Differential requirement for de novo lipogenesis in cholangiocarcinoma and hepatocellular carcinoma of mice and humans
Potential conflict of interest: Nothing to report.
Supported by the Italian Association Against Cancer (grant IG 12139, to D.F.C.); the National Institutes of Health (R01CA136606, to X.C.; R21CA187306, to X.C. and A.S.; and P30DK026743 for the University of California-San Francisco Liver Center); and the National Natural Science Foundation of China (grant 81201553, to L.L.).
Abstract
Hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (ICC) are the most prevalent types of primary liver cancer. These malignancies have limited treatment options, resulting in poor patient outcomes. Metabolism reprogramming, including increased de novo lipogenesis, is one of the hallmarks of cancer. Fatty acid synthase (FASN) catalyzes the de novo synthesis of long-chain fatty acids from acetyl-coenzyme A and malonyl-coenzyme A. Increased FASN expression has been reported in multiple tumor types, and inhibition of FASN expression has been shown to have tumor-suppressing activity. Intriguingly, we found that while FASN is up-regulated in human HCC samples, its expression is frequently low in human ICC specimens. Similar results were observed in mouse ICC models induced by different oncogenes. Ablating FASN in the mouse liver did not affect activated AKT and Notch (AKT/Notch intracellular domain 1) induced ICC formation in vivo. Furthermore, while both HCC and ICC lesions develop in mice following hydrodynamic injection of AKT and neuroblastoma Ras viral oncogene homolog oncogenes (AKT/Ras), deletion of FASN in AKT/Ras mice triggered the development almost exclusively of ICCs. In the absence of FASN, ICC cells might receive lipids for membrane synthesis through exogenous fatty acid uptake. In accordance with the latter hypothesis, ICC cells displayed high expression of fatty acid uptake-related proteins and robust long-chain fatty acid uptake. Conclusion: Our data demonstrate that FASN dependence is not a universal feature of liver tumors: while HCC development is highly dependent of FASN and its mediated lipogenesis, ICC tumorigenesis can be insensitive to FASN deprivation; our study supports novel therapeutic approaches to treat this pernicious tumor type with the inhibition of exogenous fatty acid uptake. (Hepatology 2016;63:1900-1913)
Abbreviations
-
- ACAC
-
- acetyl-coenzyme A carboxylase
-
- FA
-
- fatty acid
-
- FASN
-
- fatty acid synthase
-
- HCC
-
- hepatocellular carcinoma
-
- ICC
-
- intrahepatic cholangiocarcinoma
-
- IL-33
-
- interleukin-33
-
- KO
-
- knockout
-
- LPL
-
- lipoprotein lipase
-
- mRNA
-
- messenger RNA
-
- NICD
-
- Notch intracellular domain 1
-
- N-Ras
-
- neuroblastoma Ras viral oncogene homolog
-
- RT-PCR
-
- reverse-transcription polymerase chain reaction
Liver cancer is among the most frequent solid tumor types and a leading cause of cancer-related death worldwide. Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer.1, 2 Treatment options for HCC are limited and generally ineffective.3, 4 Sorafenib, a multikinase inhibitor and the only chemotherapeutic drug available for the treatment of unresectable HCC, has limited efficacy in improving survival of HCC patients.5, 6 Intrahepatic cholangiocarcinoma (ICC) is the second most frequent primary liver tumor, accounting for ∼10% of all liver cancers.7, 8 ICC is an aggressive malignancy and one of the most devastating cancers of the gastrointestinal tract.7 The incidence and mortality rates of ICC are increasing worldwide.9, 10 Treatment options for ICC are very limited, and there is no Food and Drug Administration-approved targeted therapy for ICC.
