Nonselective β-blockers do not affect mortality in cirrhosis patients with ascites: Post Hoc analysis of three randomized controlled trials with 1198 patients
Potential conflict of interest: Nothing to report.
Supported by the Danish Council for Independent Research under the Danish Agency for Science, Technology and Innovation (10-081838/FSS, to P.J.).
Abstract
The safety of nonselective β-blockers (NSBBs) in advanced cirrhosis has been questioned. We used data from three satavaptan trials to examine whether NSBBs increase mortality in cirrhosis patients with ascites. The trials were conducted in 2006-2008 and included 1198 cirrhosis patients with ascites followed for 1 year. We used Cox regression to compare all-cause mortality and cirrhosis-related mortality between patients who did and those who did not use NSBBs at randomization, controlling for age, gender, Model for End-Stage Liver Disease score, Child-Pugh score, serum sodium, previous variceal bleeding, cirrhosis etiology, and ascites severity. Moreover, we identified clinical events predicting that a patient would stop NSBB treatment. At randomization, the 559 NSBB users were more likely than the 629 nonusers to have a history of variceal bleeding but less likely to have Child-Pugh class C cirrhosis, hyponatremia, or refractory ascites. The 52-week cumulative all-cause mortality was similar in the NSBB user and nonuser groups (23.2% versus 25.3%, adjusted hazard ratio = 0.92, 95% confidence interval 0.72-1.18), and NSBBs also did not increase mortality in the subgroup of patients with refractory ascites (588 patients, adjusted hazard ratio = 1.02, 95% confidence interval 0.74-1.40) or in any other subgroup. Similarly, NSBBs did not increase cirrhosis-related mortality (adjusted hazard ratio = 1.00, 95% confidence interval 0.76-1.31). During follow-up, 29% of initial NSBB users stopped taking NSBBs, and the decision to stop NSBB treatment marked a sharp rise in mortality and coincided with hospitalization, variceal bleeding, bacterial infection, and/or development of hepatorenal syndrome. Conclusion: This large and detailed data set on worldwide nonprotocol use of NSBBs in cirrhosis patients with ascites shows that NSBBs did not increase mortality; the decision to stop NSBB treatment in relation to stressful events may have added to the safety. (Hepatology 2016;63:1968-1976)
Abbreviations
-
- CI
-
- confidence interval
-
- HR
-
- hazard ratio
-
- HRS
-
- hepatorenal syndrome
-
- MAP
-
- mean arterial pressure
-
- MELD
-
- Model for End-Stage Liver Disease
-
- NSBB
-
- nonselective β-blocker
-
- SBP
-
- spontaneous bacterial peritonitis
Use of nonselective β-blockers (NSBBs) as secondary prevention of variceal bleeding was introduced in 1981.1 Since then, numerous randomized trials and meta-analyses have documented NSBBs' efficacy in preventing variceal bleeding,2, 3 and today NSBBs are the standard pharmacological treatment for primary and secondary prevention of variceal bleeding.4, 5 They reduce portal pressure by decreasing cardiac output (β1-blockade) and splanchnic blood flow (β2-blockade).6
Surprisingly, in 2010 serious concerns about the safety of NSBB use in cirrhosis patients with refractory ascites were raised by a single-center observational study including 151 cirrhosis patients with refractory ascites.7 The investigators observed a median survival time of 5 months in the 77 NSBB users versus 20 months in the 74 nonusers. This study stirred an intense debate,8-11 and subsequent studies have not been able to clarify whether NSBBs are safe for use in patients with decompensated cirrhosis.11-16 In 2012, the seemingly contradictory findings concerning beneficial and harmful effects of NSBBs were united in the window hypothesis.17 It posits that NSBBs should only be administered within a certain window during disease progression that closes when the hyperdynamic circulation impairs the cardiac compensatory reserve so much that the cardioinhibitory effect of NSBBs compromises organ perfusion.17, 18 The problem now is to find a clinical event or a biomarker that tells clinicians that the window is closing. Suggestions have included refractory ascites,13 spontaneous bacterial peritonitis (SBP),16 low mean arterial pressure (MAP),12 the hepatorenal syndrome (HRS), and sepsis19; but the problem remains unsolved.
