothers. To identify highly viremic mothers at risk for transmission, a previous proposed strategy for management of HBV-infected pregnant women included two-step screening, initial qualitative HBsAg testing, with or without HBeAg, followed by HBV-DNA quantification.18, 19 Data of the natural history of serum HBsAg-level changes in pregnant women are lacking. Our preliminary data (Supporting Fig. S2) show that quantitative maternal HBsAg levels remained stable from gestational age 30 weeks until 2 months postpartum. A larger patient cohort is required to confirm this finding. We anticipate further studies to validate the value of quantitative HBsAg in prenatal maternal screening to identify mothers with HBV infection and mothers at high risk for transmission in a single test.
In conclusion, quantitative maternal HBsAg positively correlates with maternal viral load, is significantly related to infant's infection, and predicts infection as well as maternal viral load does. A maternal viral load above 6-7 log10 IU/mL (approximately 6.7-7.7 log10 copies/mL) or a maternal HBsAg above 4.0-4.5 log10 IU/mL is associated with a significant risk of infection under current immunoprophylaxis. In addition to maternal viral load, quantitative maternal HBsAg can serve as an alternative marker to identify mothers at high risk for transmission and determine eligibility for treatment. Our results provide important information for the rational design of future screening and intervention strategies to further eliminate mother-to-infant HBV transmission.
Acknowledgments
We thank Professor Jen-Pei Liu for statistical consultation. We thank Miss Chiao-Ling Chen, I-Ting Wang, Yen-Tzu Chen, and Pei-Lin Tsai for their help in following up with the subjects and administrative work. We appreciate the laboratory work done by National Taiwan University Center of Genomic Medicine, Hepatitis Research Center and Department of Laboratory Medicine, National Taiwan University Hospital.