Metabolic reprogramming is now recognized as one of the defining characteristics of cancer.11-13 Alterations in metabolic fluxes go far beyond the well-known Warburg effect and can be observed in many subnetworks of central carbon metabolism.11-13 In many cancers, aberrant fatty acid (FA) metabolism has been observed.14, 15 In particular, it is well-established that de novo lipogenesis is often up-regulated in solid tumors, and tumor cells become less dependent on exogenous FAs for growth.14, 15 Increased expression and activity of FA synthase (FASN), the central enzyme involved in de novo lipogenesis, is required for the survival and proliferation of many tumor cells; and targeting FASN has been considered a strategy for cancer treatment.16-18 In our previous study, we demonstrated that de novo lipogenesis and FASN expression increase along human hepatocarcinogenesis and are inversely associated with the length of patient survival.19 In addition, we have recently demonstrated that FASN depletion both suppresses AKT-driven hepatocarcinogenesis in mice and strongly restrains the growth of HCC cell lines in vitro.20 However, whether FASN is also overexpressed in human ICC and required for cholangiocarcinogenesis has not been investigated to date.
In the present study, we report that, different from human HCC, expression of FASN is frequently down-regulated in ICC tissues when compared with corresponding nontumorous liver tissues. Similar results were obtained in various mouse ICC models. Furthermore, ablation of FASN in the mouse liver did not affect AKT/Notch intracellular domain 1 (NICD) induced ICC formation in vivo. Similarly, deletion of FASN in AKT/Ras mice prevented development of HCC, but not ICC, leading to the predominant formation of liver tumors with cholangiocellular features. Together with the observation of robust uptake of exogenous FA by ICC, the present results suggest that up-regulation of de novo FA synthesis with a concomitant decline of exogenous FA uptake is not a universal feature of cancer.
Materials and Methods
Human Cholangiocarcinoma Samples
A collection of formalin-fixed, paraffin-embedded ICC (n = 45) samples was used in the present study. Thirty frozen ICC and corresponding nontumorous surrounding livers from the same collection were also used. The clinicopathological features of liver cancer patients are summarized in Supporting Table S1. ICC specimens were collected at the University of Greifswald (Greifswald, Germany). Institutional review board approval was obtained at the local ethical committee of the University of Greifswald.
Constructs
The plasmids used in the study, including pT3-EF1α-myr-AKT, pT3-EF1α-NICD1, pT2-Caggs-neuroblastoma Ras viral oncogene homolog (N-Ras)V12, pT3-EF1α-Cre, pT3-EF1α-miR-29, and pCMV-SB, have been described.21-23 Angptl4/pBabe was purchased from Addgene (plasmid 19156), and Angptl4 complementary DNA was cloned into pT3-EF1α vector through a Gateway cloning strategy. All plasmids were purified using the Endotoxin-free Maxi Prep Kit before injecting into mice.
Hydrodynamic Injection and Mouse Monitoring
FASNfl/fl mice in a C57BL/6 background have been described.24, 25 CD36−/− mice in a C57BL/6 background were used as described.26 AlbCre mice in a C57BL/6 background27 were obtained from the Jackson Laboratory (Bar Harbor, ME). FASNfl/fl mice were crossed with AlbCre mice to eventually generate liver-specific FASN knockout mice, the AlbCre;FASNfl/fl line. Male and female mice were used in the study, and no difference was noticed when using either male or female mice. Hydrodynamic transfection was performed as described.28 Mice were housed, fed, and monitored in accordance with protocols approved by the Committee for Animal Research at the University of California-San Francisco.
Histopathologic Analysis
Liver histopathologic analysis on mouse lesions was performed by two experienced liver pathologists (M.E. and F.D.) on tissue slides stained with hematoxylin and eosin and the periodic acid-Schiff reaction in accordance with the criteria of Frith et al.29
A detailed description of Materials and Methods is provided in the Supporting Information.
