Na+/H+ exchanger regulatory factor 1 knockout mice have an attenuated hepatic inflammatory response and are protected from cholestatic liver injury
Potential conflict of interest: Dr. Boyer consults for AbbVie.
This work was supported by National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases grants DK R37 25636 and DK P30-34989.
Abstract
The intercellular adhesion molecule 1 (ICAM-1) is induced in mouse liver after bile duct ligation (BDL) and plays a key role in neutrophil-mediated liver injury in BDL mice. ICAM-1 has been shown to interact with cytoskeletal ezrin-radixin-moesin (ERM) proteins that also interact with the PDZ protein, Na+/H+ exchanger regulatory factor 1 (NHERF-1/EBP50). In NHERF-1−/− mice, ERM proteins are significantly reduced in brush-border membranes from kidney and small intestine. ERM knockdown reduces ICAM-1 expression in response to tumor necrosis factor alpha. Here we show that NHERF-1 assembles ERM proteins, ICAM-1 and F-actin into a macromolecule complex that is increased in mouse liver after BDL. Compared to wild-type (WT) mice, both sham-operated and BDL NHERF-1−/− mice have lower levels of activated ERM and ICAM-1 protein in the liver accompanied by significantly reduced hepatic neutrophil accumulation, serum alanine aminotransferase, and attenuated liver injury after BDL. However, total bile acid concentrations in serum and liver of sham and BDL NHERF-1−/− mice were not significantly different from WT controls, although hepatic tetrahydroxylated bile acids and Cyp3a11 messenger RNA levels were higher in NHERF-1−/− BDL mice. Conclusion: NHERF-1 participates in the inflammatory response that is associated with BDL-induced liver injury. Deletion of NHERF-1 in mice leads to disruption of the formation of ICAM-1/ERM/NHERF-1 complex and reduction of hepatic ERM proteins and ICAM-1, molecules that are up-regulated and are essential for neutrophil-mediated liver injury in cholestasis. Further study of the role of NHERF-1 in the inflammatory response in cholestasis and other forms of liver injury should lead to discovery of new therapeutic targets in hepatic inflammatory diseases. (Hepatology 2015;62:1227-1236)
Abbreviations
-
- Ab
-
- antibody
-
- ALP
-
- alkaline phosphatase
-
- ALT
-
- alanine aminotransferase
-
- BDL
-
- bile duct ligation
-
- Ccl
-
- (C-C motif) ligand
-
- co-IP
-
- coimmunoprecipitation
-
- Cxcl
-
- (C-X-C motif) ligand
-
- ECs
-
- endothelial cells
-
- EBP50
-
- ERM-binding phosphoprotein 50
-
- ERM
-
- ezrin-radixin-moesin
-
- ESI
-
- electrospray ionization
-
- H&E
-
- hematoxylin and eosin
-
- HUVECs
-
- human umbilical vein endothelial cells
-
- ICAM-1
-
- intercellular adhesion molecule 1
-
- IHC
-
- immunohistochemistry
-
- IP
-
- immunoprecipitation
-
- mRNA
-
- messenger RNA
-
- MS
-
- mass spectrometry
-
- NF-κB
-
- nuclear factor kappa B
-
- NHERF-1
-
- Na+/H+ exchanger regulatory factor 1
-
- PCR
-
- polymerase chain reaction
-
- PIP2
-
- phosphatidylinositol 4,5-bisphosphate
-
- PIP5Kiβ
-
- type I PIP5 kinase beta
-
- PMHs
-
- primary mouse hepatocytes
-
- SD
-
- standard deviation
-
- TEM
-
- transendothelial migration
-
- TNF-α
-
- tumor necrosis factor alpha
-
- WT
-
- wild type
Cholestasis is characterized by rapid elevation of bile acid levels in the liver and plasma, followed by hepatocyte injury and bile duct proliferation, and, if progressive, liver fibrosis and cirrhosis.1, 2 Though various causes of cholestasis have been well described, the molecular mechanisms of cholestatic liver injury remain largely undefined. One major hypothesis notes that the accumulation of highly toxic hydrophobic bile acids within hepatocytes is the direct cause of hepatocellular death.3-5 This hypothesis is based mainly on work in cultured rat hepatocytes treated with high concentrations (50 or 100 µM) of proapoptotic bile acids, such as glycochenodeoxycholic acid and taurolithocholic acid,6-9 as well as in mice fed lithocholic acid.10 However, studies using bile duct ligation (BDL), a well-established cholestasis model, suggest that cholestatic liver injury may be caused largely by the inflammatory response involving neutrophil-mediated liver cell necrosis.11, 12 This view is supported by the infiltration of neutrophils around the site of injury in mouse liver within 8 hours after BDL and their predominance during this acute phase of liver injury.12, 13 In addition, the bile acid species that increase the most in concentration in serum after BDL in mice are relatively nontoxic hydrophilic bile acids, such as taurocholate, β-muricholic acid and tauro-β-muricholic acid. Exposure of cultured mouse hepatocytes to even millimolar concentrations of a mixture of these bile acids does not cause apoptosis or necrosis, but substantially induces expression of proinflammatory genes, such as intercellular adhesion molecule 1 (ICAM-1).14