The aim of this study was to investigate whether NSBB treatment associates with increased mortality in cirrhosis patients as well as in subgroups of decompensated cirrhosis, e.g., in patients with refractory ascites who have been the focal point of the discussion of NSBB safety. We had access to data from three large multicenter randomized trials, and these data gave us a unique opportunity to pursue our aim using high-quality data on an international cohort of 1198 cirrhosis patients with ascites.20
Patients and Methods
Patients
Between July 2006 and December 2008 three multicenter randomized controlled trials were conducted to examine the efficacy of satavaptan in treating ascites in cirrhosis patients. A total of 1198 patients were included. The trials were conducted similarly but had different target populations: patients with diuretic-manageable ascites (n = 462), patients with ascites managed with diuretics and occasional therapeutic paracentesis (n = 496), and patients with diuretic-resistant ascites managed primarily with therapeutic paracentesis (n = 240). Although the first trial included patients with diuretic-manageable ascites, the definition of “diuretic-manageable” permitted one or two paracenteses within 6 months before inclusion as long as the interval between them exceeded 3 months. The classification into refractory or diuretic-responsive ascites was done by the managing clinician at each participating center. Patients with variceal bleeding or SBP in the 10 days before randomization were excluded, as were patients with a functional transjugular intrahepatic portosystemic shunt. Other reasons for exclusion were serum creatinine >150 μmol/L, serum potassium >5.0 mmol/L, serum sodium >143 mmol/L, serum bilirubin >150 μmol/L, international normalized ratio >3.0, platelets <30,000/mm3, neutrophils <1000/mm3, systolic arterial pressure <80 mm Hg or symptomatic orthostatic hypotension, hepatocellular carcinoma exceeding the Milan criteria, use of a potent modifier of the cytochrome P450 3A pathway, or use of drugs that increase the risk of Q-T interval prolongation.20 We excluded 10 patients because data on Model for End-Stage Liver Disease (MELD) or Child-Pugh score were missing. A total of 1188 patients were included in our analyses, among them 588 with refractory ascites and 600 with diuretic-responsive ascites.
Study Design
The planned treatment duration was 52 weeks, with an additional safety visit 1-2 weeks later. Thus, the maximum planned follow-up duration was 54 weeks. Two of the studies were stopped early because the risks attributed to satavaptan exceeded the benefits,20 and some patients discontinued the trial drug prematurely, primarily due to development of cirrhosis complications. Irrespective of the reason for treatment cessation, patients were contacted after week 52 of planned treatment to determine survival or date of death. On top of that, any patient who experienced an adverse event was followed until the event resolved or stabilized, so follow-up could extend far beyond the planned 54 weeks. This means that follow-up to week 52 (for all who stopped treatment early) or 54 (for all who completed treatment) was independent of mortality risk and that follow-up beyond week 54 was limited to patients with adverse events. Therefore, we stopped follow-up after 54 weeks in the analyses presented here.
Data Collection
Data on NSBB use and dose, MELD score, serum sodium, refractory ascites, Child-Pugh score, cirrhosis etiology (alcohol only, chronic hepatitis B only, chronic hepatitis C only, nonalcoholic steatohepatitis only, cryptogenic cirrhosis, or other etiology), MAP, total bilirubin, albumin, international normalized ratio, hemoglobin, platelet count, satavaptan use, and previous SBP and variceal bleeding were collected at the time of randomization. Data on presence or absence of esophageal varices were not available. MAP was calculated as diastolic blood pressure + (systolic blood pressure - diastolic blood pressure)/3, and all blood pressures were measured in the supine position, at rest. Most of the patient data were updated at every 4-week visit, but this updating ended 1 week after premature treatment cessation. Analyses with time-updated patient data could therefore not be conducted with follow-up through week 54 (or 52), so our primary analysis used only patient data from the randomization visit.