Results
Down-Regulation of FASN Expression in Human and Mouse ICC Samples
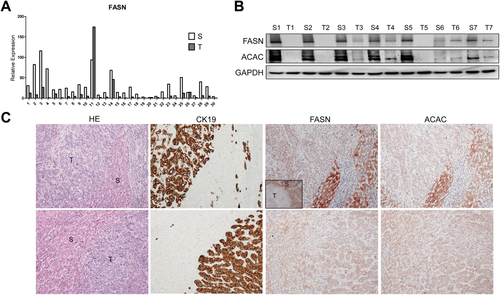
First, we determined the expression of FASN in human ICC samples. The FASN messenger RNA (mRNA) level was analyzed in 30 paired human nontumor liver tissue and ICC specimens using real-time quantitative reverse-transcription polymerase chain reaction (RT-PCR). We found that FASN mRNA expression was significantly down-regulated in ICC samples compared to respective nontumor liver samples (P = 0.002; Fig. 1A). The same result was obtained using The Cancer Genome Atlas microarray data of 36 surgically resected ICCs and 59 surrounding nontumor liver tissues (P = 1 × 10−7; Supporting Fig. S1). To further validate these findings, we examined the protein expression of FASN and its upstream inducer, acetyl-coenzyme A carboxylase (ACAC), in our collection of human ICC specimens. In accordance with mRNA data, we found that FASN and ACAC immunolabeling was significantly lower in 38 and 34 of 45 ICC samples, respectively (84.4% and 75.5%, respectively), when compared with nontumorous surrounding counterparts, as detected by immunohistochemistry (Fig. 1C, upper panel). Equivalent levels of FASN and ACAC immunoreactivity in ICC and corresponding nonneoplastic livers were detected in the remaining samples (Fig. 1C, lower panel). An equivalent trend was observed when analyzing part of the ICC collection (n = 22) by western blotting (Fig. 1B), where 19 of 22 samples (86.4%) showed lower levels of FASN in ICC when compared with nonneoplastic livers. Thus, our results indicate that FASN (and ACAC) is almost ubiquitously down-regulated in human ICC.
We have shown the coordinated activation of AKT/mammalian target of rapamycin and Notch cascades in human ICC specimens.21 In accordance with the latter findings in human ICC, hydrodynamic transfection of activated forms of AKT (myr-AKT1) and Notch1 (NICD1) drives rapid ICC development (AKT/NICD) in mice.21 Thus, we determined whether FASN expression levels in liver lesions from AKT/NICD mice were altered. Consistent with the human ICC data, FASN was expressed at a much lower level in AKT/NICD cholangiocellular tumors than in surrounding nonneoplastic liver tissues (Fig. 2A). Furthermore, these lesions did not accumulate neutral-lipid droplets (as revealed by oil red O staining) (Supporting Fig. S2). These results are in striking contrast with the remarkable lipogenic phenotype, elevated FASN expression, and extensive oil red O-positive labeling that characterize hepatocellular lesions developed in AKT mice (Supporting Fig. S2).19
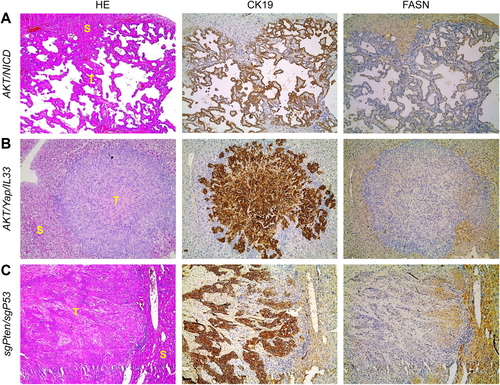
Recently, we established a murine ICC model by directly targeting biliary epithelial cells with activated AKT (myr-AKT) and Yap (YapS127A) oncogenes. In this model, ICC development requires the administration of interleukin-33 (IL-33) (AKT/Yap/IL-33).30 Similar to AKT/NICD lesions, ICCs developed in AKT/Yap/IL-33 mice exhibited down-regulation of FASN when compared to nontumorous livers (Fig. 2B).
Next, we examined FASN expression in mouse ICC induced by deletion of Pten and TP53 through CRISPR-mediated gene editing (sgPten/sgP53).31 Once again, we found that FASN protein is expressed at lower levels in ICCs than surrounding liver tissues from sgPten/sgP53 mice (Fig. 2C).
