Predictors of hepatitis B e antigen-negative hepatitis in chronic hepatitis B virus-infected patients from childhood to adulthood
Potential conflict of interest: Nothing to report.
Supported by grants from the Ministry of Science and Technology of Taiwan (102-2314-B-002-039-MY2) and National Taiwan University Hospital (NTUH.104-S2663).
Abstract
Hepatitis B e antigen (HBeAg)-negative hepatitis is a clinical indicator of poor outcome for chronic hepatitis B viral (HBV) infection. This long-term prospective cohort study aimed to elucidate the predictors of developing HBeAg-negative hepatitis in chronic HBV-infected subjects followed from childhood to adulthood. We followed 434 HBeAg-positive chronic HBV-infected patients from a median age of 7.22 years (interquartile range 4.31-10.21 years). Spontaneous HBeAg seroconversion occurred in 359 subjects at a median age of 13.93 years (interquartile range 8.76-20.59 years), and 75 subjects developed HBeAg seroconversion after antiviral therapy. These patients were followed for a median of 14.40 years (interquartile range 6.14-22.02 years) after HBeAg seroconversion. Clinical data were analyzed to delineate the predictors of developing HBeAg-negative hepatitis. The HBV basal core promoter and precore/core gene sequences were also evaluated in subjects with and without HBeAg-negative hepatitis. The overall annual incidence of HBeAg-negative hepatitis was 0.37% (95% confidence internal 0.35-0.39) in spontaneous HBeAg seroconverters. The overall annual incidence of HBeAg-negative hepatitis increased to 2.64% in lamivudine-treated subjects but did not increase in those treated with interferon-alpha (0.58%). Male gender (hazard ratio = 3.15), HBV genotype C (hazard ratio = 4.40), HBeAg seroconversion after 18 years of age (hazard ratio = 2.46), and lamivudine therapy prior to HBeAg seroconversion (hazard ratio = 1.42) were predictors of HBeAg-negative hepatitis in HBeAg seroconverters (P < 0.05). HBeAg-negative hepatitis subjects carried more A1762T/G1764A, C2063A, and A2131C HBV gene mutations than those without HBeAg-negative hepatitis. Conclusions: HBeAg seroconversion during childhood predicts a lower risk of HBeAg-negative hepatitis in later life. Interferon-alpha therapy may be an effective antiviral therapy beneficial in chronic HBV-infected children with severe inflammation that facilitates HBeAg seroconversion in earlier life. (Hepatology 2016;63:74–82)
Abbreviations
-
- ALT
-
- alanine aminotransferase
-
- anti-HBe
-
- antibody against HBeAg
-
- BCP
-
- basal core promoter
-
- CI
-
- confidence interval
-
- HBeAg
-
- hepatitis B e antigen
-
- HBsAg
-
- hepatitis B surface antigen
-
- HBV
-
- hepatitis B virus
-
- PCR
-
- polymerase chain reaction
-
- UNL
-
- upper normal limit
Hepatitis B e antigen (HBeAg) seroconversion is indicative of reduced hepatitis activity in chronic hepatitis B virus (HBV)-infected patients.1-3 HBeAg seroconversion after 40 years of age has been reported to increase the risk of chronic liver insufficiency, liver cirrhosis, and even hepatocellular carcinoma.4-6 Although HBeAg seroconversion is an important hallmark during chronic HBV infection, some patients still experience HBeAg-negative hepatitis after HBeAg seroconversion. The annual incidence of HBeAg-negative hepatitis was estimated to be 2%-4% in adult patients,2, 3, 5, 6 although this patient population may represent only the chronic HBV-infected subjects who experienced delayed HBeAg seroconversion in adulthood and the risk of HBeAg-negative hepatitis remains unclear in subjects who experience HBeAg seroconversion at a young age.6, 7
Previous studies have noted the presence of the HBV basal core promoter (BCP) and precore/core gene mutations during the immune-clearance phase before HBeAg seroconversion and hepatitis B core antigen cytoplasmic retention in hepatocytes of HBeAg-negative hepatitis patients.8-12 Chronic HBV genotype B-infected subjects experience HBeAg seroconversion earlier than those with HBV genotype C infection.13, 14 The relative risks of HBV-related hepatocellular carcinoma and liver disease-related death were consistently severalfold higher in males than in females.15-18 However, the relationship between HBV genotype, gender, and HBeAg-negative hepatitis remains controversial.5, 6, 19 Prior nucleos(t)ide analogue treatment was also reported as a risk factor for hepatitis reactivation after HBeAg seroconversion, but the differences among various antiviral agents are unclear.19
We conducted this study to investigate the impact of possible risk factors, including HBV genotype, gender, age at HBeAg seroconversion, antiviral treatment prior to HBeAg seroconversion, and relevant HBV BCP and precore/core gene mutations on incidence of HBeAg-negative hepatitis in a long-term and large-scale cohort study that followed patients from childhood to adulthood.
