Pathological functions of interleukin-22 in chronic liver inflammation and fibrosis with hepatitis B virus infection by promoting T helper 17 cell recruitment
Potential conflict of interest: Nothing to report.
This work was supported by the The National Natural Science Foundation of China (31170865), the National Key Basic Research Program of China (2012CB519005 and 2009CB522507), the National Grand Program on Key Infectious Disease (2013ZX10002001-001-003 and 2012ZX10002-007-002), and the National Science Fund for Outstanding Young Scholars (81222024).
Abstract
It is well established that interleukin (IL)-22 has hepatoprotective and antifibrotic functions in acute liver injury models; however, its function in patients with liver fibrosis and liver cirrhosis (LC) remains obscure. In the current study, we demonstrated that expression of numerous IL-22 pathway-associated genes was significantly up-regulated in hepatitis B virus (HBV)-infected liver tissues, compared to normal controls, through microarray analysis. In agreement with these findings, liver-infiltrating IL-22+ cells were largely increased in HBV-infected patients with LC, compared to those without LC or healthy subjects, and were positively associated with liver fibrosis staging scores. Immunohistochemistry and flow cytometric analyses revealed that IL-22 was produced by multiple intrahepatic immune cells and, preferentially, by T-helper (Th) 17 cells in LC patients. In an HBV transgenic (Tg) mouse model of T-cell-mediated chronic liver inflammation and fibrosis, blockade of IL-22 attenuated hepatic expression of chemokine (C-X-C motif) ligand 10 and chemokine (C-C motif) ligand 20 (CCL20) and subsequently reduced Th17 recruitment and liver inflammation and fibrosis progression. In vitro treatment with IL-22 stimulated hepatic stellate cells (HSCs) to secrete several chemokines and subsequently promoted Th17 cell chemotaxis. Blocking C-X-C chemokine receptor type 3 or CCL20 reduced Th17 cell chemotaxis by IL-22-treated HSCs. Conclusions: IL-22 plays a pathological role in exacerbating chronic liver inflammation and fibrosis by recruiting hepatic Th17 cells in HBV-infected patients and HBV Tg mice. (Hepatology 2014;59:1331-1342)
Abbreviations
-
- Ab
-
- antibody
-
- ALT
-
- alanine aminotransferase
-
- AST
-
- aspartate aminotransferase
-
- BDL
-
- bile duct ligation
-
- CCL
-
- chemokine (C-C motif) ligand
-
- CCR
-
- C-C chemokine receptor
-
- CHB
-
- chronic hepatitis B
-
- CLDs
-
- chronic liver diseases
-
- CXCL
-
- chemokine (C-X-C motif) ligand
-
- CXCR
-
- C-X-C chemokine receptor
-
- DC
-
- dendritic cell
-
- ECM
-
- extracellular matrix
-
- FCM
-
- flow cytometric
-
- FoxP
-
- forkhead box protein
-
- G
-
- grading
-
- HAI
-
- histology activity index
-
- HBV
-
- hepatitis B virus
-
- HC
-
- healthy control
-
- HCV
-
- hepatitis C virus
-
- IFN
-
- interferon
-
- Ig
-
- immunoglobulin
-
- IHC
-
- immunohistochemistry
-
- IL
-
- interleukin
-
- HSC
-
- hepatic stellate cell
-
- LC
-
- liver cirrhosis
-
- LIL
-
- liver-infiltrating lymphocyte
-
- mRNA
-
- messenger RNA
-
- NK
-
- natural killer
-
- PMA
-
- phorbol 12-myristate 13-acetate
-
- R
-
- receptor
-
- S
-
- staging
-
- α-SMA
-
- alpha-smooth muscle actin
-
- γδTCR
-
- gamma-delta T-cell receptor
-
- Tg
-
- transgenic
-
- Th
-
- T helper
-
- Treg
-
- regulatory T cell
Liver fibrosis and cirrhosis are the major causes of liver-disease-related morbidity and mortality worldwide.1, 2 Liver fibrosis, occurring as a result of chronic liver inflammation or injuries, is generally resulted from the excessive accumulation of extracellular matrix (ECM) and eventually progresses to liver cirrhosis (LC). Hepatic stellate cell (HSC) activation is considered to be the key step for liver fibrogenesis through producing a large amount of alpha-smooth muscle actin (α-SMA) and collagens.3, 4 However, the mechanisms underlying the promotion of liver fibrosis progression remain obscure.
