Creatinine- versus cystatine C-based equations in assessing the renal function of candidates for liver transplantation with cirrhosis
Potential conflict of interest: Nothing to report.
Abstract
Renal dysfunction is frequent in liver cirrhosis and is a strong prognostic predictor of orthotopic liver transplantation (OLT) outcome. Therefore, an accurate evaluation of the glomerular filtration rate (GFR) is crucial in pre-OLT patients. However, in these patients plasma creatinine (Pcr) is inaccurate and the place of serum cystatine C (CystC) is still debated. New GFR-predicting equations, based on standardized assays of Pcr and/or CystC, have been recently recommended in the general population but their performance in cirrhosis patients has been rarely studied. We evaluated the performance of the recently published Chronic Kidney Disease Epidemiology Collaboration equations (CKD-EPI-Pcr, CKD-EPI-CystC, and CKD-EPI-Pcr-CystC) and the more classical ones (4- and 6-variable MDRD and Hoek formulas) in cirrhosis patients referred for renal evaluation before OLT. Inulin clearance was performed in 202 consecutive patients together with the determination of Pcr and CystC with assays traceable to primary reference materials. The performance of the GFR-predicting equations was evaluated according to ascites severity (no, moderate, or refractory) and to hepatic and renal dysfunctions (MELD score ≤ or >15 and KDOQI stages, respectively). In the whole population, CystC-based equations showed a better performance than Pcr-based ones (lower bias and higher 10% and 30% accuracies). CKD-EPI-CystC equation showed the best performance whatever the ascites severity and in presence of a significant renal dysfunction (GFR <60 mL/min/1.73 m2). Conclusion: Pcr-based GFR predicting equations are not reliable in pre-OLT patients even when an IDMS-traceable enzymatic Pcr assay is used. Whenever a CystC-assay traceable to primary reference materials is performed and when a true measurement of GFR is not possible, CystC-based equations, especially CKD-EPI-CystC, may be recommended to evaluate renal function and for KDOQI staging. (Hepatology 2014;59:1522-1531)
Abbreviations
-
- AUC
-
- area under the curve
-
- CCC
-
- concordance correlation coefficient
-
- CKD
-
- chronic kidney disease
-
- CKD-EPI
-
- Chronic Kidney Disease Epidemiology Collaboration equation
-
- CystC
-
- serum Cystatine C
-
- eGFR
-
- estimated GFR
-
- GFR
-
- glomerular filtration rate
-
- IDMS-traceable Pcr determination
-
- traceable to an isotope dilution mass spectrometry reference measurement procedure
-
- INR
-
- international normalized ratio
-
- KDOQI
-
- Kidney Disease Outcomes Quality Initiative
-
- LOA
-
- limit of agreement
-
- MDRD-4
-
- four-variable Modification of Diet in Renal Disease equation
-
- MDRD-6
-
- six-variable Modification of Diet in Renal Disease equation
-
- MELD
-
- Model for End-Stage Liver Disease
-
- mGFR
-
- measured GFR
-
- Pcr
-
- plasma creatinine
-
- OLT
-
- orthotopic liver transplantation
-
- ROC
-
- receiver-operator characteristic
-
- Ualb/Ucr
-
- urine albumin to urine creatinine ratio
See Editorial on Page 1242
Renal dysfunction is frequently associated with liver cirrhosis and seems to be a strong predictor of early mortality.1 Thus, plasma creatinine (Pcr) has been included in the widely used Model for Endstage Liver Disease (MELD) score to prioritize patients for liver graft allocation.2 Pretransplant Pcr is also a predictor of posttransplant mortality and posttransplant renal function in orthotopic liver transplantation (OLT).3, 4 Nair et al.4 demonstrated that a pretransplant glomerular filtration rate (GFR) <40 mL/min/1.73 m2 was associated with decreased short- and long-term survivals after OLT and that a combined liver and kidney transplantation should be considered in patients with GFR values <30 mL/min/1.73 m2 (Stage ≥4). Therefore, an accurate evaluation of renal function is crucial in candidates for liver transplantation.