Patients and Methods
Study Subjects
We recruited 597 initially HBeAg-positive chronic HBV-infected patients from 1984 to 2014 from (1) six cross-sectional surveys of HBV markers conducted in 1984, 1989, 1994, 1999, 2004, and 2009 in Taiwan; (2) prospective screening programs for children of hepatitis surface antigen (HBsAg)-seropositive mothers; and (3) the outpatient clinic of the National Taiwan University Hospital's Department of Pediatrics enrolled as part of a prospective study initiated nearly 30 years ago. A physical examination, abdominal sonography, and measurements of serum alanine aminotransferase (ALT), aspartate aminotransferase, alpha-fetoprotein, and HBV seromarkers (semiquantitative HBsAg, the antibody against HBsAg, the antibody against hepatitis B core antigen, HBeAg, and the antibody against HBeAg [anti-HBe]) were performed at each 6-month interval visit. Upon detection of elevated serum ALT levels, the follow-up interval was shortened to 1-3 months. In this study, the upper normal limit (UNL) for ALT in childhood was defined as 30 IU/L in both genders based on our previous report.20 Subjects with hepatitis C virus coinfection were not included in this cohort. The prevalence of anti-hepatitis D virus positivity in chronic HBV-infected children was reported as ∼1.6%. The natural course of HBV infections in childhood in Taiwan may be more closely related to HBV rather than to hepatitis D viral infection.21 Hence, we did not assess anti-hepatitis D virus in this study.
The immune-tolerant phase of chronic HBV infection in this study was defined as HBeAg+, anti-HBe-, and serum ALT below the UNL. HBeAg seroconversion was defined as the clearance of serum HBeAg and appearance of anti-HBe exceeding 6 months in duration. The inactive phase of chronic HBV infection was defined as the normalization of ALT levels after HBeAg seroconversion. HBeAg-negative hepatitis was defined according to the Asian Pacific Association for the Study of the Liver consensus on chronic HBV management and the guideline of national health insurance in Taiwan as ALT elevation greater than or equal to two-fold UNL for 6 months with serum HBV viral load elevation ≥2000 IU/mL following HBeAg seroconversion.22 The study protocol was approved by the institutional review board of the National Taiwan University Hospital, and the patients or their guardians provided signed informed consent to collect blood samples and clinical data.