ICAM-1 is an immunoglobulin-like cell adhesion molecule that is expressed at low levels in endothelial cells (ECs) under normal conditions. After BDL, ICAM-1 is up-regulated in both hepatic endothelial and parenchymal cells.15 ICAM-1 mediates neutrophil-endothelial as well as neutrophil-hepatocellular interactions by binding to β2-integrins that are expressed on the neutrophil cell surface16, 17 (Fig. 1). Liver necrosis is dramatically reduced in ICAM-1-deficient mice after BDL and is accompanied by a decrease in hepatic infiltration of neutrophils, suggesting that liver injury induced by BDL is neutrophil dependent.15
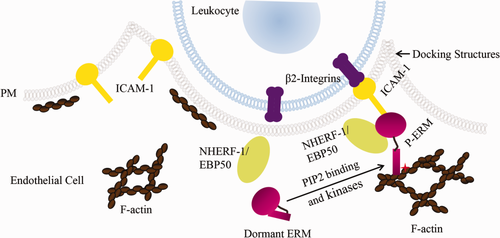
ICAM-1 and ERM proteins participate in leukocyte transendothelial migration. ICAM-1 is localized at the apical membrane of ECs and is up-regulated under inflammatory conditions. Upon leukocyte-endothelium interaction, ICAM-1 interacts with β2-integrins that are expressed on the cell surface of leukocytes and is connected to the underlying F-actin network through scaffolding proteins such as activated ERM proteins in microvilli-like membrane projections. These “docking” structures anchor and partially embrace the leukocyte to promote firm leukocyte adhesion and initiate leukocyte TEM. NHERF-1/EBP50, a PDZ protein, interacts with ERM proteins. We propose, in the current study, that NHERF-1 assembles ERM proteins, ICAM-1 and F-actin into a macromolecule complex in the cell cortex of sinusoidal ECs as well as hepatocytes in mouse liver and that this complex participates in neutrophil transendothelial and -hepatocyte migration after BDL. Abbreviation: PM, plasma membrane.
Recently, ezrin-radixin-moesin (ERM) proteins have been discovered to also influence leukocyte migration across the endothelial barrier to the site of inflammation. ERM proteins are a family of homologous proteins that interact with numerous partners, including membrane proteins, phospholipids, actin cytoskeleton, and signaling molecules, and organize multiprotein complexes that subserve diverse functions, including cell morphogenesis, endocytosis/exocytosis, adhesion and migration,18-20 in specific cellular compartments. In tumor necrosis factor alpha (TNF-α)-stimulated human umbilical vein endothelial cells (HUVECs), activated ERM proteins have been shown to interact with ICAM-1 and vascular cell adhesion molecule 1 (VCAM-1).21 Together with filamentous actin, these proteins form microvilli-like membrane projections called docking structures that anchor and partially embrace the leukocyte to promote firm leukocyte adhesion and initiate leukocyte transendothelial migration21, 22 (Fig. 1). ERM proteins play a role in ICAM-1 protein expression and increases in cellular permeability in response to inflammation. ERM knockdown inhibits ICAM-1 up-regulation in HUVECs as well as cytoskeletal rearrangement and permeability increases induced by TNF-α in pulmonary microvascular ECs.23, 24
ERM proteins exist in a dormant conformation in the cytoplasm and become activated upon binding to phosphatidylinositol 4,5-bisphosphate (PIP2) and phosphorylation by kinases. Phosphorylated ERM can bind to membrane proteins directly or indirectly by binding to the Na+/H+ exchanger regulatory factor 1 (NHERF-1). NHERF-1, also known as ERM-binding phosphoprotein 50 (EBP50), is abundantly expressed beneath apical membranes of polarized epithelial cells, such as hepatocytes and bile duct epithelial cells.25 It is a scaffolding protein that interacts with membrane proteins through its two PDZ domains and therefore assembles and coordinates functionally related signaling proteins into integrated macromolecule complexes.26 NHERF-1 interacts with ERM proteins through its ERM-binding motif and regulates expression of ERM proteins at the apical membrane of polarized cells. In NHERF-1−/− mice, ERM proteins are significantly reduced in brush-border membranes from kidney and small intestine.27 However, the roles of NHERF-1 and ERM proteins in neutrophil-mediated liver injury in cholestasis remain unclear. We hypothesized that NHERF-1 assembles ERM proteins, ICAM-1 and F-actin into a macromolecule complex that plays a role in neutrophil infiltration in mouse liver and that deficiencies in NHERF-1 disrupt the formation of this complex and affect expression of hepatic ERM proteins and ICAM-1, thereby influencing neutrophil accumulation and cholestatic liver injury induced by BDL.
