Bidirectional transendothelial migration of monocytes across hepatic sinusoidal endothelium shapes monocyte differentiation and regulates the balance between immunity and tolerance in liver
Potential conflict of interest: Nothing to declare.
Supported by the START program of the Medical Faculty of the University of Aachen/Germany (to H.W.Z.) with additional funding from a Wellcome Trust Program award (to D.H.A.).
Abstract
Monocytes are versatile cells that can fulfill proinflammatory and anti-inflammatory functions when recruited to the liver. Recruited monocytes differentiate into tissue macrophages and dendritic cells, which sample antigens and migrate to lymph nodes to elicit T-cell responses. The signals that determine monocyte differentiation and the role of hepatic sinusoidal endothelial cells (HSECs) in this process are poorly understood. HSECs are known to modulate T-cell activation, which led us to investigate whether transendothelial migration of monocytes across HSECs influences their phenotype and function. Subsets of blood-derived monocytes were allowed to transmigrate across human HSECs into a collagen matrix. Most migrated cells remained in the subendothelial matrix, but ∼10% underwent spontaneous basal to apical transendothelial migration. The maturation, cytokine secretion, and T-cell stimulatory capacity of reverse transmigrating (RT) and subendothelial (SE) monocytes were compared. SE monocytes were mainly CD16–, whereas 75%-80% of RT monocytes were CD16+. SE monocytes derived from the CD14++CD16− subset and exhibited high phagocytic activity, whereas RT monocytes originated from CD14++CD16+ and CD14+CD16++ monocytes, displayed an immature dendritic cell–like phenotype (CD11cposHLA-DRposCD80loCD86lo), and expressed higher levels of chemokine (C-C motif) receptor 8. Consistent with a dendritic cell phenotype, RT monocytes secreted inflammatory cytokines and induced antigen-specific CD4+ T-cell activation. In contrast, SE monocytes suppressed T-cell proliferation and activation and exhibited endotoxin tolerance. Transcriptome analysis underscored the functional differences between SE and RT monocytes. Conclusions: Migration across HSECs shapes the subsequent fate of monocytes, giving rise to anergic macrophage-like cells in tissue and the release of immunocompetent pre–dendritic cells into the circulation. (Hepatology 2016;63:233–246)
Abbreviations
-
- CCL
-
- chemokine (C-C motif) ligand
-
- CCR
-
- chemokine (C-C motif) receptor
-
- CMV
-
- cytomegalovirus
-
- CXCL
-
- chemokine (C-X-C motif) ligand
-
- DC
-
- dendritic cell
-
- HSEC
-
- hepatic sinusoidal endothelial cell
-
- HUVEC
-
- human umbilical vein endothelial cell
-
- IFN-γ
-
- interferon-γ
-
- IL
-
- interleukin
-
- LPS
-
- lipopolysaccharide
-
- PD-1
-
- programmed death ligand-1
-
- RT
-
- reverse transmigrating
-
- SE
-
- subendothelial
-
- TEM
-
- transendothelial migration
-
- Th1
-
- T helper 1
-
- TLR
-
- toll-like receptor
-
- TNF-α
-
- tumor necrosis factor-α
Monocytes fulfill important functions in the defense against pathogens by linking innate to adaptive immunity.1 Their plasticity and inherent heterogeneity allow monocytes to give rise to tissue-resident macrophages and dendritic cells (DCs).2-5 Macrophage precursor cells display phagocytic activity and can clear senescent cells as well as microbial and other foreign material to promote tissue repair and remodeling. During steady-state conditions monocytes traverse the endothelium to enter tissue from blood in order to replenish and augment the local pool, and this process is greatly increased with inflammation.5 A substantial minority of monocytes with a DC-like phenotype sample antigens in the subendothelial compartment and migrate to afferent lymph nodes.2 Human monocytes comprise three morphologically and functionally distinct subsets based on expression of CD14 and CD16: “classical” CD14++CD16–, “intermediate” CD14++CD16+, and “nonclassical” CD14+CD16++ monocytes.6, 7
Monocytes traffic to the liver under normal conditions, and this increases markedly in response to liver injury.8, 9 We previously showed that monocyte subpopulations differentially accumulate in the inflamed liver, although the mechanisms that control their recruitment and positioning are poorly understood.10 Hepatic sinusoidal endothelial cells (HSECs) differ morphologically and functionally from endothelial cells in other vascular beds and actively control the translocation of circulating immune cells from the sinusoids into the liver parenchyma.11, 12 Importantly, HSECs modulate local immunity by, for instance, skewing T helper 1 (Th1) and Th17 responses to induce suppressive T cells, thereby contributing to the prevailing immune regulatory hepatic microenvironment.13, 14 We hypothesized that transendothelial migration (TEM) across activated HSECs shapes monocyte fate and differentiation, thereby regulating hepatic immune responses.
Because monocytes not only migrate from blood into tissue but also undergo reverse transmigration out of tissue into the blood, we studied unidirectional and bidirectional migration of monocyte subsets across human HSECs to determine how these processes affect subsequent monocyte function. We report that CD16+ monocytes preferentially undergo reverse transmigration, after which they can activate CD4+ T cells, whereas monocytes that remain in the subendothelial space resemble anti-inflammatory macrophages that promote T-cell anergy. Functional differences following TEM are accompanied by substantial changes in the transcriptome. Thus, TEM across the endothelium contributes to hepatic immune regulation.