HBV Marker Serological Tests, HBV Viral Load, and Genotyping
Serum samples were obtained at each visit to examine HBV markers and liver function profiles. HBV markers including HBsAg, HBeAg, antibody against HBsAg, anti-HBe, and antibody against hepatitis B core antigen were assessed by enzyme immunoassay (Abbott Laboratories, North Chicago, IL). Quantitative HBsAg titers were determined in serum specimens by the commercial Architect HBsAg QT kit (Abbott Laboratories). HBV genotype and viral load were determined using real-time polymerase chain reaction (PCR) and melt curve assays.23 The HBV DNA and quantitative HBsAg titers were relatively stationary during the immune-tolerant phase in children. Thus, quantitative HBsAg and HBV DNA levels obtained from the first available specimens at the immune-tolerant phase represented the baseline virologic status of these subjects.24-26
Seminested PCR and Sequencing
We analyzed the HBV BCP and precore/core gene sequences during the inactive phase following HBeAg seroconversion in chronic HBV-infected subjects with and without HBeAg-negative hepatitis in an age-matched, gender-matched, HBV genotype-matched, and follow-up period-matched case-control design. Serum samples from the HBeAg-negative hepatitis phase in subjects with HBeAg-negative hepatitis and in control subjects without HBeAg-negative hepatitis who were matched for follow-up period were also analyzed. Viral DNA was extracted from serum samples (100 μL) using the QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer's protocol. For the first PCR round, 1-μL samples of viral DNA were used as the templates in 50-μL PCR mixtures. The first PCR mixtures contained 0.4 μM primers (forward PC1F-5′-ACATAAGAGGACTCTTGGAC-3′; reverse CoreR1- 5′-AGTTTCCCACCTTATGAGTC-3′), 50 μM deoxyribonucleotide triphosphates, 1× VAS Taq buffer, and 0.1 unit/μL VAS Taq. PCR was performed by amplification at 95°C for 1 minute, 50°C for 1 minute, and 72°C for 1 minute for 40 cycles in a thermal cycler. For the seminested PCR template, varying dilutions of the first PCR product were prepared depending on the viral load in the serum sample (usually 1/1000 for 2 × 104 IU/mL). The seminested PCR mixtures contained 0.4 μM primers (forward PC2F-5′-TACTTCAAAGACT GTGTGTTTA-3′; reverse CoreR1- 5′-AGTTTCCCA CCTTATGAGTC-3′), 50 μM deoxyribonucleotide triphosphates, 1× VAS Taq buffer, and 0.1 unit/μL VAS Taq. The seminested PCR was performed by amplification at 95°C for 1 minute, 50°C for 1 minute, and 72°C for 1 minute for 40 cycles in a thermal cycler. The PCR products of ∼800 bp were extracted using the FavorPrep Gel/PCR Purification Kit and sequenced at the Core Laboratory of the National Taiwan University Hospital.
Statistical Analysis
Stata software (StataCorp, College Station, TX) and MedCalc software (ver. 12.4.0; MedCalc Software, Ostend, Belgium) were used for statistical analyses. The mid-time point between the first detected HBeAg seroconversion and the preceding follow-up time was defined as the event time of HBeAg seroconversion. Univariable and multivariable Cox proportional-hazards regression were applied to calculate the relative hazard ratios, 95% confidence intervals (CI), and P values between the different factors. Kaplan-Meier analysis was used to assess survival. Factors achieving P values <0.05 in univariate analysis were included in the multivariate Cox proportional-hazards model. The Mann-Whitney U test was used to determine differences in continuous variables, while Fisher's exact test was used for categorical variables. Analysis of variance was used to evaluate differences among multiple groups. P < 0.05 was considered to be statistically significant. Bonferroni correction was used to adjust P values for multiple comparisons.
Results
Description of the Study Cohort
Of the initial cohort of 597 HBeAg-positive chronic HBV-infected patients, 163 were excluded (for persistent HBeAg+ and anti-HBe- in 157 subjects and for HBeAg reversion after HBeAg seroconversion during follow-up in six additional subjects). HBeAg seroconversion was confirmed in the 434 subjects followed to investigate the risk factors of HBeAg-negative hepatitis (Fig. 1). The six subjects who experienced HBeAg-reversion loss HBeAg transiently for 2-4 months all remained HBeAg+. The median HBV viral load and ALT 1 year after HBeAg reversion in these six subjects were 7.66 log10 IU/mL (range 6.18-9.45 log10 IU/mL) and 27 IU/L (range 12-29 IU/L), respectively.