Materials and Methods
Animals and Cells
For additional details on animals and cells used in this study, see the Supporting Methods. NHERF-1–/– mice were generated as previously described.28 Wild-type (WT) C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Age-matched (13-17 weeks) male mice were used for the present studies. Animals were housed in a temperature- and humidity-controlled room under a light cycle with free access to food and water. BDL was performed under sterile conditions, as previously described from this laboratory.29 Control animals underwent sham surgery in which the bile duct was exposed, but not ligated. Tissues and serum were collected 7 days postsurgery. Mice were fasted overnight and all animals were sacrificed between 8 am and 11 am. Tissues were flushed with normal saline, snap frozen in liquid nitrogen, and stored at −80°C until used. All experimental protocols were approved by the local animal care and use committee, according to criteria outlined in the Guide for the Care and Use of Laboratory Animals prepared by the National Academy of Sciences, as published by the National Institutes of Health (NIH publication 86-23, revised 1985).
Coimmunoprecipitation
Coimmunoprecipitation (co-IP) was performed using a Thermo Scientific Pierce Co-Immunoprecipitation Kit (Pierce Biotechnology, Rockford, IL), according to the manufacturer's instructions. Briefly, mouse liver tissue was homogenized in immunoprecipitation (IP) lysis/wash buffer (25 mM of Tris, 150 mM of NaCl, 1 mM of ethylenediaminetetraacetic acid, 1% NP-40, and 5% glycerol [pH 7.4], supplemented with protease and phosphatase inhibitor cocktails; Pierce), and tissue debris was removed by centrifugation at 13,000g for 10 minutes. Four milligrams of liver protein was precleared with control agarose resin and incubated overnight at 4°C with agarose resin coupled with a rat anti-mouse ICAM-1 antibody (Pierce). A co-IP reaction using inactivated agarose resin from the co-IP kit incubated with liver lysates served as a negative control for the experiment. After washing four times with IP lysis/wash buffer, the resin was eluted with elution buffer (pH 2.8) and eluate analyzed by immunoblotting.
Liver Histology and Neutrophil Staining
Formalin-fixed liver tissue was paraffin embedded and sections were stained with hematoxylin and eosin (H&E). Necrosis was assessed by blinded morphometric analysis. Immunohistochemistry (IHC) was performed using a rat monoclonal antibody (Ab) to Ly-6B.2 alloantigen (AbD Serotec, Kidlington, UK) and a peroxidase/3,3’-diaminobenzidine substrate kit (Vector Laboratories, Burlingame, CA). Neutrophil accumulation was quantitated with ImageJ software (NIH, Bethesda, MD).
Bile Acid Measurements and Liver Function Tests
Liver tissue was extracted in 75% ethanol (100 mg/mL) for bile acid measurement. Total bile acid concentration in serum and extracted liver was measured using an assay kit (Diazyme Laboratories, Poway, CA). Serum levels of alanine aminotransferase (ALT) and alkaline phosphatase (ALP) were measured using the COBAS MIRA system (Roche Diagnostics, Indianapolis, IN) by the Analytical Core of the Mouse Metabolic Phenotyping Center at Yale University (U24 DK059635).
Bile Acid Analysis
Liver extracts were analyzed by nano-electrospray ionization (ESI) mass spectrometry (MS). Bile acid analyses were performed on a mass spectrometer (PerkinElmer Sciex API-III; PerkinElmer, Edmonton, Alberta, Canada) modified with a nano-ESI source (Protana), as previously described.30
Immunoblotting
Proteins in mouse liver lysates, co-IP complex, and primary mouse hepatocyte (PMH) lysates were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes. Primary Abs (Supporting Table 1) were incubated overnight at 4°C. Densitometric scanning of protein bands was performed using TotalLab TL100 software (TotalLab Ltd, Newcastle upon Tyne, UK).
Statistical Analysis
All data were subjected to outlier detection and analyzed using the nonparametric Kruskal-Wallis’ and Mann-Whitney's tests offered in GraphPad Prism (GraphPad Software Inc., La Jolla, CA). Values are expressed as the mean ± standard deviation (SD). A P value of less than 0.05 was considered significant.