Emerging evidence indicates that host genetic,5, 6 virological,7, 8 and immunological factors influence liver fibrotic progression. Particularly, host immune components have been considered to actively modulate HSC activation and be ultimately responsible for liver fibrogenesis.1, 3, 4 It is commonly accepted that T helper (Th) 1 cytokines suppress fibrosis, whereas Th2 cytokines promote fibrosis.9 The importance of natural killer (NK)10, 11 and NKT cells12, 13 in regulating HSC activation and liver fibrosis has been demonstrated by cell depletion or inactivation in animal models. These studies suggest that liver-infiltrating lymphocytes (LILs) orchestrate highly complex immune responses, thereby regulating liver fibrogenesis. However, few studies have reported on the essential effects of these important immunological events on liver fibrosis, particularly in patients with chronic hepatitis B virus (HBV) infection.
Interleukin (IL)-22 recently has also been demonstrated to play a key role not only in controlling bacterial infection, but also in promoting tissue repair by up-regulating a variety of antiapoptotic, antioxidant, and proliferative genes in various types of epithelial cells, including hepatocytes.14-17 IL-22 is produced primarily by Th17,18 Th22,19 and activated NK and NKT cells.20 IL-22 receptors (R) including IL-22R1 and IL-10R2, is expressed exclusively on epithelial cells in various organs and ubiquitously in multiple types of cells, respectively.14, 16 By binding to IL-22 receptors, IL-22 appears to control tissue responses to inflammation. Regarding liver diseases, several lines of evidence support that IL-22 plays a beneficial role in preventing acute hepatocellular damage induced by CCl4, concanavalin A, Fas ligand,21, 22 or alcohol in animal models.23 However, IL-22 was also shown to play a pathological role in exacerbating chronic liver inflammation and injury in an HBV transgenic (Tg) mouse model.24 Additionally, IL-22 was reported to promote the proliferation of liver stem/progenitor cells and induce HSC senescence in IL-22-overexpressing mouse models with chronic liver injury.25, 26 Recent studies have revealed that IL-22 expression is up-regulated in livers of patients with chronic HBV24, 27, 28 or hepatitis C virus (HCV)28, 29 infection. These data suggest that IL-22 exhibits pro- and anti-inflammatory actions under different sets of conditions of liver diseases; however, it is illusive regarding when and why such divergent outcomes occur. Identification of the predominant cell types secreting IL-22 and how it is being regulated are also of importance in determining outcomes of chronic HBV infection.
The present study analyzed the expression profile and functional roles of intrahepatic IL-22 in HBV-infected patients and determined the effects of IL-22 blockade on progression of liver inflammation and fibrosis in HBV Tg mice. The findings uncover a pathological role of IL-22 in chronic liver inflammation and fibrosis that may facilitate the rational development of immunotherapeutic strategies targeting IL-22.
Patients and Methods
Study Subjects
Seventy-four chronic hepatitis B (CHB) patients and 36 LC patients were recruited for this study. All patients were diagnosed according to our previously described criteria30, 31 and had not taken antiviral therapies or immunosuppressive drugs within 6 months before sampling. Individuals with concurrent HCV, hepatitis D virus, or human immunodeficiency virus infection, autoimmune liver disease, or alcoholic liver disease were excluded. Forty-eight age- and sex-matched individuals were enrolled as healthy controls (HCs). The study protocol was approved by the ethics committee of our unit, and written informed consent was obtained from each subject. The basic characteristics of the enrolled subjects are listed in Table 1.