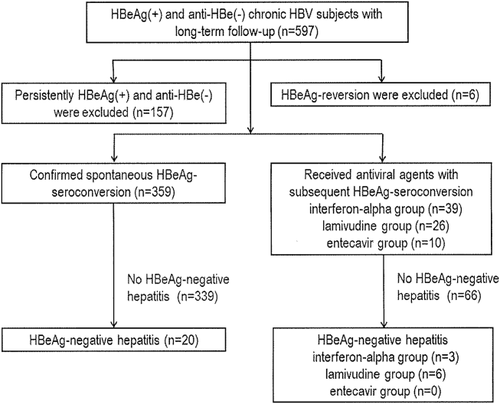
Among the 434 subjects in the cohort, the initial ALT levels at enrollment before HBeAg seroconversion were below UNL in 261 subjects (60.1%), between one-fold to two-fold UNL in 66 subjects (15.2%), and above two-fold UNL in 107 subjects (24.7%). During the follow-up period, 75 subjects experienced severe, protracted inflammation and received antiviral therapy according to the Asian Pacific Association for the Study of the Liver guidelines, including interferon-alpha (39 subjects), lamivudine (26 subjects), and entecavir (10 subjects), prior to HBeAg seroconversion. Another 359 subjects developed spontaneous HBeAg seroconversion without antiviral therapy. There were 3266 person-years of follow-up from the time of enrollment to HBeAg seroconversion and another 6579 person-years of follow-up after HBeAg seroconversion in the 434 study subjects. The median follow-up time after HBeAg seroconversion was 14.4 years (interquartile range 6.14-22.02 years) in these 434 subjects. A total of 8166 appointments were scheduled for these 434 subjects before HBeAg seroconversion, and 879 (10.8%) appointments were missed. After HBeAg seroconversion, 12,687 appointments were scheduled for these 434 subjects and 1608 (12.7%) appointments were missed.
Serum samples were available for HBV viral load determination in 315 subjects (72.6%) at the immune-tolerant phase before HBeAg seroconversion and in 313 subjects (72.1%) within 1 year of HBeAg seroconversion. Stored serum samples from 381 subjects (87.8%) were adequate for HBV genotyping, and serum samples for quantitative HBsAg titers were available from 243 (67.7%) of the 359 spontaneous HBeAg seroconverters during the immune-tolerant phase.
HBeAg-Negative Hepatitis in Spontaneous HBeAg Seroconverters
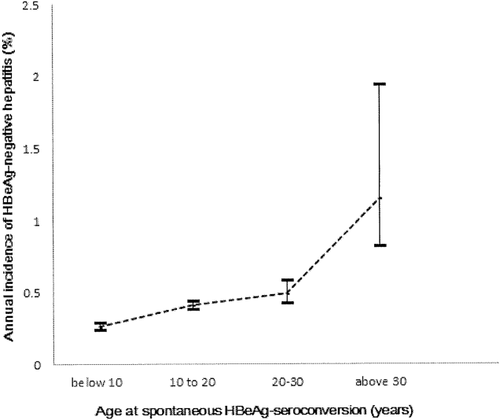
The overall HBeAg-negative hepatitis rate was 5.6% (20/359), and the overall annual incidence of HBeAg-negative hepatitis was 0.37% (95% CI 0.35%-0.39%) during the median 15.77 years (interquartile range 7.23-23.02 years) of follow-up after spontaneous HBeAg seroconversion. Among the 359 spontaneous HBeAg seroconverters, 232 (64.6%) subjects developed HBeAg seroconversion before 18 years of age. The age-specific annual incidence of HBeAg-negative hepatitis was 0.29% in subjects with spontaneous HBeAg seroconversion at childhood (before 18 years of age) and 0.64% beyond childhood (P < 0.05). The age-specific annual incidences of HBeAg-negative hepatitis after spontaneous HBeAg seroconversion were 0.26% (95% CI 0.24%-0.29%), 0.41% (95% CI 0.38%-0.44%), 0.49% (95% CI 0.42%-0.58%), and 1.15% (95% CI 0.82%-1.94%) in subjects with spontaneous HBeAg seroconversion at <10, 10-19, 20-29, and ≥30 years of age, respectively (Fig. 2).
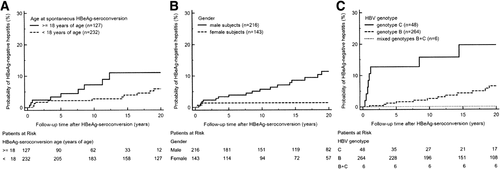
The HBV viral titers and HBsAg titers before HBeAg seroconversion were not different between subjects with and without HBeAg-negative hepatitis (Table 1). In the univariate Cox proportional-hazards survival analysis, male gender, genotype C HBV infection, and HBeAg seroconversion occurring beyond childhood (after 18 years of age) were all significant predictors of developing HBeAg-negative hepatitis (Tables 1 and 2). These phenomena were also consistent with Kaplan-Meier survival analysis and log rank testing (Fig. 3A-C). The significance of these predictors persisted in the multivariate Cox proportional-hazards survival analysis (Table 2). The peak ALT level during the immune-clearance phase before HBeAg seroconversion was not a significant risk factor for HBeAg-negative hepatitis on univariate survival analysis (P = 0.13).