Results
Expression of NHERF-1 and ERM Proteins as Well as Formation of ICAM-1/ERM/NHERF-1 Complex Are Increased in Mouse Liver After BDL
To examine the roles of NHERF-1 and ERM proteins in neutrophil-mediated liver injury in cholestasis, we first examined expression of NHERF-1 and ERM proteins in liver whole-cell lysates of sham-operated and bile duct–ligated WT mice by immunoblotting. Compared to the sham group, expression levels of both NHERF-1 and total ERM proteins in BDL mouse liver were significantly increased (P < 0.05; Fig. 2A). Given that NHERF-1 is an ERM-binding protein and ERM proteins have been shown to interact with ICAM-1 and F-actin,21 we tested potential complex formation by these molecules in WT mouse liver. Co-Ip was performed with liver lysates of sham-operated and bile duct–ligated mice using an anti-ICAM-1 Ab. We found that both NHERF-1 and ERM proteins were detected in the Co-IP complex along with ICAM-1 (Fig. 2B), confirming our hypothesis that ICAM-1, ERM proteins, NHERF-1 and F-actin form a macromolecule complex in mouse liver. We also observed that, compared to the sham group, there was a dramatic increase in abundance of ICAM-1, ERM proteins, and NHERF-1 in the co-IP complex from BDL mouse liver, suggesting that this complex plays a role in neutrophil-mediated liver injury after BDL.
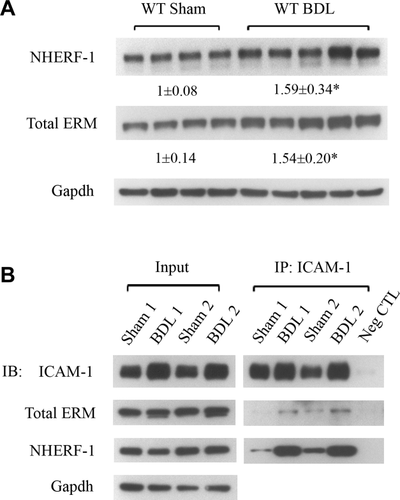
Expression of NHERF-1 and ERM proteins as well as formation of ICAM-1/ERM/NHERF-1 complex are increased in mouse liver after BDL. (A) Representative immunoblottings of whole-cell lysates from WT mouse liver tissue showing significant increase in levels of NHERF-1 and total ERM proteins in WT BDL mice, compared to sham-operated mice. The relative ratio of the respective protein bands in sham and BDL mice was determined by densitometric analysis and normalized to Gapdh. The amount of protein from the sham group is set as 1. Values represent the means ± SD of 4 or 5 animals in each group. *P < 0.05. (B) Representative immunoblottings of co-IP. Liver lysates of sham-operated or BDL WT mice were immunoprecipated with an anti-ICAM-1 Ab. In each co-IP reaction, 10 µg of the Ab was immobilized to 25 µL of the agarose resin and incubated with 4 mg of precleared liver protein. The lysates (input) and the IP complex were immunoblotted with anti-ICAM-1, anti-total ERM, anti-NHERF-1, or anti-Gapdh as indicated; n = 4 in the sham or BDL group. Note the dramatic increase in the abundance of ICAM-1, ERM proteins, and NHERF-1 in the co-IP complex from BDL liver lysates, compared to sham liver lysates. Abbreviations: Gapdh, glyceraldehyde 3-phosphate dehydrogenase; IB, immunoblotting; Neg CTL, negative control.
NHERF-1−/− Mice Have Reduced ERM and ICAM-1 Protein Expression in Liver and Hepatocytes
To test whether NHERF-1 deficiency affects ERM protein expression in the liver, we next examined expression levels of both total and phosphorylated ERM proteins in liver of WT and NHERF-1−/− mice by immunoblotting. Expression of hepatic total ERM proteins in NHERF-1−/− sham mice was reduced to 52% of WT mice (P < 0.05), and expression of phosphorylated ERM proteins in NHERF-1−/− mouse liver was further reduced to 38% of WT mice (P < 0.01; Fig. 3A). After BDL, phosphorylated ERM proteins remained reduced in NHERF-1−/− mouse liver, indicating that activated ERM proteins are reduced in liver of NHERF-1−/− BDL mice. Interestingly, expression of hepatic total ERM proteins in NHERF-1−/− BDL mice was not significantly different from WT mice, suggesting compensatory up-regulation of ERM proteins in NHERF-1−/− mouse liver after BDL.