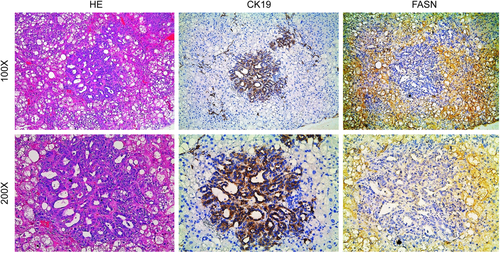
Finally, we investigated the levels of FASN in a mouse model characterized by the overexpression of activated forms of AKT and N-Ras (N-Ras-V12) in the liver (AKT/Ras). In these mice, both HCC and ICC develop, with pure ICC lesions accounting for approximately 10% of the tumors.32 Preneoplastic and neoplastic hepatocellular lesions expressed high levels of FASN (Fig. 3), while cholangiocellular lesions exhibited low expression of FASN (Fig. 3), confirming our previous findings.
Altogether, the present data indicate that FASN expression is down-regulated in human and mouse ICC when compared with nontumorous liver.
Fasn is Dispensable for AKT/NICD-Induced ICC Formation in MICE
Next, we investigated the functional role of FASN in cholangiocarcinogenesis. For this purpose, we determined whether FASN and de novo lipogenesis are required for AKT/NICD induced ICC formation, taking advantage of FASNfl/fl mice.24, 25
Two methods were employed to delete FASN in the context of overexpressing AKT and NICD oncogenes in the mouse liver (Fig. 4A). In the first approach, we hydrodynamically injected AKT/NICD together with Cre (AKT/NICD/Cre) into FASNfl/fl mice (Fig. 4A). This method allowed us to specifically delete FASN while simultaneously expressing AKT and NICD1 oncogenes in the same hepatocyte population of FASNfl/fl mice. The effectiveness of this coinjection approach has been validated.22 As a control, we injected AKT/NICD with a pT3EF1α empty vector (AKT/NICD/pT3) into FASNfl/fl mice. Of note, all AKT/NICD/Cre as well as all AKT/NICD/pT3-injected FASNfl/fl mice became moribund and were killed by 5 weeks postinjection. No statistical difference in the survival rate between the two cohorts was detected (P = 0.59). Multiple cystic lesions were present throughout the livers of AKT/NICD/Cre and AKT/NICD/pT3 mice (Fig. 4B). Microscopically, numerous cystoadenocarcinomas and ICCs, identical to those previously detected in FVB/N mice coinjected with AKT and NICD oncogenes,21 occupied most of the liver parenchyma in the two mouse groups (Fig. 4C). Subsequent immunohistochemical analysis demonstrated that cholangiocellular tumors were positive for the transfected oncogenes, namely hemagglutinin-tagged AKT (Fig. 4D) and Myc-tagged NICD1 (not shown), while being negative for FASN expression (Fig. 4D).
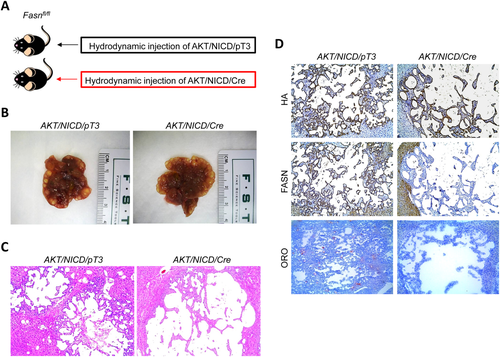
In the second approach, we generated liver-specific FASNfl/fl null mice by breeding AlbCre mice with FASNfl/fl mice to eventually create AlbCre;FASNfl/fl mice (Fig. 5). AKT/NICD oncogenes were hydrodynamically injected into AlbCre;FASNfl/fl mice as well as control FASNfl/fl mice (Fig. 5A). All AKT/NICD injected AlbCre;FASNfl/fl and FASNfl/fl mice developed a lethal burden of ICC around 5 weeks postinjection and were killed (Fig. 5B,C). No statistical difference in the survival rate between the two cohorts was found (P = 0.16). Importantly, immunostaining demonstrated a lack of FASN expression in normal and tumor liver tissues from AlbCre;FASNfl/fl mice (not shown). This was further validated using western blotting (Fig. 5D), showing the expression of ectopically injected hemagglutinin-tagged AKT in the tumor samples. In addition, FASN was strongly expressed in liver tumor tissues from FASNfl/fl mice, but its expression was very low in tumors from AlbCre;FASNfl/fl mice (Fig. 5D). The faint band of FASN protein in AlbCre;FASNfl/fl mouse livers is likely the result of FASN expression in other cell types, such as endothelial cells.