Materials and Methods
Isolation and Culture of Endothelial Cells
ECs were isolated from human explanted or resected liver and surplus donor tissue as described.15, 16 Briefly, parenchymal cells were isolated from collagenase-digested tissue over a 33%/77% Percoll gradient (Amersham Biosciences, GE Healthcare, Little Chalfont, UK), and magnetic selection with antibody HEA125 (Progen Biotechnik, Germany) was used to deplete cholangiocytes followed by anti-CD31 selection of HSECs (Invitrogen, Paisley, UK) and culture in human endothelial basal growth medium (Invitrogen), 10% (v/v) heat-inactivated AB human serum (HD Supplies, Glasgow, UK), 10 ng/mL vascular endothelial growth factor, and 10 ng/mL hepatocyte growth factor (PeproTech, Peterborough, UK) in collagen-coated culture flasks. All human tissue and blood was collected with local research ethics committee approval and patient consent.
Bidirectional Monocyte Transmigration Across HSECs
Bovine collagen I (Gibco, Life Technologies), 3 mg/mL in 10 × minimal essential medium (Life Technologies) pH 7.4, was polymerized in 24-well cell culture standing inserts (pore size 0.4 µm) at 37 °C (Merck Millipore, Watford, UK) equilibrated in supplemented HSEC medium for at least 3 days. To study phagocytosis, collagen matrix was supplemented with 1 × 107 unopsonized fluorescence-labeled Zymosan A BioParticles (Molecular Probes, Invitrogen). Collagen gels were coated with 100 μL fibronectin at 37 °C for 30 minutes (Invitrogen/Gibco; 50 µg/mL in phosphate-buffered saline) and seeded with HSECs grown until confluence following stimulation with 10 ng/mL tumor necrosis factor-α (TNF-α) (PeproTech) and 10 ng/mL/interferon-γ (IFN-γ) (PeproTech) for 24 hours. CD14+ monocytes (2 × 106) or presorted monocyte subsets in endothelial basal growth medium/0.1% bovine serum albumin were layered onto the fibronectin-coated collagen plugs. After 1.5 hours, nonadherent cells were washed off and the medium was replaced by endothelial basal growth medium containing 2.5% (v/v) heat-inactivated human serum. During the subsequent 48-hour incubation spontaneously reverse transmigrating (RT) monocytes were harvested by thoroughly washing off cells from above the endothelial layer with ice-cold phosphate-buffered saline/0.5 mM ethylene glycol tetraacetic acid/1% fetal calf serum. The collagen plugs containing retained subendothelial (SE) monocytes were gently digested in 20% (v/v) collagenase from Clostridium histolyticum (Sigma-Aldrich) and 20% (v/v) heat-inactivated fetal calf serum at 37 °C for 10 minutes. Digests were placed on ice, filtered, and resuspended in phosphate-buffered saline/2 mM ethylene diamine tetraacetic acid/1% fetal calf serum. We confirmed that surface markers were not lost during collagenase digestion (data not shown). Contaminating HSECs were depleted with biotinylated Ulex Europaeus Agglutinin I (Vector Laboratories, Burlingame, CA) and streptavidin-conjugated Dynabeads (Life Technologies, Carlsbad, CA) and magnetic depletion. In some experiments human umbilical vein endothelial cells (HUVECs) were used as endothelial cells. RT and SE monocytes were counted and subjected to further analysis or in vitro experiments. Trypan blue exclusion confirmed viability.
Statistical Analysis
The Student t test and GraphPad Prism software were used to compare numerical variables between two groups and one-way analysis of variance followed by Bonferroni's posttest, for comparisons between more than two groups. Results are expressed as mean ± standard error of the mean. P < 0.05 was considered statistically significant. *P < 0.05, **P < 0.01, ***P < 0.001.
For further information on materials and methods, please refer to Supporting Information.
Results
Intrahepatic Accumulation of Monocytes/Macrophages Is Driven by Activated Endothelial Cells
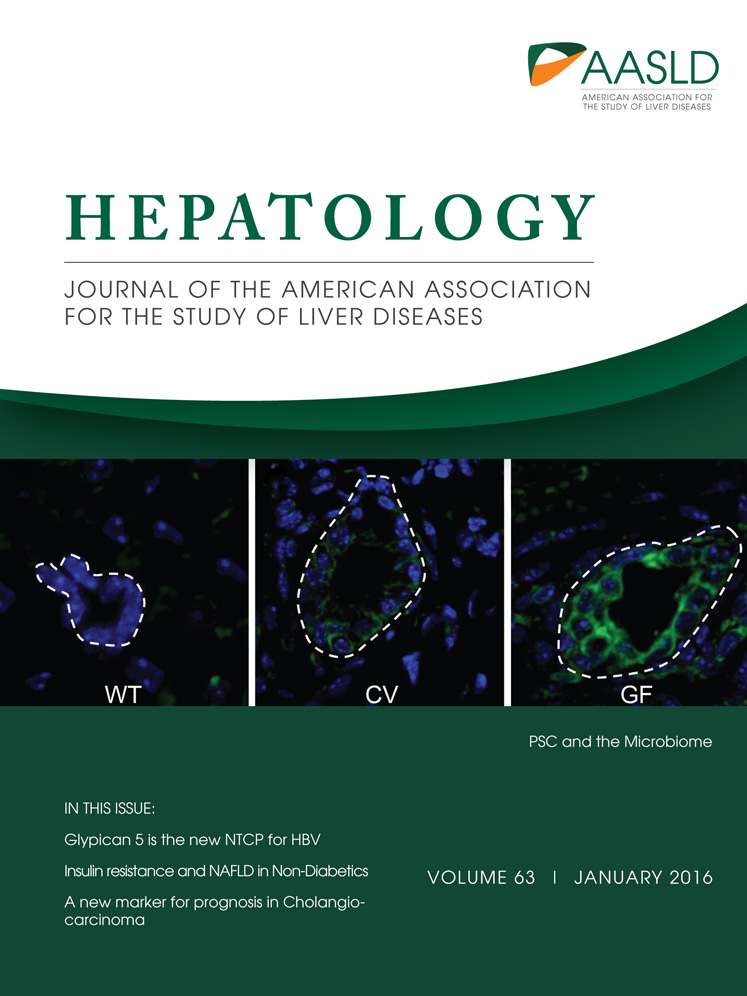