Statistical Analysis
Follow-up started at randomization and ended at death or censoring at the time of the last patient contact in week 52-54. We used Kaplan-Meier estimates to calculate the cumulative mortality for NSBB users and nonusers and Cox proportional hazards regression to estimate the effect of NSBB use on mortality. We adjusted for confounding by patient gender, age, cirrhosis etiology, MELD score, Child-Pugh score, serum sodium, history of variceal bleeding (yes or no), and severity of ascites (diuretic-responsive ascites [reference] or refractory ascites). Cirrhosis etiology was included in the regression model as a categorical variable with five categories and “alcohol only” as the reference category. We did not adjust for blood pressure (or MAP) because NSBBs lower blood pressure, and a variable (blood pressure) that changes in response to the exposure (NSBB treatment) cannot be a confounder.21
Death is a specific, but not very sensitive, marker of a poor prognosis. Therefore, we repeated the Cox regression with “hospitalization or death” as a combined endpoint. The analysis was otherwise the same.
We repeated the analyses of both outcomes in different subgroups of the patient cohort: patients with refractory or diuretic-responsive ascites, patients randomized to satavaptan or placebo, patients with previous SBP or variceal bleeding, patients with a high MELD score (≥18), patients with high-dose NSBB use (≥80 mg propranolol daily or >6.25 mg carvedilol daily), and in five subgroups of MAP (<71 mm Hg, 71-80 mm Hg, 81-90 mm Hg, 91-100 mm Hg, and >100 mm Hg). We adjusted for the same confounders in the subgroup analyses as in the analysis of the overall cohort.
Causes of death were reported by the clinicians who cared for the patients during the trials, and this information could not be supplemented. We categorized all deaths as either cirrhosis-related (from liver failure, hepatocellular carcinoma, gastrointestinal bleeding, HRS, or infection), from other known causes, or from unknown causes; and then we examined the association between NSBBs and cirrhosis-related death. In this analysis we used the same regression model and adjusted for the same confounders as in the primary analysis.
Discontinuation of NSBBs
In our primary analysis we used only patient data from the randomization visit, so this analysis could not take into account that patients might have started or stopped NSBB treatment during the follow-up period. Therefore, we conducted two analyses to explore why NSBBs were discontinued. Both of them included only those patients who used NSBBs at randomization, and follow-up ended when patients stopped taking the trial drug because only few patient characteristics were updated after that time.
In the first analysis, we evaluated the hypothesis that NSBBs were discontinued when the clinician thought that the patient was too ill to tolerate them. Under that hypothesis, discontinuation of NSBBs would be associated with a sharp rise in mortality hazard. We defined a time-dependent “NSBB discontinuation” variable indicating current use of NSBBs, and then we used Cox regression to examine the effect of discontinuation of NSBBs on mortality, adjusting for the same confounders as in our primary analysis.
The second analysis was an elaboration of the first. It sought to explore why clinicians would find it necessary to discontinue NSBBs. In this analysis, we used Cox regression with stepwise backward elimination of candidate predictors to identify a small set of very strong predictors of discontinuation (P < 0.05). The outcome was time to first discontinuation of NSBBs. We used a larger set of predictors for this purpose: sodium, bilirubin, creatinine, white blood cell count, albumin, MELD, and MAP, as well as the change in these variables since randomization (we computed Δ sodium, Δ bilirubin, etc., as the difference between the current value and the value at randomization). Other included predictors were gender, age, Child-Pugh score at randomization, hospital admission, HRS, SBP, bacterial infection other than SBP, hepatic encephalopathy, and treatment arm (satavaptan versus placebo).
Results
We analyzed data from 1188 patients, of whom 559 were NSBB users and 629 were nonusers at the time of randomization: 688 (58%) of the patients had cirrhosis due to alcoholism alone, 53 (4%) due to hepatitis B alone, and 161 (14%) due to hepatitis C alone; 97 (8%) had cirrhosis due to nonalcoholic steatohepatitis or cryptogenic cirrhosis, 83 (7%) had cirrhosis from both alcohol and hepatitis C, 33 (3%) had cirrhosis from autoimmune liver disease, 26 (2%) had cirrhosis from both alcohol and hepatitis B, 10 (1%) had cirrhosis from hemochromatosis, and the remaining 37 (3%) had cirrhosis from various other causes. The NSBB users were more likely than the nonusers to have a history of variceal bleeding (30% versus 13%) and low MAP (≤70 mm Hg, 13% versus 10%; 71-80 mm Hg, 34% versus 27%), but they were less likely to have Child-Pugh class C cirrhosis (24% versus 28%), refractory ascites (46% versus 52%), or serum sodium <135 mmol/L (28% versus 35%) (Table 1).