| With HBeAg-Negative Hepatitis (n = 20) | Without HBeAg-Negative Hepatitis (n = 339) | |
|---|---|---|
| Male gender, n (%) | 18 (90%) | 198 (51.2%) |
| HBV genotype, n (%)a | ||
| B | 12 (60%) | 252 (84.6%) |
| C | 8 (40%) | 40 (13.4 %) |
| Mixed B+C | 0 (0%) | 6 (2.0%) |
| HBV viral load, log10 IU/mL, median (25th-75th percentile) | ||
| Immune-tolerant phaseb | 7.8 (7.2-7.9) | 7.5 (6.5-8.1) |
| Within 1 year after HBeAg seroconversionc | 4.0 (3.3-5.0) | 3.2 (1.3-3.1) |
| HBsAg titer at immune-tolerant phase, log10 IU/mL, median (25th-75th percentile)d | 4.3 (3.5-4.8) | 4.3 (3.3-4.7) |
| Initial enrollment age, years, median (25th-75th percentile) | 6.0 (1.0-11.4) | 7.3 (3.7-10.0) |
| Age at final medical visit, years, median (25th-75th percentile) | 30.7 (22.2-34.2) | 31.1 (26.3-36.0) |
| Serial serum ALT levels, IU/L, median (25th-75th percentile) | ||
| ALT level at enrollment | 40 (18-131) | 22 (12-56) |
| Peak ALT level before HBeAg seroconversion | 222 (119-566) | 141 (60-376) |
| ALT level at HBeAg seroconversion | 33 (19-157) | 15 (10-27) |
| ALT level 1 year after HBeAg seroconversion | 28 (19-135) | 13 (8-19) |
| Peak ALT level after HBeAg seroconversion | 173 (142-429) | 34 (25-56) |
- a HBV genotyping data were available in 20 and 298 of the study subjects with and without HBeAg-negative hepatitis, respectively.
- b The immune-tolerant phase of chronic HBV infection was defined as HBeAg+, anti-HBe-, and serum ALT <30 IU/L. HBV viral load was available in 18 and 232 subjects with and without HBeAg-negative hepatitis, respectively, during the immune-tolerant phase. The first available serum specimen collected in the immune-tolerant phase was subjected to the analysis.
- c HBV viral load was available in 20 and 234 subjects with and without HBeAg-negative hepatitis, respectively, within 1 year of HBeAg seroconversion.
- d The HBsAg titer was available in 11 and 232 subjects with and without HBeAg-negative hepatitis, respectively, during the immune-tolerant phase. The first available serum specimen collected in the immune-tolerant phase was analyzed.
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | P | Hazard Ratio | 95% CI | P | |
| HBeAg seroconversion age ≥18 (n = 127) vs. <18 years (n = 232) | 2.39 | 1.03-5.92 | 0.04 | 2.70 | 1.08-6.75 | 0.03 |
| Male (n = 216) vs. female (n = 143) | 5.74 | 1.33-24.73 | 0.02 | 6.69 | 1.55-28.92 | 0.01 |
| HBV genotypes C (n = 48) vs. B and mixed B+C (n = 270)a | 2.11 | 1.35-3.31 | 0.001 | 2.35 | 1.49-3.70 | <0.001 |
- Cox proportional-hazards regression was applied to the survival analysis for HBeAg-negative hepatitis occurrence after HBeAg seroconversion.
- a HBV genotype data available in 318 subjects with spontaneous HBeAg seroconversion.