Several methods can be used to determine the true GFR (i.e., the urine or plasma clearance of an exogenous marker). Urinary inulin (or polyfructosan) clearance is regarded as the gold standard for evaluating GFR but is impracticable in routine to measure true GFRs in all OLT candidates. This is why kidney function is commonly estimated with Pcr- or Pcr-based equations. However, Pcr is notoriously inaccurate in the diagnosis of renal dysfunction, especially in cirrhosis patients,5 because of both a reduced production of creatinine from creatine in the liver and a significant muscle wasting.6 Unlike Pcr, CystC is a non-glycosylated 13.36-kDa basic protein produced at a constant rate by all nucleated body cells.7 CystC concentration is less influenced by sex, age, muscle mass, or serum bilirubin than Pcr and is regarded as mainly influenced by GFR.8-10 Therefore, CystC was viewed as meeting many of the characteristics of an ideal GFR marker and might provide a more accurate evaluation of GFR than Pcr, especially through the use of predictive equations.11-13
Several studies have demonstrated that the performance of Pcr-based equations (Cockcroft and Gault, the four- and six-variable Modification of Diet in Renal Disease MDRD-4 and MDRD-6, and the new Chronic Kidney Disease Epidemiology Collaboration equation CKD-EPI-Pcr) are rather inaccurate in cirrhosis patients candidates for OLT.5, 14, 15 Other studies have demonstrated comparable degrees of inaccuracy of CystC-based predictive equations in OLT recipients, bringing into question their clinical utility.16-18 However, these studies used various assay methods for CystC or Pcr determination. Indeed, numerous prediction equations based on Pcr, CystC, or both, have been developed with or without an isotope dilution mass spectrometry (IDMS)-standardization of Pcr measurement and with or without immunoturbidimetric or nephelometric CystC quantitation. This method heterogeneity has led to difficult extrapolation of the formulas to the currently used laboratory methods (IDMS standardized enzymatic Pcr measurement and CystC measurement with standardization to an international calibrator).
In 2009, Levey et al.19 proposed a new Pcr-based formula (CKD-EPI-Pcr) for more accurate estimation of GFR, particularly when Pcr is in the lower ranges. Later, in 2012, Inker et al.20 proposed new GFR estimating equations based on CystC alone (CKD-EPI-CystC) or in combination with Pcr (CKD-EPI-Pcr-CystC); these equations use Pcr and/or CystC assays traceable to primary reference materials. Some authors claimed that CKD-EPI-Pcr would not have a better performance than other Pcr-based formulas in cirrhosis patient awaiting OLT,15 but there are few available reports concerning the performance of these new formulas (CKD-EPI-CystC and CKD-EPI-Pcr-CystC) comparatively to Hoek et al.21 and to 4- and 6-variable-MDRD22 formulas.10
The aim of the present study was to compare the performance of the newly published equations with that of older ones (i.e., MDRD-4, MDRD-6, Hoek) in a population of cirrhosis patients referred to our department for renal evaluation by a reference method before OLT.
Materials and Methods
Patients
To evaluate the performance of GFR estimating equations, we used a dataset on 202 consecutive cirrhosis patients, candidates for OLT, referred to our center between May 2010 and September 2012 to undergo inulin clearance tests as a routine procedure for GFR measurement before transplantation. All patients had concomitant determinations of Pcr and plasma CystC and evaluations of ascites severity and hepatic gravity score (Child-Pugh class A/B/C and MELD).
To evaluate the influence of ascites, hepatic dysfunction, or renal dysfunction on the performance of GFR-estimating equations, the patients were divided: 1) into three groups according to ascites severity (n = 81 patients, moderate: 85, and refractory: 36); 2) into two groups according to the MELD score (≤15: 169 patients and >15: 33 patients); and, 3) into four groups according to the Kidney Disease Outcomes Quality Initiative classification (KDOQI); that is, stage I (normal renal function, mGFR ≥90 mL/min/1.73 m2, 75 patients), stage II (mild renal dysfunction, 60 ≤mGFR <90 mL/min/1.73 m2, 75 patients), stage III (moderate renal dysfunction, 30 ≤mGFR <60 mL/min/1.73 m2, 42 patients) and stage IV-V (severe renal dysfunction, mGFR <30 mL/min/1.73 m2, 10 patients).
GFR Determination
mGFR was determined by renal inulin clearance (polyfructosan, Inutest, Fresenius Kabi, Graz, Austria). Briefly, a standard technique was used in fasting patients (except diabetic ones) with a continuous infusion of polyfructosan (0.33 mg/kg/min, diluted in 10% mannitol) after a load dose of polyfructosan (30 mg/kg in 10 minutes). The continuous infusion of polyfructosan consisted of an initial equilibration period of 45 minutes followed by at least three control periods of 30 to 45 minutes. Water diuresis was induced by oral absorption of 5 mL/kg of water followed by 3 mL/kg every 30 minutes when possible (when oral hydration was not restricted) combined with an intravenous infusion of 10% mannitol (1 mL/min). This enabled bladder voiding every 30 minutes.