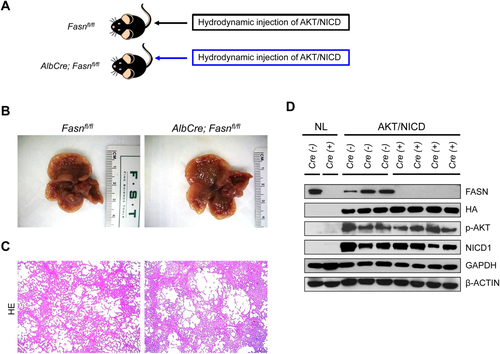
Altogether, the present results indicate that FASN and its mediated de novo lipogenesis are not required for ICC development driven by AKT and NICD oncogenes.
FASN Expression is Required for AKT/RAS-Driven HCC, but Not ICC, Formation in MICE
Because AKT/Ras coexpression induces the development of both HCC and ICC in the mouse liver,32 we investigated whether FASN is required for AKT/Ras-driven hepatocarcinogenesis. For this purpose, we hydrodynamically injected AKT/Ras together with Cre (AKT/Ras/Cre) into FASNfl/fl mice (Fig. 6A). AKT/Ras plasmids were coinjected with pT3 into FASNfl/fl mice as control (AKT/Ras/pT3). All AKT/Ras/pT3 mice developed a lethal burden of liver tumors by 7-9 weeks postinjection, in accordance with our previous findings (Fig. 6B).33 In contrast, AKT/Ras/Cre mice developed a high burden of liver tumors ∼10 weeks postinjection, and virtually all mice succumbed to liver tumors 14 weeks postinjection (Fig. 6B). Histologically, liver tumors from AKT/Ras/pT3 mice appeared to be similar to those detected when AKT/Ras was injected into FVB/N wild-type mice,32 with >80% of tumor lesions consisting of pure HCC and the remaining lesions consisting of pure ICC or mixed HCC/ICC. Indeed, sporadic cytokeratin 19-positive ICC lesions could be found in AKT/Ras/pT3 liver tissues (Fig. 6C, left panel). Intriguingly, AKT/Ras/Cre mice showed a striking difference in the HCC/ICC ratio, with >90% of the lesions being pure ICC and very few (<5%) consisting of pure HCC (Fig. 6C, right panel).
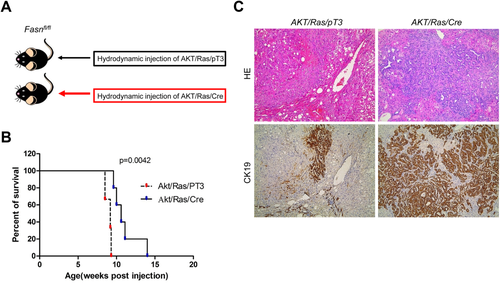
Thus, FASN loss significantly affects HCC formation, but not ICC development, in AKT/Ras-driven hepatocarcinogenesis.