In order to study the fate of monocytes after transmigration to the subendothelial compartment, we established a model of monocyte transmigration and reverse transmigration involving primary human HSECs, adapted from Randolph et al.17 Monocytes were allowed to migrate across activated HSECs and a thin layer of fibronectin into a three-dimensional collagen scaffold. After depletion of nonmigrating cells and 2 days of continued coculture, resident monocytes from the subendothelial compartment (SE monocytes) and monocytes that underwent spontaneous reverse transmigration (RT monocytes) from the subendothelial compartment back through the endothelial layer were harvested. To verify that RT monocytes had sampled the subendothelial compartment and had not simply detached from the endothelial monolayer, we supplemented the collagen scaffold with fluorescein isothiocyanate–labeled zymosan particles and tracked the localization of phagocytic monocytes/macrophages. Migration into zymosan-supplemented collagen increased the number of retained subendothelial cells, most of which were CD16low. The RT monocytes contained zymosan, albeit at lower levels than the SE monocytes, and were predominantly CD16high (Fig. 1B). Longtime imaging could visualize forward and reverse transmigration of monocytes across HSECs under cell culture conditions (Supporting Video). In order to confirm that the TEM was an active process, we reduced migration in both directions by inhibiting Janus kinase–signal transducer and activator of transcription signaling, which is required for macrophage TEM (Supporting Fig. S1).18 Interestingly, direct comparison of cell compartmentalization after monocyte TEM across HSECs or HUVECs revealed that HSECs clearly favor subendothelial retention (90% SE monocytes), whereas HUVECs permitted approximately 50% of monocytes to reverse transmigrate (Fig. 1C). To mimic the inflammatory environment present in the subendothelial compartment during liver disease, the collagen matrix was supplemented with conditioned medium of activated primary liver myofibroblasts. TNF-α/IFN-γ stimulation of activated primary liver myofibroblasts led to a profound increase in the total number of transmigrating cells, though the ratio of RT/SE was not significantly altered (Supporting Fig. S2). These data demonstrate that after active recruitment across HSECs monocytes differentiate into either sessile macrophage-like cells with high phagocytic capacity or mobile DC-like cells. Recruitment is augmented by cell-derived chemotactic stimuli.
HSECs favor recruitment and subendothelial accumulation of CD14+ monocytes. (A) Schematic illustration of a tissue model to study TEM of monocytes. Primary HSECs isolated from explanted livers were seeded onto a collagen three-dimensional matrix with an interposed fibronectin thin layer and grown to confluence. The HSEC monolayer was stimulated with 10 ng/mL of TNF-α/IFN-γ for 24 hours to enhance immune cell recruitment, followed by addition of freshly purified CD14+ monocytes. Nonmigrating cells were discarded, and the coculture was continued for a total of 48 hours. During this period spontaneous reverse transmigration across HSECs of a minor fraction of monocytes occurred, with the majority arising from resident SE monocytes. (B) Collagen matrix was supplemented with fluorescein isothiocyanate-labeled zymosan particles. Representative contour plots indicate the differential phagocytic capacity of RT and SE monocytes after 48 hours of cultivation and the impact on CD16 expression. (C) Comparison of quantitative RT/SE monocyte distribution after bidirectional TEM across either HSECs or HUVECs. Mean and standard error of the mean from three independent experiments; P values from unpaired t test. Abbreviation: FITC, fluorescein isothiocyanate.
RT Monocytes Express CD16 and Can Be Derived From All Monocyte Subsets
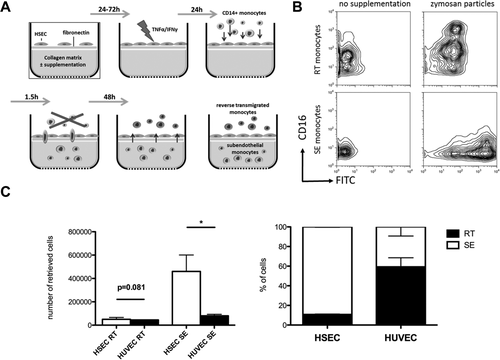
The majority of SE (mean 78.9 ± 9.8%) monocytes were classical CD14++CD16– monocytes, whereas 69.4% (±12.6%) of RT monocytes were intermediate CD14++CD16+ and few were classical monocytes. Very few cells in either compartment were nonclassical CD14+CD16++ cells, suggesting that this subset does not readily undergo TEM (Fig. 2A). Monocytes are highly plastic cells, and different subsets represent various states of maturity and differentiation, prompting us to determine how the different subsets in blood contributed to either SE or RT monocytes. When classical monocytes were used as the starting cell type, >80% were retained in the SE compartment (80.7 ± 12.6%) and fewer cells underwent reverse TEM compared with intermediate and nonclassical subsets (Fig. 2B,C), suggesting that CD16 expression is associated with the ability to undergo RT. Most RT monocytes were CD16+, indicating that these cells gain CD16 expression either during TEM or in the subendothelial space and that this confers on some cells the ability to undergo reverse TEM (Fig. 2C).