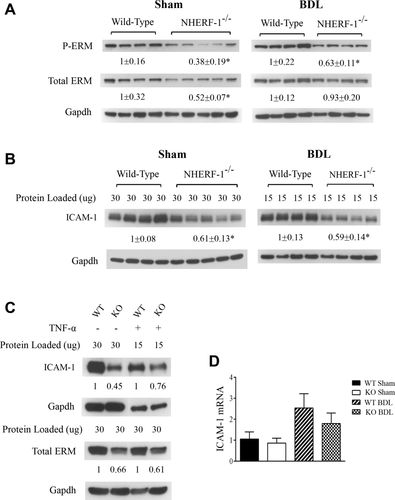
NHERF-1−/− mice have significantly reduced ERM and ICAM-1 protein expression in liver and hepatocytes. Representative immunoblottings of whole-cell lysates from mouse liver tissue (A and B) and PMHs (C) demonstrating significant reduction in total ERM, phosphorylated ERM, and ICAM-1 proteins in NHERF-1−/− sham and BDL mice, compared to WT sham and BDL mice. In (A), (B), and (C), the relative ratio of the respective protein bands in WT and NHERF-1−/− mice was determined by densitometric analysis and normalized to Gapdh. The amount of protein from the WT is set as 1. In (A) and (B), values represent the means ± SD of 4 or 5 animals in each group. In B, 30 µg of protein from each of the sham samples was loaded onto the gel, whereas only 15 µg of protein from each of the BDL samples was loaded in order to avoid overloading. In (C), primary hepatocytes were isolated from WT and NHERF-1−/− mice and cultured as described in the Supporting Methods. Whole-cell lysates of untreated primary hepatocytes or primary hepatocytes treated with TNF-α (10 ng/mL) for 6 hours were subjected to immunoblotting; n = 2 in each group. (D) Relative levels of ICAM-1 mRNA in liver of WT and NHERF-1−/− mice quantified by real-time PCR. Data are normalized to Gapdh, and the amount of hepatic ICAM-1 mRNA from WT sham mice is set as 1. Values represent the means ± SD of 6-9 individual animals in each group. KO, NHERF-1−/−. *P < 0.05. Abbreviation: Gapdh, glyceraldehyde 3-phosphate dehydrogenase.
Given that ERM proteins have been shown to play a role in ICAM-1 expression in nonhepatic ECs,23, 24 we then quantified protein expression of ICAM-1 in both sham-operated and BDL mouse liver. As expected, ICAM-1 protein expression was significantly up-regulated in both the WT and NHERF-1−/− mouse liver 7 days after BDL (note that in Fig. 3B, compared to sham samples, only half the amount of total protein was loaded onto the gel for BDL samples to avoid overloading). However, compared to WT mice, hepatic ICAM-1 expression level was 40% lower in both sham-operated and BDL NHERF-1−/− mice (P < 0.01; Fig. 3B). Using primary hepatocyte cultures, we also found that both ERM and ICAM-1 protein levels were dramatically reduced in NHERF-1−/− mouse hepatocytes compared to WT mouse hepatocytes, and these proteins remained reduced after TNF-α stimulation (Fig. 3C), indicating that NHERF-1 has a direct role in expression of ERM proteins and ICAM-1 as well as inflammatory response in hepatocytes. Further analysis of ICAM-1 messenger RNA (mRNA) expression by real-time polymerase chain reaction (PCR) showed that, compared to WT mice, hepatic ICAM-1 mRNA levels were not significantly different in NHERF-1−/− mice (Fig. 3D), suggesting that post-transcriptional regulation plays a primary role in reduced expression of hepatic ICAM-1 protein in sham-operated and BDL NHERF-1−/− mice. Together, these data demonstrated that NHERF-1 deficiency leads to reduction in expression of ERM proteins and ICAM-1 in hepatocytes as well as sham and BDL mouse liver.
Neutrophil Accumulation in Liver of NHERF-1−/− Mice Is Significantly Reduced After BDL
To examine whether disruption of formation of ICAM-1/ERM/NHERF-1 complex as well as decreased hepatic ERM protein and ICAM-1 expression in NHERF-1−/− mice result in reduced neutrophil infiltration in the liver, we assessed neutrophil accumulation in mouse liver by IHC staining. Whereas sham-operated WT and NHERF-1−/− mice had very few neutrophil infiltrates in liver parenchyma, a large number of neutrophils accumulated in and around necrotic foci of hepatocytes in the liver of WT BDL mice (Fig. 4A). In contrast, only a limited number of neutrophils were detected in the liver of NHERF-1−/− BDL mice and very few infiltrated into parenchyma. Statistical analysis with ImageJ on stained liver sections revealed positive staining in 0.47 ± 0.25% of the area in NHERF-1−/− BDL mice, a 47% reduction compared to the WT control that had 0.89 ± 0.32% area of positive staining (P < 0.05; Fig. 4B). Because β2-integrins on neutrophils interact with ICAM-1 and participate in neutrophil infiltration as well, we also examined β2-integrin expression on neutrophils from WT and NHERF-1−/− mice by flow cytometry. No significant difference was detected between the two groups (Supporting Fig. 1), suggesting that it is unlikely that reduced neutrophil infiltration in liver of NHERF-1−/− BDL mice was the result of a decrease in β2-integrin expression on the neutrophils of these mice.