| Category | HC | CHB | LC |
|---|---|---|---|
| Cases | 48 | 74 | 36 |
| Age, years | 29 (21-48) | 31 (19-52) | 48 (25-68) |
| Gender, M/F | 38/10 | 56/18 | 28/8 |
| ALT, IU/L | ND | 72 (6-627) | 35 (8-218) |
| AST, IU/L | ND | 43 (14-481) | 47 (15-278) |
| Serum albumin, g/L | ND | 39 (32-50) | 33 (23-44) |
| Total bilirubin, μmol/L | ND | 14.4 (0.82-414) | 26.7 (7.1-181) |
| Direct bilirubin, μmol/L | ND | 5.4 (1.6-236) | 12.9 (4.0-301) |
| Prothrombin activity, % | ND | 92 (68-190) | 66.4 (22-101) |
| HBeAg, positive/negative | ND | 51/23 | 18/18 |
| Serum HBV levels, IU/mL | ND | 3.5 × 107 | 2.8 × 105 |
| (100-4.6 × 109) | (100-1.1 × 108) |
- Abbreviations: M, male; F, female; HBeAg, hepatitis B e antigen; ND, not determined.
Liver biopsies were collected from 30 CHB and 16 LC patients, and 10 healthy liver tissue samples were obtained from donors whose livers were used for transplantation. The degree of hepatic inflammation and fibrosis was graded using the modified histology activity index (HAI) described by Scheuer.32 In addition to the tissues used for pathological evaluation, liver biopsy specimens were employed for extraction of total RNA for hepatic messenger RNA (mRNA) expression profiling analysis or were homogenized for the isolation of LILs and/or embedded in Tissue Tek for in situ immunohistochemical (IHC) staining.33
Animal Experiments
HBV Tg mice C57BL/6J-TgN (Alb1 HBV) 44Bri, which express S, pre-S, and X genes under the mouse albumin promoter, were used in this study as previously reported.34 To induce chronic liver inflammation and fibrosis, mice were treated with anti-mouse CD137 agonist monoclonal antibody (Ab; clone 2A, 200 μg/dose) five times at 7-day intervals, according to our previous protocols,35 and administered with anti-IL-22 (100 μg/dose; 8E11.9; Genentech, South San Francisco, CA) or isotype control 3 days before each injection of anti-CD137. On day 5 after the final anti-CD137 injection, mice were sacrificed and livers were collected for pathological and immunological evaluation. All mice were maintained in the animal facility at the Institute of Biophysics, Chinese Academy of Sciences (Beijing, China). All studies involving animals were approved by the institutional laboratory animal care and use committee.
Statistical Analysis
All data were analyzed using SPSS 13.0 for Windows software (SPSS Inc., Chicago, IL). Multiple comparisons were made among the different groups using Kruskal-Wallis' H nonparametric test. Comparisons between various individuals were performed using Mann-Whitney's U test, whereas comparisons between the same individual were performed using Wilcoxon's matched-pairs t test. Correlations between variables were evaluated using Spearman's rank-correlation test. For all tests, a two-sided value of P < 0.05 was considered significant.
All other materials and methods are given in the Supporting Information.
Results
Expression of IL-22 Signaling Pathway-Associated Genes Was Up-Regulated in Liver Tissues From CHB Patients
Liver tissues from 3 HCs and 4 CHB patients were initially used for screening differentially expressing genes using human Genome U133 Plus 2.0 Array (22,668 genes; Affymetrix, Santa Clara, CA). All CHB patients had similar disease background and inflammatory grading (G) scores, except for fibrotic staging (S): CHB2 and CHB3 had low S scores (G2S1 and G3S2), and CHB1 and CHB4 had high S scores (both G3S4; Supporting Table 1). Four hundred and eighty-four genes were up-regulated and 631 were down-regulated in liver tissues from CHB patients, compared to HC subjects (the screening standard for differentially expressed genes was defined as P < 0.05; fold change, >2). Hierarchical clustering indicated that these differentially expressed genes segregated into two main groups (HC and CHB; Fig. 1A). We then searched the GeneGo database to identify potentially relevant pathways in these differentially expressed genes. The IL-22-signaling pathway was the second-highest scoring pathway among the top 10 maps (Fig. 1B), suggesting that the IL-22 pathway was significantly up-regulated in CHB patients. We initially chose liver tissues from these 4 CHB patients for mRNA screening and identified that the IL-22 pathway was significantly up-regulated in these patients. Subsequently, many other CHB and LC patients were enrolled for further studies described below.
