Statins and portal hypertension: A tale of two models
Potential conflict of interest: Nothing to report.
Portal hypertension (PTH) is a leading cause of death and liver transplantation. Whereas the most common cause of PTH is liver cirrhosis, noncirrhotic causes of PTH, such as prehepatic portal vein occlusion and schistosomiasis, are also well-known etiologies, especially in non-Western parts of the world.1 At the present time, most treatments effective for cirrhotic PTH are extrapolated to be of benefit for noncirrhotic PTH as well.
Our knowledge of the pathophysiology of PTH has evolved during the past decades. New concepts have emerged from a better knowledge of its molecular pathobiology. In particular, the role of sinusoidal endothelial cells (ECs) and hepatic stellate cells (HSCs) in modulating the development of increased intrahepatic resistance and PTH is now better delineated. The concept of endothelial dysfunction, an impaired release of vasoactive molecules from ECs during hepatic injury, is better understood. Additionally, HSC contraction and matrix deposition contributions to the dynamic and fixed component of increased intrahepatic resistance, respectively, have been defined. Specific vasoregulatory molecules have been implicated in this process, including nitric oxide and endothelin, and, more recently, hedgehog pathway has been implicated as well.1 More recent studies now also implicate angiogenesis in PTH pathogenesis through effects both in the liver and outside of the liver.2, 3 Several molecules and processes that have been identified in the pathogenesis of PTH are thought to be targets for the statin class of drugs.
Statins are broadly used medications. In the United States, more than one quarter of adults age 40 and over use cholesterol-lowering medications, as do almost half of those over age 75.4 Interestingly, the potentially beneficial effects of statins in PTH appear to be independent of their cholesterol-lowering activity. Statins decrease fibrosis and lower PTH in animals and humans through multiple putative mechanisms, including blunting the RhoA/Rho-kinase pathway in myofibroblastic HSCs, activating hepatic nitric oxide production, and regulating angiogenesis.2, 3 Thus, statins are emerging as an exciting potential therapy for human PTH and its complications.5, 6
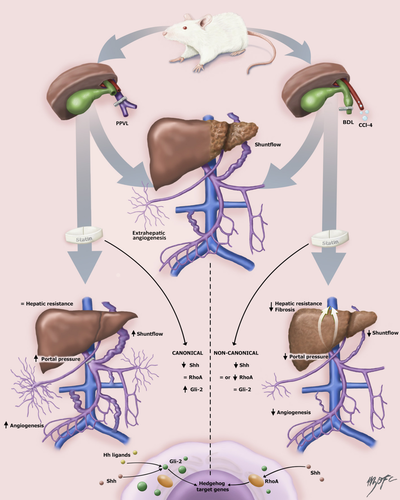
A recent study by Uschner et al. investigated the effect of atorvastatin in cirrhotic and noncirrhotic PTH.7 Investigators evaluated the differences of angiogenesis and various hemodynamic and vascular biological parameters in cirrhotic and noncirrhotic PTH with particular emphasis on the canonical (Sonic hedgehog [Shh] and Glioma-associated oncogene family zink finger-2 [Gli-2] [Shh/Gli]) and noncanonical (Sonic hedgehog and RhoA/Rho-kinase [Shh/RhoA]) hedgehog pathway. In order to assess the differences of angiogenesis, Uschner et al. compared animal models of cirrhotic (bile duct ligation [BDL]; CCl4 intoxication) and noncirrhotic (partial portal vein ligation; PPVL) PTH. Rats received either atorvastatin (15 mg/kg; 7 days) or control chow before death to ascertain effects of statins in PTH development. The investigators conducted invasive hemodynamic measurements and assessed angiogenesis in vitro and in vivo in samples of liver and vessel from animals and also humans with PTH. The results led the investigators to propose that statins cause divergent effects in cirrhotic and noncirrhotic PTH.