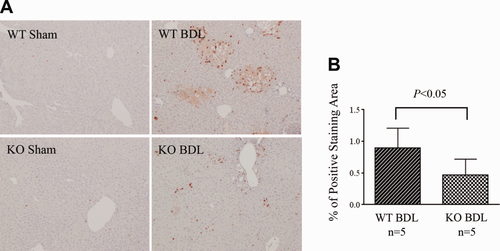
Neutrophil accumulation in the liver is significantly reduced in NHERF-1−/− BDL mice. (A). IHC staining of neutrophils on liver sections. Note that a large number of neutrophils accumulated in liver in WT BDL mice, mostly detected in and around the necrotic foci of hepatocytes. In contrast, only a limited number of neutrophils were detected in liver parenchyma of NHERF-1−/− BDL mice. (B) Morphometry of neutrophil accumulation in BDL mouse liver by ImageJ. Data represent means ± SD of percentage of positive staining area on 10 random images (magnification, ×10) per animal from 5 animals in each group. KO, NHERF-1−/−.
NHERF-1−/− Mice Are Protected From Liver Injury After BDL
Given that neutrophils play an important role in BDL-induced liver injury, we then tested whether reduction of neutrophil infiltration in livers of NHERF-1−/− mice results in reduced liver injury after BDL. Liver function analysis revealed that whereas serum levels of ALT in sham-operated WT and NHERF-1−/− mice were similar (24.7 ± 5.6 vs. 23.9 ± 11.9 U/L), NHERF-1−/− BDL mice had 70% lower serum ALT activities compared to WT BDL mice (238.1 ± 67.9 vs. 890.0 ± 325.9 U/L; P < 0.001; Table 1). In contrast, serum ALP levels were similar to WT BDL mice (780.0 ± 391.6 vs. 737.8 ± 204.6 U/L), consistent with the finding of comparable levels of bile duct proliferation on histological analysis (data not shown), even though NHERF-1−/− BDL mice had reduced levels of liver necrosis (as noted in Fig. 5). No injury was detected in sham-operated WT and NHERF-1−/− mice. Seven days after BDL, the WT group had 13.2 ± 5.0% of the area of necrotic foci. In contrast, the NHERF-1−/− BDL group had only 3.3 ± 3.8% of the area of necrotic foci, which is 75% less than WT BDL mice (P < 0.05; Fig. 5A,B).
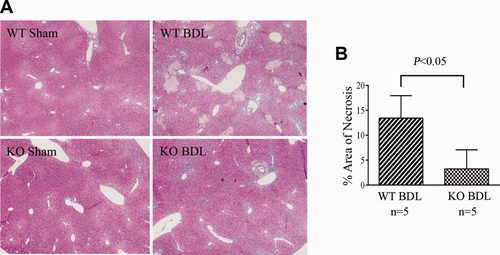
Liver necrosis was significantly lower in NHERF-1−/− BDL mice compared to WT BDL mice. (A) H&E staining of liver sections of sham-operated and BDL mice. (B) Morphometry of liver necrosis in BDL mice. Data represent means ± SD of 5 animals in each group. Note that no necrosis was detected in both WT and NHERF-1−/− sham mouse liver. However, the WT BDL group had 14% area of liver necrosis foci, whereas the NHERF-1−/− BDL group had only 3% area of liver necrosis foci. KO, NHERF-1−/−.
| Parameter | WT Sham | NHERF-1–/– Sham | WT BDL | NHERF-1–/– BDL |
| Serum ALT (U/L) | 24.7 ± 5.6 | 23.9 ± 11.9 | 890.0 ± 325.9 | 238.1 ± 67.9* |
| Serum ALP (U/L) | 73.7 ± 8.5 | 72.2 ± 10.2 | 737.8 ± 204.6 | 780.0 ± 391.6 |
| Serum bile acids (µM) | 8.15 ± 7.3 | 30.7 ± 31.4 | 278.3 ± 75.3 | 303.4 ± 78.0 |
| Liver bile acids (nmol/g liver) | 136.5 ± 46.0 | 152.1 ± 58.6 | 402.1 ± 127.2 | 347.5 ± 63.8 |
- Data represent mean ± SD of n = 5-10.
- *P<0.001, NHERF-1−/− BDL mice versus WT BDL mice.
To test whether the protective effects observed in NHERF-1−/− BDL mice were related to less cholestasis, we measured total bile acid levels in serum and liver extracts. Surprisingly, total bile acid concentrations in either serum or liver tissues of both sham and BDL NHERF-1−/− mice were not significantly different from WT controls (Table 1), suggesting that the reduced liver injury in NHERF-1−/− BDL mice was not the result of differences in bile acid concentrations in serum or liver. However, MS analysis of hepatic bile acid composition revealed that whereas sham-operated WT and NHERF-1−/− mice had similar percentages of di- (9.47 ± 2.54% vs. 10.71 ± 2.45%), tri-(86.23 ± 2.45% vs. 86.23 ± 2.45%), and tetrahydroxylated (4.3 ± 0.95% vs. 2.78 ± 1.27%) bile acids in the liver (P > 0.05), compared to 8.53 ± 2.62% in WT BDL mice, the percentage of tetrahydroxylated bile acids increased to 14.3 ± 0.74% in NHERF-1−/− BDL mouse liver (P < 0.01; Fig. 6A). Real-time PCR also demonstrated that hepatic Cyp3a11 mRNA levels in NHERF-1−/− BDL mice were significantly increased compared to WT BDL mice (P < 0.01; Fig. 6B), consistent with the change in bile acid profile and suggesting that up-regulation of Cyp3a11 provided an additional mechanism in the adaptive response to cholestatic liver injury.