| NSBB users | Nonusers | |
|---|---|---|
| Number of patients | 559 | 629 |
| Age (median, IQR) | 57 (51-64) | 57 (50-64) |
| Men (%) | 394 (70%) | 432 (69%) |
| Cirrhosis etiology | ||
| Alcohol alone (%) | 312 (56%) | 376 (60%) |
| Hepatitis B alone (%) | 23 (4%) | 30 (5%) |
| Hepatitis C alone (%) | 79 (14%) | 82 (13%) |
| NASH alone or cryptogenic (%) | 43 (8%) | 54 (9%) |
| Other (%) | 102 (18%) | 87 (14%) |
| Refractory ascites (%) | 258 (46%) | 330 (52%) |
| MELD score (median, IQR) | 12 (8-15) | 11 (8-15) |
| MELD score ≥18 (%) | 64 (11%) | 69 (11%) |
| Child-Pugh score (class A/B/C, %) | 8%/68%/24% | 8%/64%/28% |
| Child-Pugh score (mean) | 8.45 | 8.57 |
| Serum sodium, mmol/L (median, IQR) | 137 (134-140) | 136 (133-139) |
| Serum sodium, mmol/L (mean) | 137 | 136 |
| Serum sodium <135 mmol/L (%) | 156 (28%) | 220 (35%) |
| Serum sodium ≥135 mmol/L (%) | 403 (72%) | 409 (65%) |
| MAP, mm Hg (median, IQR) | 83 (73-90) | 85 (76-93) |
| MAP <71 mm Hg (%) | 70 (13%) | 63 (10%) |
| MAP 71-80 mm Hg (%) | 189 (34%) | 171 (27%) |
| MAP 81-90 mm Hg (%) | 169 (30%) | 197 (31%) |
| MAP 91-100 mm Hg (%) | 102 (18%) | 148 (24%) |
| MAP >100 mm Hg (%) | 29 (5%) | 49 (8%) |
| Previous or current SBP (%) | 89 (16%) | 87 (14%) |
| Previous or current variceal bleeding (%) | 168 (30%) | 82 (13%) |
| Previous or current hepatocellular carcinoma | 19 (3%) | 24 (4%) |
| Randomized to satavaptan (%) | 330 (59%) | 383 (61%) |
| International normalized ratio (median, IQR) | 1.4 (1.2-1.6) | 1.3 (1.2-1.5) |
| Platelet count, *1000/μL (median, IQR) | 115 (79-167) | 130 (89-187) |
| Total bilirubin, μmol/L (median, IQR) | 25 (15-41) | 24 (13.5-43.1) |
| Albumin, g/L (median, IQR) | 33 (29-38) | 34 (30-38) |
| Hemoglobin g/L (median, IQR) | 118 (103-131) | 117 (102-132) |
- Abbreviations: IQR, interquartile range; NASH, nonalcoholic steatohepatitis.
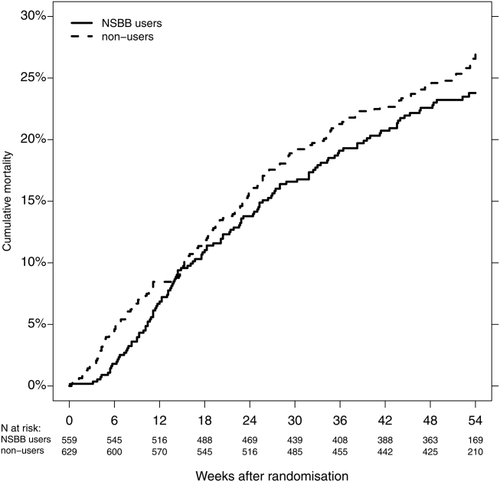
In total, 286 patients died during the follow-up (median follow-up for survival = 52.5 weeks), and the 52-week cumulative mortality was 23.2% for those who used NSBBs at randomization versus 25.3% for those who did not (Fig. 1). The impression that NSBB use was not associated with mortality persisted after confounder adjustment; the adjusted hazard ratio (HR) for NSBB use versus nonuse at randomization was 0.92 (95% confidence interval [CI] 0.72-1.18) (Table 2). When we examined the combined endpoint hospitalization or death, NSBB treatment appeared even more favorable: the 1-year cumulative risk was 57.1% for those who used NSBBs at randomization versus 63.9% for the others, and the adjusted HR was 0.83 (95% CI 0.71-0.97).