The investigators found that atorvastatin reduced portal pressure in cirrhotic rats attributable to significantly decreased hepatic vascular resistance. Interestingly, the opposite effect on portal pressure was observed in noncirrhotic PPVL rats, where also a worsening in both systemic and splanchnic hyperdynamic circulation was observed. Given that atorvastatin decreased hepatic vascular resistance in cirrhotic PTH, the investigators evaluated the expression of Hh signaling in these animals. Uschner et al. observed that Hh signaling was up-regulated in experimental (and human liver) cirrhosis and this phenomenon was blunted by atorvastatin. Moreover, the messenger RNA (mRNA) levels of the profibrotic markers, alpha smooth muscle actin, collagen-1, and vimentin, were enhanced in cirrhosis, and atorvastatin-treated cirrhotic rats exhibited attenuated increases of these fibrotic markers. The investigators also cultured primary rat HSCs and incubated cells with varying concentrations of atorvastatin. They observed that atorvastatin blunted HSC migration, as measured by scratch assay, and blocked the noncanonical Hh pathway in a RhoA-dependent manner.
Given that shunt flow was reduced in cirrhotic rats and was enhanced in noncirrhotic PTH, the role of angiogenesis in extrahepatic vessels was explored. To that end, the investigators used intraperitoneal and subcutaneous Matrigel (collagen plug) implantation. New vessels were assessed immunohistochemically with specific staining for ECs and vascular smooth muscle cells. Of note, sprouting of new vessels in these Matrigels was blunted in cirrhotic animals treated with atorvastatin, but enhanced in noncirrhotic PTH. Given that statins have been shown to attenuate fibrosis development and PTH through modulation of the Hh-signaling pathway, the investigators lastly investigated the role of this pathway in the extrahepatic vessels of human subjects with cirrhosis and healthy subjects and from the animal models. They observed that the noncanonical Hh was up-regulated in cirrhotic hepatic vessels compared to arteries from subjects without cirrhosis whereas Gli-2 mRNA levels were not affected. Interestingly, the canonical Hh pathway (Gli-2) was highly up-regulated in PPVL rats treated with atorvastatin. These results indicate a pivotal role of Shh/RhoA signaling in extrahepatic angiogenesis in the cirrhotic liver. Meanwhile, Gli-2 is a primary mediator of the canonical Hh pathway in noncirrhotic PTH in PPVL rats.
The study has several notable strengths. It gives both clinically relevant and mechanistic data regarding the effect of statins on angiogenesis in cirrhotic and noncirrhotic PTH. The question about why statins have different effects in cirrhotic and noncirrhotic PTH is a clinically important one. It appears that the beneficial consequences of statins may be explained through their hepatic effects on ECs and HSCs, leading to an improvement in PTH in the cirrhotic model. However, in extrahepatic PTH, the dominant effects of statins are on extrahepatic angiogenesis, which is exacerbated, leading to further increase in portal pressure (see Fig. 1). The investigators should be commended given that the study used a multimodel approach with animal and human data, as well as complementary in vivo and in vitro experiments to address an important clinical and experimental question. However, caution is needed before broadly extrapolating the results of this study. The PPVL model does not necessarily equate to the heterogeneous group of noncirrhotic PTH syndromes that span from portal vein thrombosis to nodular regenerative hyperplasia. Indeed, the acute/subacute changes observed in the liver after a 2-week period of PPVL reflects an uncommon clinical situation and may not represent the pathophysiological mechanisms at play in patients with noncirrhotic PTH. Thus, noncirrhotic PTH should be understood as a mixture of disorders with very different underlying pathophysiological mechanisms not fully represented by PPVL.
Preliminary results from a double-blind, randomized, controlled trial regarding the use of simvastatin in patients with cirrhosis suggest a survival benefit in advanced stages of the disease.8 Further studies on this subject are eagerly needed to expand the therapeutic armamentarium of PTH. Indeed, a broader landscape of new therapies for PTH are under development, including a variety of antifibrogenic, antiangiogenic, microbiome altering, and kinase-regulating drugs. The current study helps us to understand potential clinical benefits and pitfalls of statin therapy in human PTH.
Acknowledgment
The authors thank Dr. Nicolás Triantafilo for his invaluable help with the figure.
-
Juan P. Arab, M.D.1,2
-
Vijay H. Shah, M.D.1
-
1Division of Gastroenterology and Hepatology
-
Mayo Clinic
-
Rochester, MN
-
2Department of Gastroenterology
-
School of Medicine
-
Pontificia Universidad Católica de Chile
-
Santiago, Chile