FA Uptake in Mouse ICC Lesions
Our data demonstrate that ICC lesions induced by AKT/NICD or AKT/Ras do not fully depend on de novo lipogenesis for their growth. As FAs are fundamentally required for tumor cell proliferation to provide new phospholipids for plasma membranes,14-16, 18, 34 the lack of FASN dependence suggests an increased role for the uptake of exogenous FA. As a first step to investigate this possibility, we analyzed the expression of the major FA transporters through endocytosis mechanisms as well as of lipoprotein lipase (LPL) and CD36 in AKT/NICD tumor cells. LPL is the enzyme responsible for extracellular lipolysis of triglycerides into FAs, which are then intracellularly translocated through CD3635 and/or FA transporters of the SLC27 family.36 We found that three members of the FA transport protein family, namely SLC27A1, SLC27A2, and SLC27A5, as well as LPL and CD36 were expressed in AKT/NICD cells (Fig. 7A). In particular, while SLC27A2 and SLC27A5 were down-regulated in AKT/NICD ICC compared to surrounding nontumorous liver, SLC27A1, LPL, and CD36 were up-regulated in ICC (Fig. 7A). In addition, mRNA levels of SLC27A2, SLC27A5, and CD36 were found to be down-regulated in human ICC samples from The Cancer Genome Atlas data set, whereas SLC27A1 and LPL levels were higher in ICC samples than nontumorous liver tissues in the same data set (Supporting Fig. S3). Up-regulation of LPL and down-regulation of CD36, respectively, in human ICC when compared with corresponding nontumorous surrounding livers was further validated in our sample collection by real-time RT-PCR (Supporting Fig. S4).
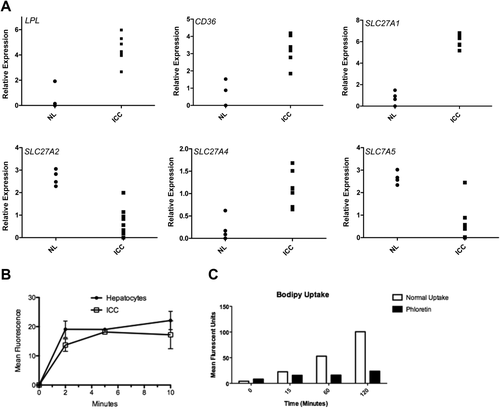
Next, we isolated normal hepatocytes as well as primary murine ICC cells from AKT/NCID injected mice and compared their FA uptake activity using a fluorescence-activated cell sorting based assay. We found that AKT/NICD tumor cells took up a fluorescent FA analogue (C1-Bodipy-C12) at a comparable rate to normal hepatocytes (Fig. 7B), which are known to have robust transporter-mediated uptake.37, 38 Furthermore, the HUCCT1 human ICC cell line also displayed robust uptake of exogenous FA, which was inhibited by the polyphenol phloretin, indicating a transporter mediated process (Fig. 7C).38
Thus, the present data suggest that AKT/NICD tumor cells might use FA released by extracellular lipolysis and/or endocytosis in a transporter-dependent manner.
AKT/NICD Tumors are Resistant to CD36 Deprivation
Finally, we assessed if the CD36/LPL axis is the major FA uptake route required for AKT/NICD-dependent cholangiocarcinogenesis. Being that efficient use by cancer cells of FA released by extracellular lipolysis requires the activity of both LPL and CD36, we determined whether the deprivation of CD36 affects AKT/NICD-driven liver carcinogenesis. For this purpose, we hydrodynamically transfected AKT/NICD oncogenes into CD36 knockout mice (referred to as AKT/NICD/CD36KO mice; Fig. 8).39 Surprisingly, we found that cholangiocarcinogenesis was neither inhibited nor delayed in AKT/NICD/CD36KO mice when compared with wild-type mice injected with AKT/NICD (AKT/NICD mice). Indeed, similar to AKT/NICD mice, AKT/NICD/CD36KO mice developed multiple ICCs and were killed 5 weeks postinjection (Fig. 8). Histologically, the lesions developed in AKT/NICD/CD36KO mice consisted of ICCs (Fig. 8), and they were undistinguishable from those described in AKT/NICD (Fig. 2), AKT/NICD/pT3 (Fig. 4), and AKT/NICD/Cre mice (Fig. 4). Furthermore, we inhibited LPL activity by coinjection of AKT/NICD with Angptl4 (AKT/NICD/Angptl4) or miR-29 (AKT/NICD/miR29) (Supporting Fig. S5A). Angptl4 is a well-known inhibitor of LPL,40 whereas miR-29 inhibits LPL expression in the liver.41 Compared with control AKT/NICD/pT3 injected mice, coinjection of Angptl4 or miR29 slightly delayed the development of ICC (Supporting Fig. S5A). Nevertheless, by 6-7 weeks postinjection, all mice developed a lethal burden of liver tumor and were required to be euthanized. Histologically, the lesions developed in AKT/NICD/Angptl4 or AKT/NICD/miR29 mice consisted of ICCs, similar to ICCs induced by AKT/NICD/pT3 (Supporting Fig. S5B).