HBeAg-Negative Hepatitis in Subjects With and Without Antiviral Therapy Prior to HBeAg Seroconversion
The cumulative incidence of HBeAg-negative hepatitis was significantly higher in those administered lamivudine therapy before HBeAg seroconversion (23.1%) than in those with the other therapy types (Table 3). The mean overall annual incidence of HBeAg-negative hepatitis was higher in the lamivudine group (mean = 2.64%, 95% CI 2.04%-3.72%) than the interferon-alpha group (mean = 0.58%, 95% CI 0.50%-0.69%) and the spontaneous HBeAg seroconverters (mean = 0.37%, 95% CI 0.35%-0.39%).
| Spontaneous HBeAg Seroconverters (n = 359) | Lamivudine Group (n = 26) | Interferon-alpha Group (n = 39) | Entecavir Group (n = 10) | P | |
|---|---|---|---|---|---|
| Male gender, n (%) | 216 (60.2%) | 22 (84.6%) | 21 (53.8%) | 5 (50%) | 0.14 |
| HBeAg-negative hepatitis, n (%) | 20 (5.6%) | 6 (23.1%) | 3 (7.7%) | 0 (0%) | 0.02 |
| HBV genotypes, n (%)a | |||||
| B and mixed B+C | 270 (84.9%) | 15 (71.4%) | 34 (91.9%) | 3 (60%) | |
| C | 48 (15.1%) | 6 (28.6%) | 3 (8.1%) | 2 (40%) | 0.07 |
| Initial enrollment age, years, median (25th-75th percentile) | 7.3 (3.4-10.2) | 7.3 (4.1-9.9) | 6.9 (2.9-9.2) | 6.8 (2.7-15.7) | 0.65 |
| HBeAg seroconversion age, years, median (25th-75th percentile) | 13.3 (8.3-19.6) | 19.8 (9.7-25.5) | 16.0 (13.0-22.9) | 11.3 (6.0-16.7) | 0.07 |
| Serum ALT levels, IU/L, median (25th-75th percentile) | |||||
| ALT level at enrollment | 23 (12-60) | 22 (13-78) | 14 (10-24) | 91 (30-106) | 0.85 |
| Peak ALT level before HBeAg seroconversion | 154 (67-395) | 339 (191-593) | 269 (101-481) | 256 (147-313) | 0.001 |
| Peak ALT level after HBeAg seroconversion | 38 (26-60) | 61 (32-127) | 44 (27-80) | 41 (32-58) | 0.02 |
| HBV viral load at immune-tolerant phase, log10 IU/mL, median (25th-75th percentile)b | 7.4 (6.6-8.1) | 8.1 (7.3-8.4) | 7.8 (7.1-8.2) | 7.9 (6.7-8.2) | 0.05 |
- a HBV genotyping data were available in 318, 21, 37, and five subjects from the spontaneous HBeAg seroconverters, lamivudine, interferon-alpha, and entecavir groups, respectively.
- b HBV viral load was available in 250, 22, 34, and nine subjects during the immune-tolerance phase before HBeAg seroconversion from the spontaneous HBeAg seroconverters, lamivudine, interferon-alpha, and entecavir groups, respectively.
Male gender, HBeAg seroconversion occurring after childhood, HBV genotype C infection, and lamivudine therapy prior to HBeAg seroconversion were independent and significant predictors of HBeAg-negative hepatitis after HBeAg seroconversion in the entire cohort (Supporting Fig. S1A-D; Table 4). The significance of lamivudine therapy on HBeAg-negative hepatitis incidence remained apparent after adjusting for gender, HBV genotype, HBeAg seroconversion age, and peak serum ALT levels before HBeAg seroconversion (P = 0.03).
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | P | Hazard Ratio | 95% CI | P | |
| HBeAg seroconversion ≥18 (n = 162) vs. <18 years of age (n = 272) | 3.06 | 1.44-6.51 | 0.004 | 2.46 | 1.07-5.64 | 0.03 |
| Male (n = 264) vs. female (n = 170) | 4.01 | 1.39-11.51 | 0.01 | 3.15 | 1.06-9.32 | 0.04 |
| HBV genotype C (n = 59) vs. B and mixed B+C (n = 322)a | 3.88 | 1.83-8.22 | <0.001 | 4.40 | 1.94-9.98 | <0.001 |
| Prior lamivudine therapy (n = 26) vs. spontaneous HBeAg seroconverters (n = 359) | 1.80 | 1.32-2.46 | <0.001 | 1.42 | 1.02-1.98 | 0.03 |
| Prior interferon-alpha therapy (n = 39) vs. spontaneous HBeAg seroconverters (n = 359) | 1.47 | 0.44-4.97 | 0.53 | — | — | — |
- Cox proportional-hazards regression was applied to the survival analysis for the occurrence of HBeAg-negative hepatitis after HBeAg seroconversion. Because the number of entecavir-treated patients was small (n = 10), we did not further analyze the effect of entecavir in this statistical model.