| Adjusted HR (95% CI) | |
|---|---|
| NSBB use versus nonuse | 0.92 (0.72-1.18) |
| Age, per year | 1.03 (1.02-1.04) |
| Male versus female | 1.03 (0.79-1.35) |
| Serum sodium, per mmol/L | 0.94 (0.91-0.96) |
| Child-Pugh score, per point | 1.28 (1.18-1.39) |
| MELD score, per point | 1.05 (1.02-1.08) |
| History of variceal bleeding, yes versus no | 1.43 (1.09-1.88) |
| Cirrhosis etiology | |
| Alcohol only (reference) | 1 |
| Hepatitis B only | 1.24 (0.75-2.06) |
| Hepatitis C only | 1.20 (0.85-1.69) |
| NASH or cryptogenic | 1.61 (1.08-2.39) |
| Other etiology | 1.09 (0.78-1.53) |
| Refractory ascites versus diuretic-responsive ascites | 1.29 (1.01-1.65) |
- Abbreviation: NASH, nonalcoholic steatohepatitis.
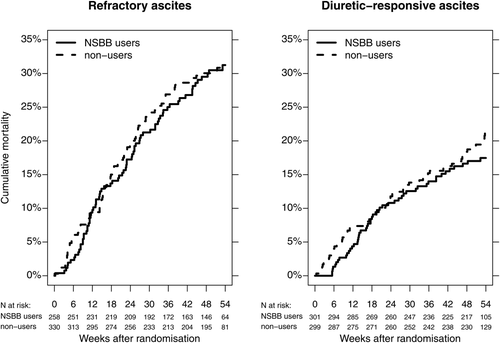
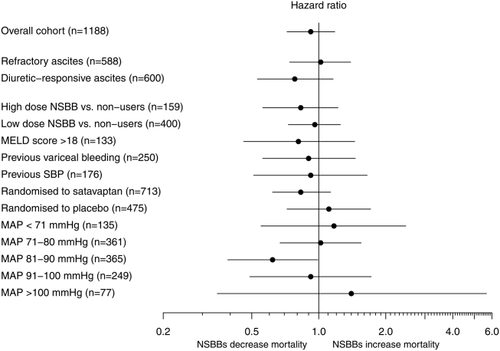
Among the 588 patients with refractory ascites, 258 used NSBBs at randomization and 330 did not. The 52-week cumulative mortality was 30.5% for NSBB users versus 30.9% for nonusers (adjusted HR = 1.02, 95% CI 0.74-1.39). In the group with diuretic-responsive ascites it was 17.0% for NSBB users versus 19.5% for nonusers (adjusted HR = 0.78, 95% CI 0.53-1.16) (Fig. 2). NSBBs were also not associated with increased mortality in any of the other subgroups we examined (Fig. 3). The pattern was the same when we examined the combined endpoint, i.e., hospitalization or death.
Of the 286 decedents, 226 (79%) died from cirrhosis-related causes, 33 (12%) from other known causes, and 27 (9%) from unknown causes. The adjusted HR of cirrhosis-related mortality for NSBB users versus nonusers was 1.00 (95% CI 0.76-1.31) (Table 3). In the subgroup of cirrhosis patients with refractory ascites it was 1.20 (95% CI 0.84-1.72), and in the subgroup of patients with diuretic-responsive ascites it was 0.75 (95% CI 0.48-1.15).
| Adjusted HR (95% CI) | |
|---|---|
| NSBB use versus nonuse | 1.00 (0.76-1.31) |
| Age, per year | 1.03 (1.01-1.04) |
| Male versus female | 1.01 (0.75-1.36) |
| Serum sodium, per mmol/L | 0.93 (0.91-0.95) |
| Child-Pugh score, per point | 1.31 (1.20-1.44) |
| MELD score, per point | 1.04 (1.01-1.08) |
| History of variceal bleeding, yes versus no | 1.50 (1.11-2.03) |
| Cirrhosis etiology | |
| Alcohol only (reference) | 1 |
| Hepatitis B only | 1.24 (0.69-2.23) |
| Hepatitis C only | 1.31 (0.89-1.91) |
| NASH or cryptogenic | 1.57 (0.99-2.50) |
| Other etiology | 1.27 (0.89-1.84) |
| Refractory ascites versus diuretic-responsive ascites | 1.16 (0.89-1.53) |
- Abbreviation: NASH, nonalcoholic steatohepatitis.