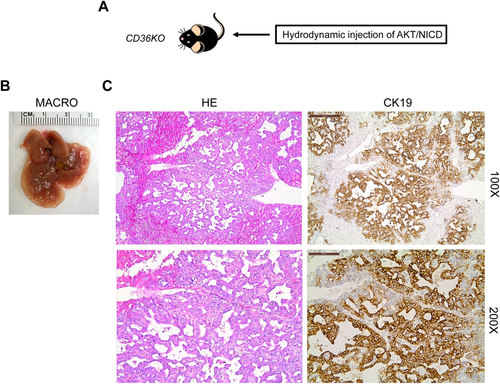
Altogether, the present data indicate that disruption of the LPL/CD36 axis does not significantly affect AKT/NICD-driven cholangiocarcinogenesis.
Discussion
A significant amount of evidence indicates that metabolic alterations such as aberrant glucose, glutamine, and lipid metabolism are indicative of cancer. The Warburg effect, which refers to the fact that tumor cells rely on aerobic glycolysis instead of oxidative phosphorylation for survival, is perhaps the most studied cancer metabolic phenotype.42 Aberrant de novo FA biosynthesis is another important feature of malignant transformation.16 Most normal human tissues use dietary (exogenous) lipids for synthesis of new structural lipids, while de novo FA synthesis is generally suppressed. Consequently, enzymes involved in lipogenesis, including FASN, ACAC, and stearoyl-coenzyme A desaturase 1, are expressed at negligible levels in normal cells.43 In contrast, in highly proliferating cancer cells, the exacerbated lipogenesis is reflected by the increased activity and expression of these lipogenic enzymes.16 FASN is the key lipogenic enzyme as it catalyzes the synthesis of palmitate from acetyl-coenzyme A and malonyl-coenzyme A.16 Up-regulation of FASN has been reported in multiple tumor types, including breast, prostate, and renal cancer, as well as in HCC.16, 18 Inhibition of FASN by either small molecules (C75, orlistat) or small interfering RNA can efficiently suppress tumor cell growth in vitro and xenograft models.16, 18 Importantly, a recent study showed that transgenic expression of FASN in mice results in prostate intraepithelial neoplasia,44 supporting a direct oncogenic role of FASN and lipogenesis in prostate carcinogenesis. In the liver, although overexpression of FASN by hydrodynamic injection did not trigger tumor development, FASN suppression completely abolished AKT-dependent hepatocarcinogenesis.20 In addition, strong growth restraint and massive apoptosis followed FASN silencing in HCC cell lines.19 Altogether, the present data seem to underline a crucial role of FASN in carcinogenesis and the dependence of tumors on FASN activity to grow and progress.
In human ICC, a rising and highly aggressive primary tumor of the liver,7, 9, 10 the role of FASN has not been investigated to date. In the current study, we show that in both human and mouse ICC, FASN expression is down-regulated when compared with nontumorous surrounding liver tissues. In addition, the consequent functional analyses demonstrated that FASN expression is dispensable for AKT/NICD-driven cholangiocarcinogenesis in mice. Thus, the present data highlight the tissue type-specific metabolic needs along carcinogenesis. The different requirement of FASN for HCC and ICC is well exemplified by AKT/Ras mice, which are characterized by the development of both HCC and ICC lesions.32 In these mice, indeed, we found that while FASN deprivation led to inhibition of HCC development, ICC normally formed, further confirming the need of FASN for hepatocarcinogenesis but not cholangiocarcinogenesis.