RT monocytes are mainly composed of CD14++CD16+ monocytes and originate from CD16+ and CD16– precursor cells (A). Composition of RT and SE monocytes according to differential CD14 and CD16 expression. The percentage of classical CD14++CD16–, intermediate CD14++CD16+, and nonclassical CD14+CD16++ monocytes among RT and SE monocytes is shown for each experiment (n = 7 independent experiments with HSECs and monocytes from different donors; P values from paired t test). (B) Representative zebra plots of peripheral monocyte subset distribution prior to fluorescence-activated cell sorting (left) and CD14/CD16 expression of sorted monocyte subsets after 48 hours of bidirectional TEM across HSECs (right). (C) Stacked columns depicting percentages of SE and RT fractions for each monocyte subset (mean and standard error of the mean from n = 3 independent experiments). Abbreviation: n.s., not significant.
Reverse Transmigration of Monocytes Across HSECs Imparts a Phenotype Consistent With Immature DCs
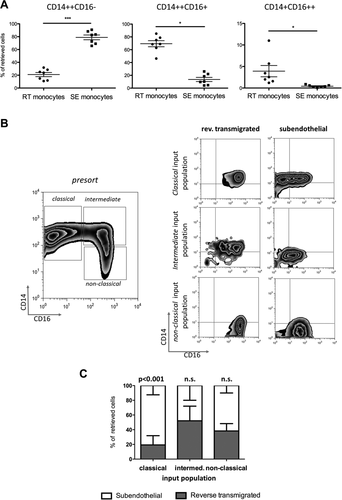
We analyzed the different subsets for features of DC differentiation to see if reverse transmigration selects monocytes with the capability to become DCs and to reenter the vasculature before being recruited to lymph nodes through high endothelial venules.17 Both RT and SE monocytes expressed high levels of major histocompatibility class 2 and CD40 (Fig. 3). CD86 (B7-2) was expressed at higher levels on SE monocytes with little CD80 (B7-1) or CD83 detected on either subset. RT monocytes expressed higher levels of two scavenger receptors, the mannose receptor (CD206) and CD163, both of which are associated with alternatively activated macrophages, while CD206 is also present on immature DCs. Neither RT nor SE cells expressed high levels of DC-SIGN (CD209). The classical macrophage marker CD68 was expressed at similar levels on both SE and RT monocytes. CD11b (Mac-1) and CD11c are integrin alpha chains that form heterodimers with CD18 and bind complement and intercellular cell adhesion molecule 1. CD11c expression is characteristic of DCs and was detected at high levels on RT monocytes. Chemokine (C-C motif) receptor 7 (CCR7) and CCR8 regulate homing of DCs to the afferent lymph node.19 CCR8 was expressed at higher levels on RT monocytes, suggesting that it might be involved in reverse transmigration. There were no detectable differences in expression of CCR7 or CCR5 on RT versus SE cells, and neither subset expressed CCR2 (data not shown). RT monocytes tended to express higher levels of CX3CR1 and macrophage colony-stimulating factor receptor (CD115), both of which are characteristic of DCs.20 Thus SE and RT cells are similar in terms of costimulatory molecule expression, although RT monocytes have characteristics of promigratory, DC-like cells.
RT cells exhibit a phenotype reminiscent of immature DCs. Depiction of representative histograms/density plots from flow cytometry of various surface markers implicated in the macrophage/DC phenotype on RT (black line) and SE monocytes (light gray). Isotype-matched controls highlighted in dark gray. Bar graphs represent mean fluorescence intensity (mean, standard error of the mean, P value of paired t test; *P value <0.05, **P value <0.01). Abbreviation: MFI, mean fluorescence intensity.
RT, but not SE, Monocytes Induce Robust T-Cell Proliferation
We compared RT monocytes with SE cells for their ability to induce CD4+ T-cell proliferation, a defining feature of DCs. While SE monocytes had little effect on T-cell proliferation, RT monocytes induced potent proliferative responses after pulsing with pp65 (Fig. 4A). We saw a similar although weaker effect in an allogeneic T-cell response (Fig. 4B). Thus, DC-like function resides in the RT population. In the presence of SE monocytes T-cell proliferation in response to either OKT3 or CD3/CD28 stimulation was significantly suppressed at T-cell ratio/monocyte ratios of 1:1 and below, whereas RT monocytes amplified baseline proliferation of CD4+ T cells (Fig. 4C). The inhibitory effect of SE monocytes was not due to the induction of apoptosis in CD4+ T cells (Fig. 4D).
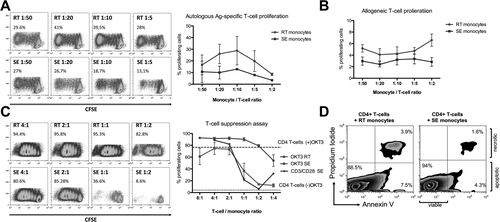
RT monocytes mount efficient CD4+ T-cell proliferative responses, whereas SE monocytes elicit T-cell suppression. (A) Representative carboxyfluorescein succinimidyl ester plots of proliferating autologous CD4+ T cells after antigen-specific stimulation with RT versus SE monocytes at monocyte/T-cell ratios of 1:50 to 1:5 (left). Quantification of proliferating autologous CD4+ T cells after antigen-specific stimulation with RT monocytes and SE monocytes given as percentage of total cells (mean and standard error of the mean from n = 3 independent experiments) (right). (B) Quantification of proliferating allogeneic CD4+ T cells after stimulation with RT monocytes versus SE monocytes in a T-cell suppression assay given as percentage of total cells (mean and standard error of the mean from n = 5 independent experiments). (C) Representative carboxyfluorescein succinimidyl ester plots of proliferating CD4+ T cells after stimulation with immobilized α-CD3 antibody (OKT3) in the presence of RT versus SE monocytes at T-cell/monocyte ratios of 4:1 to 1:4 (left). Quantification of n = 3 independent experiments (mean, standard error of the mean) comparing proliferation capacity of OKT3-stimulated CD4+ T cells in the presence of RT versus SE monocytes. CD3/CD28 bead stimulated CD4+ T cells cocultured with SE monocytes are highlighted. Dotted line represents baseline CD4+ T-cell proliferation without OKT3 in the absence of monocytes. Dashed line indicates OKT3-stimulated CD4+ T-cell proliferation without monocytes (right). (D) Representative fluorescence-activated cell sorting plot of annexin V assay after 5 days of antigen-specific autologous CD4+ T-cell stimulation with RT versus SE monocytes, gated on CD4+ T cells. Lower right quadrant represents early apoptotic cells; cells in top right quadrant are late apoptotic/necrotic. Abbreviations: Ag, antigen; CFSE, carboxyfluorescein succinimidyl ester.