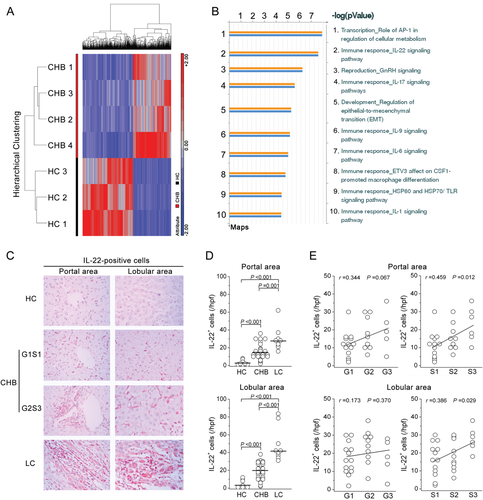
IL-22+ Cells Were Accumulated in Liver Tissues From CHB and LC Patients and Were Correlated With Liver Injury and Fibrosis Severity
IHC staining was subsequently used to investigate IL-22 protein expression in liver tissues from a larger cohort including 8 HC subjects, 30 CHB patients, and 9 LC patients (Fig. 1C-E). Few IL-22+ cells were observed in livers of healthy donors. By contrast, a large number of IL-22+ cells infiltrated livers of HBV-infected subjects, including the inflamed portal area and the lobular sinusoids (Fig. 1C,D). In addition, CHB patients with higher G and S scores had more IL-22+ cells in their livers, compared to those with lower G and S scores (Fig. 1E). Further analysis indicated that both lobular and portal hepatic IL-22+ cell numbers were positively associated with S score and showed a trend of positive association with G score in these patients (Fig. 1E).
IL-22 Is Produced by Multiple LILs
Next, we determined which types of cells produce IL-22. IHC double staining showed that IL-22 was coexpressed by CD4+ and CD8+ T cells, CD68+ macrophages, CD56+ NK/NKT cells, and gamma-delta T-cell receptor (γδTCR)+ T cells as well as NKp46+ NK cells (Fig. 2A). This observation was further confirmed using multicolor flow cytometric (FCM) analyses. In peripheral lymphocytes, IL-22 was produced mainly by CD4+ T cells, whereas among the LILs, IL-22 was produced not only by CD4+ and CD8+ T cells, but also by CD3−CD56+ NK, CD3+CD56+ NKT, and CD3+γδTCR+ T cells under stimulation with phorbol 12-myristate 13-acetate (PMA)/ionomycin in vitro (Fig. 2B and Supporting Fig. 1A). These liver-infiltrating IL-22-expressing cell profiles were similarly distributed in the three groups of subjects, except CD3−CD8+ and CD3−CD56+ NK cells, which accounted for the greater proportion of hepatic IL-22+ cells in LC patients, compared to HC subjects and CHB patients (Supporting Fig. 1B).
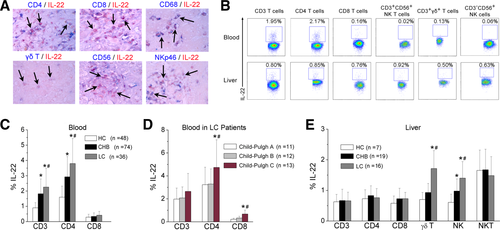
We further found that peripheral IL-22 production by CD3 and CD4, but not CD8, T cells was increased in LC patients, compared to CHB and HC subjects (Fig. 2C). Particularly, IL-22 production by CD4 and CD8 T cells was higher in LC patients with Child-Pugh C score, compared to those with Child-Pugh A or B scores (Fig. 2D). By contrast, intrahepatic IL-22 production by γδT cells and NK cells was significantly elevated in HBV-infected patients, compared to HC subjects, especially in LC patients, who displayed a greater potential for IL-22 production (Fig. 2E).