Discontinuation of NSBBs
During the follow-up, 29% of the 559 patients who used NSBBs at randomization discontinued them, and 13% of the 559 discontinued them even before their first hospitalization during the follow-up. There were a total of 486 hospitalizations of current NSBB users during the follow-up, and the NSBBs were discontinued in 15% of those hospitalizations. Discontinuation of NSBBs was associated with a very sharp rise in mortality hazard (adjusted HR = 5.13, 95% CI 2.28-11.55) (Supporting Table S1). Predictors of NSBB discontinuation included admission to hospital, variceal bleeding, bacterial infection, HRS, high Child-Pugh score, and refractory ascites (Supporting Table S2).
Discussion
This large observational study reflects actual clinical practice with respect to use of NSBBs among patients with decompensated cirrhosis. It showed that use of NSBBs did not increase the risk of death or hospitalization, either in the overall cohort or in the subgroup of patients with refractory ascites or in any of the other patient subgroups we considered.
The major strength of our study is the rigorous and detailed collection of clinically relevant data. These data showed that NSBB users and nonusers were very similar at the time of randomization with respect to cirrhosis severity. However, it is a limitation of our study that we do not know patients' hepatic venous pressure gradient. Nor do we know whether they had gastroesophageal varices or whether those who had varices were treated with ligation, but we can speculate that a large proportion of our NSBB users had large nonbleeding varices as only 30% of them had ever had variceal bleeding. By contrast, those who did not use NSBBs were more likely to have small nonbleeding varices or no varices. As a consequence of the lack of data on hepatic venous pressure gradient or gastroesophageal varices, we are unable to control for confounding by severity of portal hypertension. However, any uncontrolled confounding by portal hypertension is likely to bias our findings toward a higher mortality HR because NSBB users likely have more severe portal hypertension than nonusers. We cannot rule out that NSBB users were healthier in ways that are not described by our data; for example, the similar MAPs in NSBB users and nonusers could indicate that the NSBB-free MAP was higher in the NSBB users, who were therefore healthier. However, this is speculation, and we do not believe that a “healthy user bias” exists.22 Therefore, based on our data, it is more likely that NSBBs do not in fact increase mortality in advanced cirrhosis. Thus, our data support the use of NSBBs as described in the American Association for the Study of Liver Diseases guidelines from 2007,23 and they cannot confirm the recommendation in the newly published UK guidelines that NSBBs are discontinued at the time of SBP, renal impairment, and hypotension.24 Nor can they confirm that NSBBs can safely be continued despite SBP, renal impairment, and hypotension. Clinicians must weigh the risks and benefits of continued NSBB treatment in each patient, and we should emphasize that there was only 1 year of follow-up in the trials that we analyze here.