RT Monocytes Are Capable of Inducing CD4+ T-Cell Activation, Whereas SE Macrophage-Like Monocytes Dampen Activation
Having observed that RT, but not SE, monocytes can induce antigen-specific CD4+ T-cell proliferation, we next compared the ability of SE and RT monocytes to drive T-cell activation using CD14+ monocytes from cytomegalovirus (CMV)–seropositive donors (Fig. 5A). RT monocytes activated CD4+ T cells as demonstrated by up-regulation of the late-activation markers CD25 and HLA-DR, whereas SE monocytes had no effect on activation and suppressed expression of the mid-early T-cell activation marker CD71 (Fig. 5B,C). We observed similar effects in an allogeneic system (data not shown). To obtain insights into the consequences of T-cell activation by RT monocytes, we quantified changes in genes involved in T-cell differentiation. SE monocytes increased expression of NFATC2 (NFAT1), which is linked to CD4+ T-cell anergy and suppression along with Fas ligand (FASLG) that triggers activation-induced T-cell apoptosis.21-23 In contradistinction CD4+ T cells activated by RT monocytes expressed T-box transcription factor EOMES (Eomesodermin) and TNFRSF9 (CD137) (five-fold and 4.76-fold increases, respectively, compared with cells stimulated with SE monocyte) (Fig. 5D). These genes are associated with T-cell memory and effector function and promote clonal expansion and T-cell survival.21, 22 Cells coincubated with RT monocytes also increased expression of several genes that drive Th2 commitment, including IL13, IRF4, GFI1, IL4R, and IL13RA1 (Fig. 5D), whereas genes that counteract Th2 polarization (POU2F2 and RUNX1) were more highly expressed in T cells cocultivated with SE monocytes.
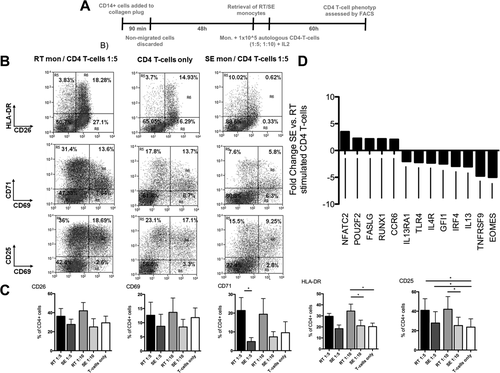
RT monocytes are capable of inducing CD4+ T-cell activation, whereas SE counterparts promote anergy in autologous T cells. (A) Schematic illustration of experiments assessing autologous CD4+ T-cell activation by RT and SE monocytes. After retrieval of SE and RT monocytes of CMV-seropositive donors, cells were cultivated with pp65 CMV antigen for 24 hours and autologous CD4+ T cells were added and cocultured for 5 days at monocyte/T-cell ratios of 1:5 and 1:10 in the presence of recombinant IL-2 (50 IU/mL). (B) Representative fluorescence-activated cell sorting dot plots of CD25, CD26, CD69, CD71, and HLA-DR expression on CD4+ T cells after 60 hours of coculture with RT and SE monocytes or without monocytes. (C) Quantification of T-cell activation marker expression. Bar graphs represent percentage of positive cells (mean and standard error of the mean of three or four independent experiments, P values from Bonferroni's post hoc test; *P value <0.05, **P value <0.01). (D) Transcriptional regulation of genes involved in T-helper cell polarization in CD4+ T cells from a CMV-seropositive donor stimulated with autologous CMV pp65 bearing SE versus RT monocytes. Genes with at least two-fold regulation are indicated. Abbreviations: FACS, fluorescence-activated cell sorting; HLA, human leukocyte antigen; mono, monocytes.
RT Monocytes Induce Effector T Cells With Th1-Like Features
To further characterize the CD4+ T-cell antigen–specific response driven by RT monocytes, we assessed the release of cytokines by activated T cells upon coculture with CMV pp65 exposed autologous RT/SE monocytes. RT monocytes induced a significantly higher proportion of IFN-γ producing T cells than SE monocytes, suggesting Th1 polarization (Fig. 6A), although responding CD4+ T cells also increased CTLA-4 and FOXP3 expression, genes that are linked to regulatory T cells but also up-regulated during early T-cell activation. In line with the proliferation assays described above, we suggest that RT monocytes induce activated effector CD4+ T cells rather than a suppressive phenotype (Fig. 6B,C). Despite a genetic profile compatible with Th2 commitment, we failed to detect interleukin-4 (IL-4) in CD4+ T cells stimulated with RT monocytes (Fig. 6C). Consistent with local IFN-γ secretion, the RT monocytes in the cocultures showed significantly increased levels of programmed death ligand-1 (PD-L1, which is strongly induced by IFNs) compared with SE monocytes (Fig. 6D), whereas its cognate ligand PD-1 was not regulated on cocultivated CD4+ T cells (Fig. 6E). Comparing HSEC-shaped and HUVEC-shaped monocytes, we observed that SE monocytes derived from HUVEC interaction were able to elicit IFN-γ secretion by autologous CD4+ T cells, indicating that failure to induce a trans-Th1 response is a unique feature of liver-specific endothelium (Fig. 6F). We conclude that RT monocytes effectively activate CD4+ T cells and potentially prime T-helper cell function, whereas SE monocytes suppress T-cell activation and blunt their differentiation.