Th17 Cell Subsets Are Responsible for Hepatic CD4 Th Cell-Derived IL-22 Production in LC Patients
The above data show that CD4 T-cell subsets are the major IL-22 producers in peripheral lymphocytes and are one of the main IL-22 producers in intrahepatic lymphocytes, which have been involved in several types of human diseases.16, 17 Therefore, we examined the production of IL-22 by CD4+ Th1, Th22, and Th17 cells15, 18, 19 and found that both peripheral and intrahepatic IFN-γ-expressing Th1 and IL-17A-expressing Th17 cells produced IL-22 in response to PMA/ionomycin stimulation, whereas few forkhead box protein (FoxP)3-expressing regulatory T (Treg) cells or IL-4-expressing Th2 cells produced IL-22 (Fig. 3A and Supporting Fig. 2). We also confirmed that IL-17A-expressing Th17 cells were mainly characterized by high levels of CD26, CD161, CC chemokine receptor (CCR) 6, and retinoic acid-related orphan receptor-γt expression,36 which proved that IL-22 was secreted by Th17 cells (Supporting Fig. 3).
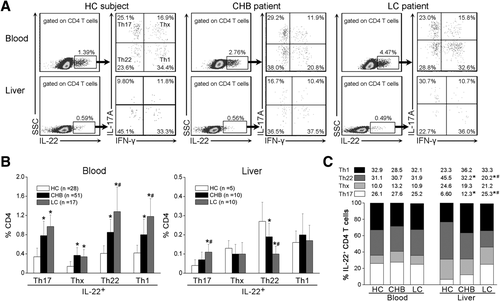
We further categorized IL-22+ CD4 T cells into four subsets according to interferon (IFN)-γ and IL-17A expression: IL-17A+IFN-γ− Th17 cells; IL-17A−IFN-γ+ Th1 cells; IL-17A−IFN-γ− Th22 cells; and IL-17A+IFN-γ+ Thx cells (Fig. 3A). It was found that the percentages of these IL-22+ Th subsets within the peripheral CD4 T cells were higher in CHB and LC patients than in HC subjects, whereas among the liver-infiltrating CD4 cells, LC patients showed a significant increase in IL-22+Th17 cells and a reduction in Th22 cells, compared to HC and CHB individuals (Fig. 3B). Further analysis indicated that distribution of these Th subsets within peripheral IL-22+ CD4 T cells was similar among the three groups. By contrast, within intrahepatic IL-22+ CD4 T cells, Th17 cell proportion was increased, whereas Th22 cell proportion was reduced in CHB patients, compared to HC subjects. This change was more pronounced in LC patients, compared to CHB patients (Fig. 3C). These results signify that increased hepatic IL-22 expression was mainly derived from Th17 cells in LC patients, as compared to that in CHB and HC subjects.
Blockade of IL-22 Attenuates Th17 Cell Infiltration and Liver Fibrosis in an HBV Tg Mouse Model
To further explore the function of IL-22 in chronic liver inflammation and fibrosis, we used an HBV Tg mouse model with repeated anti-CD137 Ab injection.35 HBV Tg mice were repeatedly administered anti-CD137 Ab to activate T cells and induce liver inflammation and fibrosis (Fig. 4A). In consistent with our previous study,35 repeated injection of anti-CD137 Ab induced significant liver fibrosis and inflammation (indicated by increased alanine aminotransferase [ALT] and aspartate aminotransferase [AST]) in HBV Tg mice, whereas blockade of IL-22 reduced liver injury (Supporting Fig. 4), inflammatory cell infiltration, and Sirius Red and α-SMA staining, compared to the immunoglobulin (Ig) control group (Fig. 4B,C). This was also further confirmed by quantifying liver necroinflammation and fibrotic scores. Blockade of IL-22 ameliorated Ishak staging and grading scores (Fig. 4C).
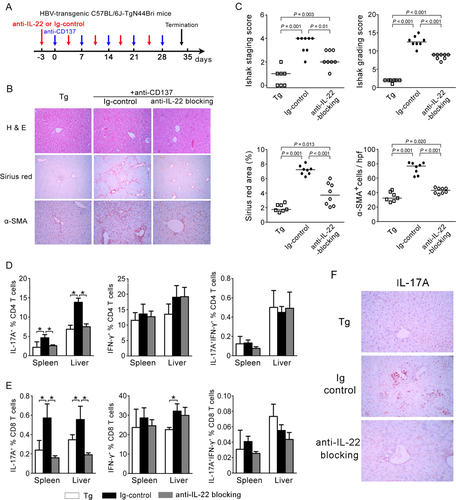
We then determined which lymphocyte population was preferentially reduced after IL-22 blockade in the anti-CD137-treated HBV Tg mouse model. We found that anti-CD137 treatment enhanced CD3 T-cell (especially CD8 T cells) and granulocyte recruitment into the liver, but reduced infiltration of B, NK, and dendritic cells (DCs) in the mouse model (Supporting Fig. 5). Similar observation also occurred in the spleen. Interestingly, blockade of IL-22 failed to alter the anti-CD137 treatment-induced hepatic and splenic lymphocyte proportion mentioned above.