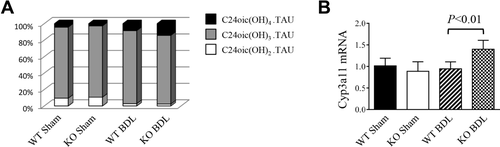
(A) Analysis of hepatic bile acid composition by MS. Dihydroxylated, trihydroxylated, and tetrahydroxylated bile acids from liver extract was quantified and normalized to the total detectable bile acids for each animal. Note that WT and NHERF-1−/− sham mice had similar percentages of dihydroxylated, trihydroxylated, and tetrahydroxylated bile acids. After BDL, the percentage of tetrahydroxylated bile acids increased to 14.3 ± 0.74% in NHERF-1−/− mice, which is significantly higher than the 8.53 ± 2.62% in WT BDL mice (P < 0.01); n = 5 or 6 in each group. (B) Quantification of hepatic Cyp3a11 mRNA levels by real-time PCR. Data are normalized to glyceraldehyde 3-phosphate dehydrogenase (Gapdh), and the amount of hepatic Cyp3a11 mRNA from WT sham mice is set as 1. Values represent the means ± SD of 5 or 6 animals in each group. KO, NHERF-1−/−.
Induction of Other Proinflammatory Genes in NHERF-1−/− Mice After BDL
Numerous studies indicate that other proinflammatory genes besides ICAM-1 are also up-regulated in mouse liver and hepatocytes after BDL or bile acid treatment.11, 14 To determine whether expression levels of cytokines TNF-α and interleukin-1β, as well as chemokines (C-X-C motif) ligand (Cxcl)−2, (C-C motif) ligand (Ccl)−2 and Ccl-7 are also affected in NHERF-1−/− mice, levels of hepatic mRNA were assessed in sham-operated and BDL mice by real-time PCR. Our results showed that all these genes were greatly induced after BDL in livers of both WT and NHERF-1−/− mice. Additionally, sham-operated NHERF-1−/− mice had significantly higher levels of hepatic TNF-α, Cxcl-2, Ccl-2, and Ccl-7 mRNA levels than WT control (P < 0.05; Supporting Fig. 2). However, NHERF-1−/− BDL mice showed a trend to lower Cxcl-2, Ccl-2, and Ccl-7 mRNA levels in the liver compared to WT BDL mice, although the reduction did not reach statistical significance (P > 0.05; Supporting Fig. 2).
Discussion
In this study, we tested the hypothesis that NHERF-1 assembles ERM proteins, ICAM-1 and F-actin into a macromolecule complex in mouse liver and deficiencies in NHERF-1 may affect expression of hepatic ERM proteins and ICAM-1, leading to reduced neutrophil accumulation and cholestatic liver injury induced by BDL. Our findings demonstrate that hepatic NHERF-1 and ERM proteins indeed co-immunoprecipitate with ICAM-1 and that formation of ICAM-1/ERM/NHERF-1 complex is increased after BDL. In addition, NHERF-1−/− mice have lower ERM and ICAM-1 protein expression in the liver and hepatocytes, in association with reduced hepatic neutrophil accumulation and attenuated liver injury after BDL. This attenuation in liver injury was also accompanied by an increase in synthesis of tetrahydroxylated bile acid in the liver that was independent of total serum or hepatic bile acid concentrations.