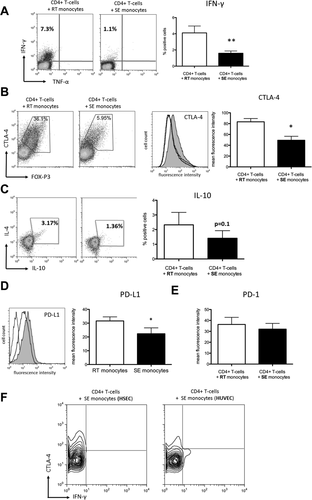
RT monocytes induce multifaceted T-cell response patterns, whereas SE monocytes promote CD4+ T-cell anergy. (A) Representative fluorescence-activated cell sorting plot of intracellular IFN-γ expression after antigen-specific stimulation of autologous CD4+ T cells with RT versus SE monocytes (left). Bar graphs depicting quantification of n = 9 experiments (mean, standard error of the mean, P value from paired t test) (right). (B) Exemplary fluorescence-activated cell sorting plots of intracellular CTLA-4 (y axis) and FOXP3 (x axis) in CD4+ T cells coincubated with either RT or SE monocytes (left). Representative histogram of intracellular CTLA-4 expression (gray line denoting isotype control, dark line denoting CD4+ T cells stimulated by SE monocytes, gray fill denoting CD4+ T cells stimulated by RT monocytes) and respective statistical analysis (mean of mean fluorescence intensity values, standard error of the mean, n = 4 experiments) (right). (C) Representative fluorescence-activated cell sorting plots of intracellular IL-4 (y axis) and IL-10 (x axis) expression after antigen-specific stimulation of autologous CD4+ T cells with RT versus SE monocytes (left). Bar graphs illustrating percentage of IL-10-positive CD4+ T cells (mean, standard error of the mean, n = 5 experiments). (D) Representative histogram of PD-L1 surface expression on RT versus SE monocytes (left) and respective statistical analysis (mean of mean fluorescence intensity values, standard error of the mean, n = 5, P value from paired t test) (right). (E) Statistical analysis of PD-1 expression on CD4+ T cells stimulated with RT versus SE monocytes (mean, standard error of the mean, n=6 experiments, P value from paired t test). (F) Representative contour plot of intracellular CTLA-4 (y axis) and IFN-γ (x axis) expression in CD4+ T cells that were stimulated with SE monocytes after TEM across HSECs versus HUVECs. *P value <0.05, **P value <0.01.
Resident SE Monocytes Fail to Mount a Proinflammatory Response Upon Toll-Like Receptor Engagement
Having shown differences in the ability of SE and RT monocytes to induce T-cell activation, we compared their ability to respond to toll-like receptor (TLR) engagement. Under baseline conditions after 24 hours in vitro culture, RT and SE monocytes secreted low levels of macrophage migration inhibitory factor, IL-8, and the anti-inflammatory IL-1 receptor antagonist. SE monocytes also secreted the chemokine (C-X-C motif) ligand 1 (CXCL1), chemokine (C-C motif) ligand 2 (CCL2), and CCL5. Neither RT nor SE monocytes spontaneously secreted the proinflammatory cytokines IL-1β, TNF-α, or IL-6 (Fig. 7A,B). Upon lipopolysaccharide (LPS) stimulation, both subsets secreted IL-6, showing the potential to respond to TLR-4 engagement; but while RT monocytes showed a proinflammatory response with increased IL-1β, TNF-α, IL-6, granulocyte colony-stimulating factor, and granulocyte-macrophage colony-stimulating factor secretion, SE monocytes were relatively refractory to LPS stimulation and failed to produce IL-1β, IL-10, IL-13, or TNF-α (Fig. 7A-C). In contrast, RT monocytes responded to LPS with increased secretion of CCL3, CCL4, CCL2, and CCL5 as well as a marked increase in IL-1β and TNF-α secretion. Thus, SE monocytes display phagocytosis, poor T-cell stimulation, and resistance to LPS, whereas RT monocytes are proinflammatory and able to activate T cells (Fig. 7A-C).
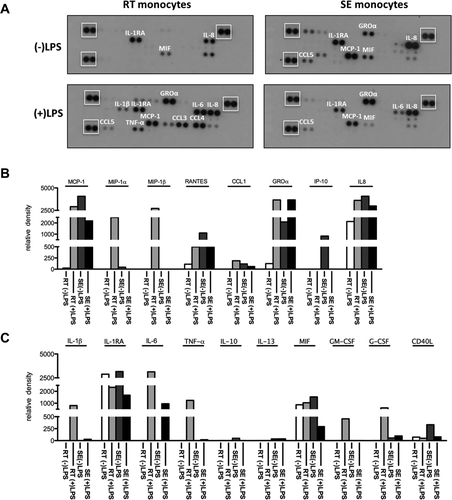
Differential release of cytokines/chemokines upon LPS stimulation highlights endotoxin resistance of SE monocytes. SE or RT monocytes (1.2 × 105) were either stimulated with 10 ng/mL of LPS or left unstimulated for 24 hours in serum-free medium. Conditioned medium was analyzed for the secretion of chemokines and cytokines. (A) Representative images of nitrocellulose membranes with spotted antibodies against several cytokines and densitometric quantification (normalized mean pixel density of chemiluminescence) of those (B) cytokines and (C) chemokines in supernatants from unstimulated RT (white) and SE (dark gray) and stimulated RT (light gray) and SE (black) monocytes. (A) Reference spots for normalization are highlighted by white boxes.