We also analyzed the liver-infiltrating Th1 and Th17 cell subsets. Anti-CD137 treatment significantly increased hepatic and splenic CD4- and CD8-derived IL-17A and IFN-γ production (Fig. 4D,E). Blockade of IL-22 markedly reduced hepatic and splenic IL-17A+ Th17 cells, compared to Ig controls. These findings were also confirmed by IHC staining (Fig. 4F), showing that the number of IL-17A+ cells was lower in the anti-IL-22-treated group than in the control Ab-treated group. By contrast, the number of IFN-γ+ Th1 and IL-17A+IFN-γ+ cells was comparable between the three groups (Fig. 4D,E).
IL-22 Preferentially Promotes Th17 Cell Migration by Stimulating HSCs to Secrete Chemokine (C-X-C Motif) Ligand 10 and Chemokine (C-C Motif) Ligand 20
To gain insight into the mechanism through which blockade of IL-22 reduced Th17 cell migration into liver of the HBV Tg model, we examined the expression of hepatic chemokines, including chemokine (C-X-C motif) ligand (CXCL) 9, 10, and 11 and chemokine (C-C motif) ligand (CCL) 2, 3, 4, 5, 19, 20, and 21, which are known to promote hepatic lymphocyte migration.37 Anti-CD137 treatment increased hepatic expression of CXCL9 and 10 and CCL2, 5, 19, and 20 (Fig. 5). Blockade of IL-22 significantly decreased expression of CXCL10 and CCL20, which are the ligands for C-X-C chemokine receptor (CXCR) 3 and CCR6, respectively, that are expressed by Th17 cells.38
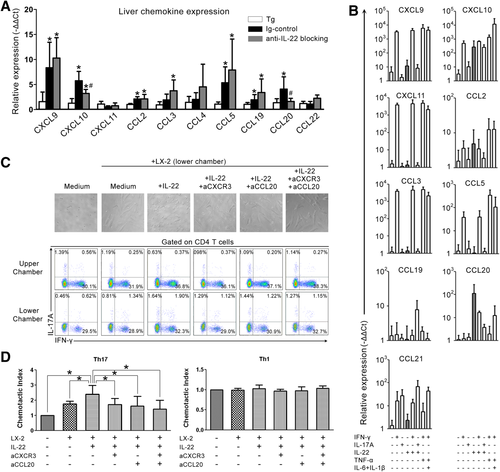
It was reported that IL-22 stimulates hepatocytes to produce acute-phase proteins, thereby promoting liver inflammation.39 Here, we evaluated the potential of IL-22 to stimulate chemokine production by HSCs and HepG2 cells and found that IL-22 alone induced HSCs to secrete high levels of CXCL10 and CCL20 (Fig. 5B), but fail to stimulate HepG2 cells to produce CXCL10 and CCL20 (Supporting Fig. 6). By contrast, IFN-γ mainly stimulated both HepG2 and LX-2 cells to produce high levels of CXCL9, CXCL10, and CXCL11, and IL-17A just stimulated HepG2, but not LX-2, cells to produce these chemokines (Supporting Table 4). These data indicated that IL-22-induced CXCL10 and CCL20 by LX-2 cells possibly promote Th17 cell recruitment from blood into the liver and further position near HSCs.40
We then performed a transwell chemotactic assay in vitro to investigate lymphocyte migration from the upper chamber into the lower chamber containing IL-22-treated HSCs. IL-22-treated HSCs had greater lymphocyte chemoattractant potential than medium-cultured HSCs, whereas blockade of CXCR3, CCL20, or both significantly reduced lymphocyte migration into the lower chamber (Fig. 5C, top). We further found that the CD4 T cells from both the upper and lower chambers had the potential to produce IL-17A and IFN-γ on PMA/ionomycin stimulation (Fig. 5C, bottom). Particularly, IL-22-treated HSCs had greater Th17 chemoattractant potential than medium-cultured HSCs; whereas blockade of CXCR3, CCL20, or both significantly reduced Th17 cell migration into the lower chamber (Fig. 5D). Taken together, these results demonstrate that IL-22 stimulates HSCs to secrete CXCL10 and CCL20, thereby promoting intrahepatic Th17 recruitment.