- a HBV genotype data were available in 381 subjects.
Differences in HBV BCP and Precore/Core Gene Sequences
Of the 20 HBeAg-negative hepatitis subjects, 15 had paired serum samples available in both the inactive phase and HBeAg-negative hepatitis phase after HBeAg seroconversion (Supporting Table S1). Serum HBV viral load increased significantly from the inactive phase to the HBeAg-negative hepatitis phase (3.92 ± 1.71 versus 5.44 ± 1.43 log10 copies/mL; P = 0.04). The prevalence of the HBV BCP gene C1773T mutation decreased with borderline significance (46.7% to 0%, P = 0.006) from the inactive phase to the HBeAg-negative hepatitis phase after HBeAg seroconversion in these 15 subjects after Bonferroni correction. The BCP and precore/core gene mutation patterns remained unchanged in subjects without HBeAg-negative hepatitis.
Serum was collected during the HBeAg-negative hepatitis phase for 18 of these 20 HBeAg-negative hepatitis subjects and used to compare the HBV BCP and precore/core gene sequences with HBeAg-negative hepatitis and 36 age-matched, gender-matched, HBV genotype-matched, and follow-up time-matched subjects without HBeAg-negative hepatitis after spontaneous HBeAg seroconversion (Supporting Table S2). The serum viral titers during the HBeAg-negative hepatitis phase in these 18 HBeAg-negative hepatitis subjects were significantly higher than those of the 36 matched controls (5.41 ± 1.40 versus 2.90 ± 1.02 log10 copies/mL; P < 0.001).
During the HBeAg-negative hepatitis phase, chronic HBV-infected subjects with HBeAg-negative hepatitis harbored more mutations (A1762T/G1764A, C2063A, and A2131C) than did the age-matched, gender-matched, HBV genotype-matched, and follow-up time-matched control subjects without HBeAg-negative hepatitis (P < 0.003; Supporting Table S2). We also found no significant difference in HBV gene sequence in subjects who developed HBeAg-negative hepatitis spontaneously or following lamivudine-related and interferon-alpha-related HBeAg seroconversion (Supporting Table S3).
Discussion
This large-scale, long-term, prospective follow-up cohort study (from young children to adults) indicated that the age-specific annual incidences of HBeAg-negative hepatitis were 0.29% and 0.64% in subjects with spontaneous HBeAg seroconversion before versus after 18 years of age, respectively. Data from chronic HBV-infected patients with spontaneous HBeAg seroconversion at <16 years of age related to HBeAg-negative hepatitis have not been reported in the literature. In a prior study from Taiwan, the estimated incidences of HBeAg-negative hepatitis were 2.9%, 3.8%, and 8.5% in subjects with spontaneous HBeAg seroconversion at 16-30, 30-40, and >40 years of age, respectively.5, 6 These differences may be attributable to differences in the studied cohorts. For example, the majority of subjects (70.5%) in the previous cohorts had serum ALT levels greater than two-fold UNL at enrollment. In contrast, the majority of our study population (>75%) had ALT levels that were normal or less than two-fold UNL at the time of enrollment, and the median enrollment age in our cohort was 7.22 years. In addition, all subjects in the previous cohorts were recruited after 16 years of age, with an approximate mean enrollment age of 30 years. Furthermore, prior studies defined HBeAg-negative hepatitis as HBV DNA ≥20,000 IU/mL with ALT greater than two-fold ULN; however, in this study, we used a cutoff of HBV DNA ≥2000 IU/mL with ALT greater than two-fold ULN for 6 months as defined by the 2012 Asian Pacific Association for the Study of the Liver consensus statement.22 The definition of HBeAg-negative hepatitis has changed in recent years based on reports describing the long-term clinical outcomes of HBV,22, 23, 27-29 which may account for differences in reported incidences of HBeAg-negative hepatitis.5, 6, 27, 28 Moreover, the previously reported age-specific annual incidence of HBeAg-negative hepatitis in adults may reflect a population more prone to inflammation with delayed HBeAg seroconversion in adulthood.5, 6 In contrast, our study provided an estimation of the risk for HBeAg-negative hepatitis that more closely resembled the natural history in young HBeAg seroconverters in Taiwan.