Given the irreplaceable role of FA for tumor cell proliferation, the decreased reliance of ICC cells on de novo FA synthesis implies a pivotal role for the uptake of exogenous FA. Indeed, we found that growing the HUCCT1 human ICC cell line in lipoprotein-deficient medium strongly inhibited cell growth (Supporting Fig. S6A). Consistently, we found that HepG2 (an HCC cell line) and HUCCT1 exhibit comparable FA uptake in culture (Supporting Fig. S6B). The importance of the protein-mediated FA uptake in HUCCT1 cells was further validated by treating cells with phloretin (Fig. 7C). Clearly, additional FA uptake experiments using primary human HCC and ICC cells are necessary to determine the importance of this phenomenon in human hepatocarcinogenesis.
In accordance with the hypothesis that exogenous FA uptake is required for ICC tumor growth, we found that LPL, CD36, and SLC27A1 were up-regulated in AKT/NICD lesions (Fig. 7A). Importantly, functional uptake studies showed that ICC derived cells were fully competent in FA uptake assays compared to hepatocytes (Fig. 7B), further supporting our hypothesis that ICC cells mainly rely on exogenous rather than newly synthesized FA to fuel their growth. In an intriguing study, it has been recently demonstrated that selected breast and prostate cancer and liposarcoma cell lines possess an active LPL/CD36 system, whose activity promotes cell proliferation and/or resistance to cytotoxic effects induced by FA inhibition of the same cells.45 In addition, elevated expression of LPL and CD36 was detected in the majority of breast, liposarcoma, and prostate tumor tissues, thus suggesting a crucial role played by lipolysis of exogenous/dietary FA in cancer cells in vivo.45 Following this pioneering study, we determined whether the same applies to mouse ICC. However, our data suggest that FA uptake occurs through a mechanism independent of CD36 as AKT/NICD-driven cholangiocarcinogenesis is not affected by CD36 loss (Fig. 8). Consistently, suppression of LPL through miR-29 or Angtpl4 has rather limited effects on AKT/NICD-driven cholangiocarcinogenesis (Supporting Fig. S5). However, as human ICCs display up-regulation of LPL, further studies are needed to better understand the relevance of the LPL/CD36 axis in the human disease.
Another candidate that might explain the resistance of ICC cells to FASN deprivation is the FA transporter SLC27A1. Expression array analysis demonstrated that SLC27A1 expression is up-regulated in human ICC samples (Supporting Fig. S3). This observation was validated by real-time quantitative RT-PCR analysis (Supporting Fig. S7). Furthermore, in a pilot study, we found that suppression of SLC27A1 by specific small interfering RNA decreases the in vitro growth of the HUCCT1 and HuH28 ICC cell lines (Supporting Figs. S8 and S9). Even more importantly, suppression of SLC27A1 synergized with FASN silencing to induce growth restraint of HUCCT1 and HuH28 cells (Supporting Figs. S8 and S9). Clearly, additional investigations are necessary to clarify the role of SLC27A1 in cholangiocarcinogenesis.
Besides the insights on the metabolic requirements of ICC cells, the present findings might have important clinical implications. Indeed, although FASN inhibitors have shown promising antineoplastic effects in many experimental tumor models, the same drugs might be ineffective against ICC, at least when used alone. Of note, it has been shown that FA uptake is an inherently druggable process, and inhibitors have been described for SLC27A1.46 Based on the preliminary data from the present study, it is conceivable that inhibitors of FA uptake, either alone or in association with FASN inhibitors, might be beneficial for the treatment of human ICC.
Acknowledgment
We are grateful to Dr. Wen Xue (Massachusetts Institute of Technology, Cambridge, MA) for providing sgPten/sgP53 mouse ICC blocks and to Dr. Clay F. Semenkovich (Washington University, St. Louis, MO) for providing FASNfl/fl mice.
References
Author names in bold denote shared co-first authorship.