Discussion
IL-22 has been implicated in the regulation of different set of conditions of liver diseases.24-29 The current study presents several key points regarding the effects of IL-22 on chronic liver inflammation and fibrosis: (1) Multiple intrahepatic cells, especially Th17 cells, are main producers of IL-22 in HBV-infected LC patients; (2) hepatic IL-22 expression is positively correlated with liver inflammation and fibrosis in LC patients; and (3) hepatic increased IL-22 exacerbates chronic liver inflammation and fibrosis by promoting Th17 cell recruitment in vitro and in vivo of an HBV Tg mouse model.
Although up-regulation of IL-22 has been found in CHB patients in recent studies,24, 27, 28 the increased extent and cellular origin of IL-22 remain obscure in chronic HBV-infected patients. The current study has demonstrated that the IL-22-signaling pathway is the second-highest scoring pathway in chronic HBV-infected livers, as compared to healthy livers, through the human mRNA expression profile screening. Furthermore, this study clearly indicates that multiple intrahepatic immune cells, including CD4 and CD8 T cells, NK/NKT cells, γδT cells, and macrophages, have the potential to generate IL-22 independent of disease status. Notably, IL-22 is also produced by Th1 cells, Th17 cells, and Th22 cells, but not by FoxP3+ Treg cells or Th2 subsets, in both the liver and peripheral blood, which is consistent with previous reports.15, 18, 19 As compared with our previous study,41 the present study demonstrated a clearer expression of IL-22 in Th17 population and a preferential polarization of intrahepatic IL-22-producing cells toward Th17 cells in LC patients, compared to HC and CHB subjects. The different anti-IL-22 Abs and the protocols used for IL-22 intracellular staining within Th17 cells may explain the differences between our previous and current studies. Finally, although the mechanisms underlying the Th17 cell skew in LC patients remain obscure, the intrahepatic inflammatory environment may be responsible for facilitating the generation of IL-22-producing cells in LC patients.42
The hepatoprotective effect of IL-22 has been well documented in acute liver injury;21, 22 however, there is also evidence that IL-22 plays a detrimental role in exacerbating chronic liver injury and inflammation.24, 43 These studies indicated that the protective or pathogenic role of IL-22 in liver disease remains contradictory, in part related to the models that were used, but also to liver injured conditions being examined (i.e., acute vs. chronic).43 The clinical studies regarding the correlation between hepatic IL-22 expression and liver fibrosis in patients are also controversial. One clinical study revealed that hepatic IL-22 expression inversely correlates with inflammation grading and fibrosis staging in patients with chronic HBV infection,27 whereas another study reported that elevation of systemic IL-22 levels are predictive for reduced survival in patients with advanced liver cirrhosis.44 The current study demonstrated that intrahepatic IL-22-producing cells were positively associated with severity of liver fibrosis in HBV-infected patients. In mouse models, IL-22 overexpression or treatment with IL-22 ameliorates liver fibrosis induced by acute CCl4 administration or bile duct ligation (BDL),25, 45 suggesting that IL-22 acts as an antifibrotic cytokine. Although these two models have been widely used to study liver fibrogensis, they do not mimic the immune-mediated liver fibrotic progression of human HBV infection. Therefore, we developed a T-cell-mediated liver disease mouse model in HBV Tg mice through repeated injection of anti-CD137 Ab, which potentially induced T-cell immune responses, and subsequently mimics human HBV-associated liver disease progression from chronic liver inflammatoty injury, fibrosis, and cirrhosis to hepatocellular carcinoma.35 By using this immune-mediated liver fibrosis model, we demonstrated that IL-22 appears to be a profibrotic cytokine in the HBV Tg mouse.35 Thus, it is imperative to determine how IL-22 exerts its effects (either positively or negatively) in various liver diseases in future study.