As many as 29% of patients stopped using NSBBs temporarily or permanently, and those who stopped taking NSBBs were clearly sicker than those who continued taking them. The major limitation of this study is that we do not know what would have happened if all patients had continued using NSBBs. There is no way to answer that question with our data—or, indeed, with any data, considering that our current data reflect actual clinical practice. Only the already existing double-blinded trials of patients randomized to NSBBs or placebo could provide an answer because clinicians are reluctant to discontinue a trial drug. None of them suggested that NSBBs increase mortality. For example, Pascal and Cales randomized 230 cirrhosis patients, 46% of whom had Child-Pugh class C cirrhosis, to propranolol or placebo.25 During the 2-year follow-up, 29 of 118 patients randomized to propranolol stopped treatment: 12 on their own initiative, two at the physician's request, 13 due to adverse effects, and the last two stopped inadvertently. The 2-year mortality risk was 28% in patients randomized to propranolol and 49% in patients randomized to placebo, and this difference was statistically significant. However, Pascal and Cales did not report the proportion of patients who had ascites, so we cannot rule out that a subgroup analysis of their patients with refractory ascites would have shown higher mortality in patients randomized to propranolol. Likewise, the Italian Multicentre Project for Propranolol in Prevention of Bleeding conducted a randomized trial including 174 cirrhosis patients with large varices to investigate the effect of propranolol versus placebo in primary prevention of variceal bleeding.26 Of the 85 patients randomized to propranolol, 25 (29%) stopped taking propranolol during the 30-month follow-up, 23 because of side effects and two because of noncompliance. The investigators concluded that there was no difference in 30-month mortality in the overall cohort or in subgroups of patients who had ascites (44% of the total cohort) or Child-Pugh class C cirrhosis (7% of the total cohort). Subgroup analyses indicated that propranolol did reduce bleeding risk in patients with Child-Pugh class A cirrhosis, but a later individual-patient meta-analysis of this and three other trials found that the NSBB effect on bleeding risk was greater in patients with a Child-Pugh score ≥8 than in patients with a lower Child-Pugh score.27 Nine other trials of NSBBs versus placebo have been conducted, but none of them included a greater proportion of Child-Pugh class C patients than the Pascal and Cales trial or conducted a subgroup analysis of patients with refractory ascites.28 A meta-analysis of the total 11 trials concluded that NSBBs reduce mortality by a nonsignificant amount.28 Trials of NSBBs versus variceal ligation have yielded similar results: patients offered ligation have the same mortality risk as those who are offered NSBBs,3 although they are not at risk of the possible adverse effects of NSBBs. Thus, NSBBs are generally safe in patients with decompensated cirrhosis, but there are no data to determine the safety of NSBBs in the most fragile patients. Therefore, we cannot claim to have shown that NSBBs are safe for all cirrhosis patients, but we can say that the way NSBBs were administered to patients in actual clinical practice was indeed safe.
Our findings conflict with those by Serste et al.7 and Mandorfer et al.,16 who found a higher mortality among NSBB users with refractory ascites and patients with SBP, respectively. These were single-center cohorts with longer follow-up and patients with more advanced disease evidenced by higher median MELD scores of 18 and 19, respectively. We can speculate that their patients continued using NSBBs past the suggested points where the effects of NSBBs may increase mortality. Another possibility is that their NSBB users had more severe cirrhosis to begin with than their nonusers did, and that was clearly the case in the study by Serste et al.7 It included 151 cirrhosis patients with refractory ascites, and the 1-year survival was only 19% in NSBB users versus 64% in nonusers. Our 1-year survival in patients with refractory ascites was 69% in both NSBB users and nonusers. In their study, NSBB users were more likely than nonusers to be Child-Pugh class C and to have a history of variceal bleeding and hepatic encephalopathy. The NSBB users also had a higher total bilirubin and a lower serum sodium, and, like others before us,8, 11, 15, 29-31 we question whether the investigators successfully managed to adjust for the substantial confounding disfavoring NSBB use. Their decision to use significance testing to identify confounding factors is not recommended,32 and it likely resulted in substantial residual confounding by cirrhosis severity. The study by Mandorfer et al. may also suffer from confounding,16 and they do not explain why they adjusted for presence of varices and Child-Pugh class but not for gender, cirrhosis etiology, and other markers of cirrhosis severity, although they were different for NSBB users and nonusers. Child-Pugh class has only three possible levels—A, B, and C—so there can be substantial residual confounding in their analysis. Finally, it must be remembered that, as a result of the randomized trials of NSBBs for primary or secondary prevention of variceal bleeding, NSBBs are nowadays given to cirrhosis patients with severe portal hypertension. These patients have higher mortality than patients with lesser portal hypertension,33 so our default expectation should be that NSBB users have higher mortality than nonusers. That expectation should temper the urge to change treatment recommendations on the basis of singular observational studies.
In conclusion, NSBBs did not increase all-cause or cirrhosis-related mortality in our overall cohort of cirrhosis patients with ascites or in any of the subgroups of more fragile patients we examined. NSBBs were frequently discontinued, primarily in patients who experienced stressful clinical events. We cannot clarify whether the decision to discontinue NSBBs saved these patients or had no impact at all, but our findings suggest that clinicians can continue to use NSBBs like they did when these randomized trials were conducted in 2006-2008.