As a versatile scaffolding protein that binds multiple partners and recruits functionally related proteins into integrated macromolecule complexes, NHERF-1 has been shown to participate in expression, membrane localization and functional regulation of numerous membrane proteins, such as cystic fibrosis transmembrane conductance regulator and β2-adrenergic receptor.32, 33 In mouse liver, NHERF-1 has been detected beneath the apical membrane of hepatocytes and cholangiocytes and has been shown to regulate expression and function of multidrug resistance-associated protein 2, an adenosine triphosphate–binding cassette transporter that plays an important role in bile formation and detoxification.25, 31 Similarly, ERM proteins also function as cross-linkers between cortical actin filaments and membrane proteins, including ICAM-1. ICAM-1 plays a key role in adhesion and locomotion of adhered leukocytes to vascular ECs during leukocyte extravasation across the endothelial barrier and their recruitment to the site of inflammation.20 In normal liver, ICAM-1 is barely detectable along the sinusoids and portal venules. In both BDL mice and patients with extrahepatic obstructive cholestasis, ICAM-1 is markedly up-regulated in sinusoidal ECs and is induced in hepatocytes in areas of parenchymal injury where the intensity of this induction correlates with degree of liver damage.15, 34 Upon leukocyte-endothelium interaction, ICAM-1 becomes enriched in microvilli-like membrane projections and connects to the underlying F-actin through ERM proteins, forming a docking structure that anchors and partially embraces the leukocyte21 (see Fig. 1). Interaction between ICAM-1 and activated ERM proteins is essential for leukocyte transendothelial migration (TEM) given that deletion of a five-amino-acid segment in the intracellular domain of ICAM-1 abrogates its interaction with ERM proteins and significantly decreases leukocyte adhesion and subsequent TEM.35 Given the fact that increased formation of ICAM-1/ERM/NHERF-1 complex was detected in BDL mouse liver (Fig. 2) and that hepatocytes constitute approximately 80% of liver parenchymal cells, it is highly plausible that this macromolecule complex is formed in the cell cortex of sinusoidal ECs as well as hepatocytes and participates in neutrophil transendothelial and -hepatocyte migration after BDL. Deletion of NHERF-1 results in disruption of the formation of this complex as well as destabilization of ERM proteins and ICAM-1, leading to decreased cortical expression of these proteins, thereby reducing neutrophil transendothelial and -hepatocyte migration in the liver of NHERF-1−/− mice after BDL. On the other hand, deficiency in NHERF-1 might also attenuate response of neutrophils to chemoattractants in BDL mice. Mañes et al. showed that interaction between NHERF-1/EBP50 and type I PIP5 kinase beta (PIP5KIβ) is necessary for PIP5KIβ localization and for chemoattractant-induced polarization of neutrophils.36 NHERF-1 assembles the CXC chemokine receptor 2 and phospholipase C β2 in neutrophils into a macromolecular signaling complex critical for neutrophil migration and infiltration.37 It is possible that NHERF-1 also affects the function of neutrophils in a similar manner during cholestatic liver injury.
The nuclear factor kappa B (NF-κB) transcription factor plays an essential role in regulation of immune and inflammatory responses.38 Recently, Leslie et al.39 showed that NHERF-1/EBP50 expression increased upon TNF-α treatment in primary macrophages and vascular smooth muscle cells and that this induction was NF-κB dependent. Conversely, deletion of NHERF-1 resulted in impaired activation of NF-κB in these cells. In NHERF-1−/− mice, macrophage activation and recruitment to vascular lesions as well as vascular inflammation were reduced after lipopolysaccharide treatment. They also found that inflammatory stimuli promote formation of an NHERF-1/protein kinase C zeta complex at the cell membrane that induces NF-κB signaling. Therefore, they concluded that NHERF-1 and NF-κB participate in a feed-forward loop leading to increased macrophage activation and enhanced response of vascular cells to inflammation. It remains to be determined whether this feed-forward loop also applies to the inflammatory response during BDL-induced cholestasis. In the present study, we also observed that, compared to sham mice, WT BDL mice had increased NHERF-1 expression in the liver (Fig. 2), accompanied by up-regulation of total NF-κB p65 as well as phosphorylated NF-κB p65 (data not shown). In addition, expression of total NF-κB p65 as well as phosphorylated NF-κB p65 was significantly reduced in the liver of NHERF-1−/− BDL mice compared to WT BDL mice (data not shown). Activation of NF-κB has been detected in mouse hepatocytes and rat bile duct epithelium after BDL, although it was reported that bile acids do not activate NF-кB in primary rat hepatocytes.40-42 However, the role of NF-κB in cholestatic liver injury remains controversial. Miyoshi et al. reported that inhibition of NF-κB activation potentiated hepatocyte apoptosis and liver injury in BDL mice and concluded that activated NF-κB functions to reduce liver injury.40 In contrast, Demirbilek et al. showed that NF-κB inhibitors attenuated liver injury in BDL rats.41 Future studies on the function of NF-κB in the inflammatory response as well as the relationship between NHERF-1 and NF-κB in BDL mice would be of interest in further understanding of roles of NHERF-1 and NF-κB in cholestatic liver injury.
In summary, our study demonstrates that NHERF-1 assembles ERM proteins, ICAM-1 and F-actin into a macromolecule complex in mouse liver and deletion of NHERF-1 in mice leads to a reduced expression of hepatic ERM proteins and ICAM-1 molecules that are up-regulated and are essential for neutrophil migration and infiltration after BDL. NHERF-1 deficiency also increased synthesis of tetrahydroxylated bile acid in the liver after BDL. These multiple mechanisms protect NHERF-1−/− mice against cholestatic liver injury induced by BDL. Further study of the role of NHERF-1 in the inflammatory response in cholestatic and other forms of liver injury should lead to discovery of new therapeutic targets in cholestasis and other hepatic inflammatory diseases.
Acknowledgement
The authors thank Kathy Harry for excellent technical assistance.
References
Author names in bold designate shared co-first authorship