The obvious question is why IL-22 has antifibrotic effects in CCl4 and BDL models, but profibrotic functions in the HBV Tg mouse model. HSCs, which are considered to be the primary source of ECM in liver fibrosis, were found to express high levels of IL-22R1 in the present study (Supporting Fig. 7). Notably, we have previously demonstrated that IL-22 treatment strongly activates phosphorylated signal transducer and activator of transcription 3 and induces HSC senescence.25, 27 The ultimate effect of IL-22 on liver fibrosis is determined by its effect on hepatocyte protection and liver inflammatory damage. Both CCl4 and BDL induce severe hepatocyte damage. IL-22 overexpression or treatment prevents hepatocyte damage, thereby ameliorating injury-driven liver fibrosis in these models. By contrast, repeated injection of anti-CD137 activated immune cells in HBV Tg mice, resulting in chronic liver inflammation. Thus, endogenous IL-22 promotes liver inflammation and subsequently exacerbates liver fibrosis in anti-CD137-treated HBV Tg mice. Indeed, IL-22 has been implicated as a proinflammatory cytokine in human diseases, such as psoriasis, rheumatoid arthritis, and Crohn's disease.16, 17 The balance between HSC senescence and inflammation-promoting fibrosis may determine the outcome of the action of IL-22 in liver fibrosis.
This study also identified a novel mechanism by which IL-22 exacerbates liver fibrosis by enhancing intrahepatic Th17 migration, which was reported to promote liver fibrosis by targeting multiple types of cells.45 The present findings supported the notion that blockade of IL-22 in vivo preferentially reduced intrahepatic Th17 cell infiltration and treatment of IL-22 in vitro stimulated HSCs to promote Th17 chemotaxis. Indeed, a study on airway inflammation has indicated that IL-22 has a proinflammatory effect in the presence of IL-17 and may be regulated by IL-17, whereas IL-22 appears to be protective in the absence of IL-17.46 Thus, the increased Th17 cells in HBV-infected livers produce more IL-22, forming a positive feedback loop that ultimately promotes liver fibrosis during chronic liver injury and inflammation (Fig. 6). Thus, in combination with our previous findings that CD8 T-cell-derived IFN-γ actively recruited hepatic macrophages to produce fibrosis-promoting cytokines and chemokines in the early phase of chronic liver disease,35 CD4 T-cell-derived IL-22 likely plays important roles in promoting liver inflammation and fibrosis at late stages of chronic liver diseases (CLDs) by activating HSCs. A key point in future study is to understand the differential role of IL-22, IL-17, and IFN-γ in promoting liver inflammation and fibrosis at various stages of CLDs with HBV infection.
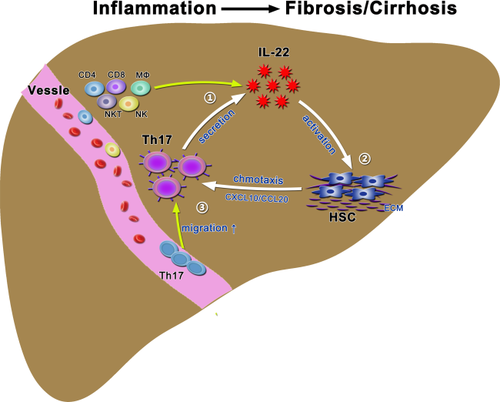
In summary, our findings suggest that increased hepatic IL-22 may promote liver fibrosis progression in chronically HBV-infected patients, possibly through induction of intrahepatic Th17 migration. Thus, inhibition of IL-22 would be a potential therapeutic option for treatment of liver fibrosis and cirrhosis in patients with chronic HBV infection. A better understanding of the relative function of various IL-22-producing cells in liver disorders may shed light on the pathogenic mechanisms and help in the development of appropriate immune interventions.
Acknowledgment
All authors appreciate Mr. Songshan Wang for his skillful technical assistance in IHC staining and they thank all patients, study-site staff, and all participating consultants for their contributions, which made this study possible.