Functional Disparities Between RT and SE Monocytes Are Reflected by Extensive Transcriptional Differences
To get a more profound insight into the effects of TEM on monocytes, we analyzed transcriptional differences between RT and SE monocytes belonging to the different subsets. Intermediate CD14++CD16+ contained the most distinct gene signature with 432 genes more than 10-fold up-regulated and 1179 genes more than 10-fold down-regulated when comparing SE and RT monocytes, whereas around 200 genes were differentially expressed (>10-fold) between SE and RT monocytes in both CD14++CD16– and CD14+CD16++ monocytes. RT monocytes originating from all subsets displayed increased CCL3, CCL4, CXCL1, CXCL2, and IL-8, and proinflammatory genes including TNF family members and IL-1 compared with SE monocytes. CD14, which is typically maintained in macrophages that do not follow the DC pathway, was consistently up-regulated on SE monocytes of all subsets. In line with the immunosuppressive phenotype of SE monocytes, they expressed 15-fold higher levels of SIGLEC-7, a marker of anti-inflammatory macrophages (Supporting Tables S1-S3).
Discussion
Liver macrophages are a heterogeneous population derived from monocytes recruited through the blood and a sessile population of resident Kupffer cells that arise from local precursors.24 In inflammatory liver disease SE monocytes are recruited in increased numbers from the blood and differentiate into distinct functional subsets of DCs that activate adaptive responses and macrophages that regulate inflammation, fibrogenesis, and resolution.25 In order to be recruited from the blood, monocytes must undergo TEM across hepatic endothelium before entering tissue; and because sinusoidal endothelium can modulate T-cell activation, we proposed that TEM might affect the subsequent differentiation and function of monocytes. Recent evidence shows that monocytes can exit inflamed tissues into blood by reverse transmigration from the abluminal to the luminal side of the endothelium, a process that may also have a major effect on differentiation and function and which has been proposed as a route for DC emigration from the liver into lymph nodes.26
We used a model system incorporating human HSECs to show for the first time that monocytes are able to undergo bidirectional migration through hepatic endothelium, with a significant number of transmigrated monocytes migrating back in the abluminal to luminal direction (Fig. 8). Migratory stimuli from activated liver stromal cells presumably augment monocyte recruitment. Migration in both directions was partly dependent on Janus kinase–signal transducer and activator of transcription signaling, confirming that it is an active process. We suggest that these processes are likely to be highly relevant to immune surveillance and inflammatory responses because they determine the function of the cells and give rise to important differences between tissue monocytes and those that exit the liver through reverse transmigration and may then enter draining lymph nodes.
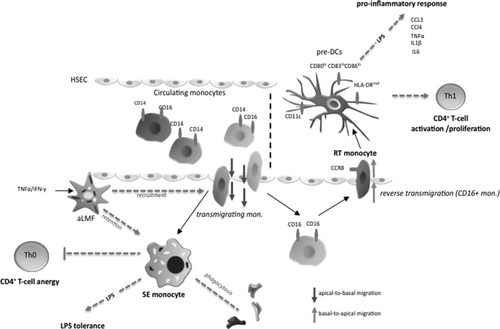
Diagram of the proposed mechanism. Diapedesis across HSECs shapes functional outcome of monocytes that enter the liver through the bloodstream. All three monocyte subsets can transmigrate, with the CD14+CD16– subset being mostly confined to the subendothelial layer (space of Disse), while CD16+ monocytes can undergo reverse transmigration. Activated liver myofibroblasts support both recruitment of monocytes as well as intrahepatic retention of monocytes through soluble factors. SE monocytes are highly phagocytic and display a macrophage-like phenotype. Upon LPS stimulation, SE monocytes fail to mount a relevant proinflammatory response (LPS tolerance) and promote CD4+ T-cell anergy by restraining cell activation and proliferation, thereby dampening local immune reactions. In turn, RT monocytes exhibit features of immature DCs and probably employ CCR8 to exit the liver. RT monocytes are potent antigen-presenting cells and release a broad spectrum of proinflammatory cytokines and chemokines upon LPS exposure, which enables a robust induction of CD4+ T-cell proliferation/activation governing a Th1-prone immune response and microenvironment. Abbreviations: aLMF, activated primary liver myofibroblast; HLA, human leukocyte antigen; mono, monocyte.