Host genetic factors are linked with HBeAg seroconversion age and might be associated with different HBeAg-negative hepatitis risks.30-32 Recent animal studies have shown that androgen increased HBV gene transcription, which was repressed by the estrogen pathway.33-35 Serum testosterone level in chronic HBV-infected males was also associated with the severity of hepatocyte damage in our previous study.36 The difference in HBeAg-negative hepatitis risk between genders is potentially linked to differences in risks of chronic liver insufficiency, liver cirrhosis, and hepatocellular carcinoma.
A recent study analyzed nucleos(t)ide analogue-induced HBeAg seroconverters and concluded that all carried a higher risk of HBeAg-negative hepatitis.19 Because 93% of the studied adults received lamivudine in that study, the observed effects may not be applicable to other nucleos(t)ide analogues with higher genetic barriers.19 In this study, only lamivudine was associated with the risk of HBeAg-negative hepatitis. Because the subjects receiving antiviral therapy experienced more severe inflammation with higher peak ALT levels than spontaneous HBeAg seroconverters, host factors and host-virus interactions may differ between subjects with and without antiviral treatment before HBeAg seroconversion. The increased risk of HBeAg-negative hepatitis in lamivudine-treated patients remained evident in a statistical model adjusted for HBV genotype, gender, HBeAg seroconversion age, and peak ALT levels before HBeAg seroconversion. There was no difference in the risk of HBeAg-negative hepatitis between the interferon-alpha group and the spontaneous HBeAg seroconverters in this study. Hence, interferon-alpha may be a better first-line therapy than lamivudine for avoiding HBeAg-negative hepatitis. The number of entecavir-treated patients in this cohort study was small, and the insignificance of entecavir may be associated with potential type II errors. Future larger-scale studies with adequate sample sizes are needed to better evaluate the effects of entecavir. Our data implied that optimal management prompting HBeAg seroconversion in childhood might minimize the lifelong risk of HBeAg-negative hepatitis in chronic HBV-infected patients.37
HBV BCP and precore/core gene mutations resulting from host immune selection pressure have been reported in the immune-clearance phase.7, 8, 37-39 In this study, we documented that, before the development of HBeAg-negative hepatitis, HBeAg-negative hepatitis subjects carried more HBV BCP and precore/core gene mutations after spontaneous HBeAg seroconversion than subjects without HBeAg-negative hepatitis. In addition, because all subjects included in this cohort study were infected by HBV genotype B or C, the results may not be generalized to subjects from different ethnic groups and with different HBV genotypes. Future studies of other ethnic groups and HBV genotypes are necessary to compensate for this limitation.
In conclusion, male gender, HBV genotype C infection, postchildhood HBeAg seroconversion, and prior lamivudine therapy were predictors of HBeAg-negative hepatitis after HBeAg seroconversion in our study cohort. HBeAg-negative hepatitis subjects carried more HBV BCP and precore/core gene mutations immediately after HBeAg seroconversion. Selecting for these mutations during HBeAg seroconversion might lead to breakthroughs in our understanding of alterations from the inactive phase to the HBeAg-negative hepatitis phase after HBeAg seroconversion. HBeAg seroconversion in childhood predicts a lower risk of HBeAg-negative hepatitis in later life. In chronic HBV-infected subjects suffering from the severe inflammatory phase in childhood, an adequate and effective antiviral therapy, such as interferon-alpha-achieved HBeAg seroconversion in childhood, may be beneficial in adulthood.
Acknowledgment
We thank Ms. Hui-Fang Lee from the Department of Pediatrics, National Taiwan University Children's Hospital, for data management and laboratory assistance.