The phenotype of SE monocytes (CD163lo, HLA-DRhi, CD86med, and CD83neg) was reminiscent of subsets of hepatic monocyte-derived macrophages described earlier.10 Furthermore, monocytes that remained in the subendothelial compartment demonstrated high phagocytic activity, were largely refractory to LPS treatment, and failed to stimulate T-cell activation, whereas RT monocytes were proinflammatory and induced robust CD4+ T-cell activation and proliferation. This is consistent with previous experiments using umbilical vein endothelial cells in which CD16+ migratory pre-DCs undergo reverse transmigration and postmigratory monocytes exhibit features of foam cell macrophages.17, 27 In our study the RT monocytes were CD16+ and experiments in which the starting population consisted of highly pure subsets confirmed that some CD16– cells can became CD16+ during the process of RT. This suggests that CD16 expression is associated with a migratory phenotype, and transcriptome analysis of these cells showed that they secrete a range of chemokines which could be involved in autocrine or paracrine migratory responses. The CD14++CD16– monocytes that failed to undergo reverse TEM expressed higher levels of STAT2, which is known to counteract differentiation of monocytes to DCs; and these cells engulfed more zymosan particles than the CD16+ cells that underwent RT, consistent with a macrophage phagocytic function.18
We found that TEM across HSECs modulates cellular function and phenotype rather than simply selecting preexisting subsets. CD14++CD16– monocytes gained CD16 expression during reverse transmigration from the basal to the apical side of the endothelium and showed reduced inflammatory cytokine secretion when residing in the subendothelial matrix. Nonclassical CD16hi monocytes have been reported to be highly motile, crawling along blood vessels, and have the highest capacity to elicit T-cell proliferation.28 We found that a considerable proportion of this subset undergoes reverse transmigration and gains the ability to activate T cells, indicating DC functions. This suggests that after capturing tissue antigens they undergo reverse TEM and migrate to draining lymph nodes either through the parasinusoidal route described by Matsuno et al.26 or through blood and high endothelial venules where they activate T-cell responses.
Many nonparenchymal hepatic cells, including DCs, HSECs, and Kupffer cells, display immunosuppressive properties.29, 30 Our data suggest that monocytes that cross sinusoidal endothelium and are then retained in the subendothelial space contribute to the tolerogenic liver environment because they were unable to efficiently activate T cells in response to viral or alloantigens. In fact, they suppressed CD4+ T-cell proliferation and were refractory to activation through TLR4, similar to endotoxin-tolerant liver myeloid DCs.31 In terms of their poor ability to activate T cells, SE monocytes in our model share some properties with human liver CD11c+CD11b+BDCA1+ myeloid DCs.32 Nevertheless, we did not observe induction of IL-10 producing regulatory T cells or Th2 skewed immune response as has been described for liver DCs but, rather, suppression of activation and proliferation associated with increased expression of NFATC2.29, 30, 32 These results provide more evidence to support the hypothesis that efficient T-cell activation in the liver occurs in draining lymph nodes whereas activation in the liver itself leads to tolerance.33 Such a mechanism could contribute to the exhaustion and disappearance of CD4+ T-cell responses seen in viral hepatitis.34 CD14++CD16– SE monocytes showed a 70-fold up-regulation of HLA-E messenger RNA transcription compared to RT monocytes. HLA-E is a ligand for the inhibitory natural killer cell ligand CD94/NKG2A, suggesting another potential anti-inflammatory mechanism employed by SE monocytes.35
Unlike myeloid-derived suppressor cells, SE monocytes were major histocompatibility class 2hi and expressed high cell surface levels of the costimulatory molecule CD86 and low levels of PD-1 ligand, which can induce T-cell apoptosis. Consistent with this PD-1 and CTLA-4 were not up-regulated in CD4+ T cells stimulated with SE monocytes, and the T cells did not show evidence of apoptosis. The molecular mechanisms through which SE monocytes suppress CD4+ T-cell activation warrant further investigation.
In contrast to their subendothelial counterparts RT monocytes induced a robust autologous and allogeneic CD4+ T-cell response characterized by IFN-γ secretion, T-cell activation, and proliferation. Our model recapitulates the translocation of migratory hepatic DCs to the draining afferent secondary tissue that has been described in rats.26 That HSECs can induce such functionally different responses suggests that monocyte/HSEC interactions contribute to the balance between systemic immune activation and local suppression of inflammatory processes within the liver. In some respects RT monocytes from our experiments resemble monocyte-derived TNF-α/inducible nitric oxide synthase–producing inflammatory DCs that take up antigens in peripheral tissue before migrating to the lymph node and releasing inflammatory cytokines.36, 37 RT monocytes secreted proinflammatory mediators after LPS stimulation, consistent with a proinflammatory phenotype, and expressed high levels of MYD88, which links TLR-mediated signals to nuclear factor κB activation. Of note, some genes were expressed by all RT monocytes regardless of their CD14/CD16 status. These included CCL3, CCL4, and IL1B, suggesting that reverse transmigration itself activates a specific pattern of genes in originally distinct subpopulations.
Functionally RT and SE monocytes resembled CD16– and CD16+ subsets, respectively, due to overlapping patterns of cytokine response upon LPS challenge, for example.38, 39 However, meticulous transcriptome analysis of highly pure sorted subsets indicated that the monocyte phenotypes described here are not a consequence of selection of preexisting subsets by HSECs but driven by the transmigration process itself, given the vast differences between RT and SE monocytes derived from the same subset.
In summary, the outcome of monocyte/HSEC interactions is dichotomous and yields immunogenic DC-like cells that potentially drive systemic immune response but also cells that resemble regulatory macrophages that dampen local inflammation. We provide evidence that the retention of immunosuppressive macrophages might be a unique feature of the hepatic endothelium. Thereby, we provide a novel mechanism through which sinusoidal endothelial cells can regulate the balance between immunity and tolerance.
Acknowledgment
We thank the staff at the Liver Transplant Unit, Queen Elizabeth Hospital Birmingham, UK, for their help with sample collection and the patients for donating blood and tissue. We also thank Gill Muirhead and Janine Youster for their help with HSEC isolation and culture, Miroslava Blahova for the excellent general technical support, and Stephen Kissane for performing SAGE analysis.
References
Author names in bold designate shared co-first authorship.