A blood sample was drawn midway through each collection period. The stability of plasmatic polyfructosan concentration throughout the test was checked before calculation. In fact, when a stable plasmatic concentration is obtained, renal clearance (continuous infusion of polyfructosan associated with urinary collection) can be determined even in the presence of ascites. The clearance values, calculated by the standard UV/P formula, were obtained from the mean values of the three to four clearance periods. The measurements of plasma and urine polyfructosan concentrations were performed using an enzymatic method.23 The results were adjusted to 1.73 m2 body surface area according to the Dubois and Dubois formula.24
Pcr was obtained with the Siemens enzymatic method (on the Dimension Vista System) traceable to the National Institute of Standards and Technology, Creatinine Standard Reference Materials 914 (verified with NIST SRM 967). Forty-five CystC samples were assessed with the Siemens N-latex Cystatin C kit using the BN systems before the use of the international reference material for Cystatin C (ERM-DA471/IFCC) and the values obtained were recalculated according to the recommendations of the manufacturer (which required a correction factor of 1.11) to obtain standardization to reference materials.
For all other measurements, CystC was measured on the Siemens Dimension Vista Nephelometer System with a method traceable to the International Federation of Clinical Chemistry Working Group for Standardization of serum CystatinC and the IRMM certified reference materials. Hepatic parameters (international normalized ratio [INR]; prothrombin index; serum albumin; and serum bilirubin) were determined in an on-site laboratory.
Statistical Analysis
The agreement between measured GFR (mGFR) by inulin clearance and estimated GFR (eGFR) by Hoek et al.,21 MDRD-4 and MDRD-6,22 CKD-EPI-Pcr,19 CKD-EPI-CystC,20 and CKD-EPI-Pcr-CystC equations20 (Table 1) was assessed as recommended by Earley and al.25
| Formula | Full Expression |
|---|---|
| Hoek formula (Hoek) (21) | Hoek (mL/min/1.73 m2) = −4.32 + 80.35 x 1/CysC |
| Adapted four-variable Modification of Diet in Renal Disease equation (MDRD-4) (22) | MDRD-4 (mL/min/1.73 m2) = 175 x (Pcr)−1.154 x age (years) −0.203 x 0.742 (if female) x 1.21 (if black) |
| Adapted six-variable Modification of Diet in Renal Disease equation (MDRD-6) (22) | MDRD-6 (mL/min/1.73 m2) = 161.5 x (Pcr)−0.999 x age (years) −0.176 x (urea nitrogen)−0.17 x (albumin)0.318 |
| [× 0.762 if female] and [× 1.18 if black] | |
| CKD-EPI creatinine equation (2009) (CKD-EPI-Pcr) (19) | CKD-EPI-Pcr (mL/min/1.73 m2) = 141 × min(Pcr/κ, 1)α × max(Pcr/κ, 1)−1.209 × 0.993age |
| [× 1.018 if female] and [× 1.159 if black] | |
| κ is 0.7 for females and 0.9 for males | |
| α is −0.329 for females and −0.411 for males | |
| “min” is the minimum of Pcr/κ or 1 and “max” the maximum of Pcr/κ or 1 | |
| CKD-EPI cystatin C equation (2012) (CKD-EPI-CystC)(20) | CKD-EPI-CystC (mL/min/1.73 m2) = 133 × min(CystC/0.8, 1)−0.499 × max (CystC/0.8, 1)−1.328 × 0.996age |
| [× 0.932 if female] | |
| “min” indicates the minimum of CystC/0.8 or 1 and “max” the maximum of CystC/0.8 or 1 | |
| CKD-EPI creatinine–cystatin C equation (2012) | CKD-EPI-Pcr-CystC (mL/min/1.73 m2) = 135 × min(Pcr/κ, 1)α × max(Pcr/κ, 1)−0.601 × min(CysC/0.8, 1)−0.375× max(CysC/0.8, 1)−0.711 × 0.995age |
| (CKD-EPI-Pcr-CystC) (20) | [× 0.969 if female] and [× 1.08 if black] |
| κ is 0.7 for females and 0.9 for males; α is −0.248 for females and −0.207 for males, | |
| “min” indicates either the minimum of Pcr/κ or 1 or the minimum of CystC/0.8 or 1 and “max” either the maximum of Pcr/κ or 1 or the maximum of CystC/0.8 or 1. | |
| MELD score (2) | MELD = 10 x [(0.957 x Ln (Pcr (mg/dL))) + (0,378 x Ln(Total Bilirubin (mg/dL))) + (1.12 x Ln (INR))+ 0.643](laboratory values <1 mg/dL were rounded up to 1) |
- Pcr: plasma creatinine (mg/dL) - CystC: serum Cystatine C (mg/L).
Each mGFR-eGFR agreement was assessed using the mean absolute bias (average difference between eGFR and mGFR), the standard deviation of the bias, and the concordance correlation coefficient (CCC). The CCC adjusts the Pearson correlation coefficient downward whenever there is a systematic bias between the methods being compared.26, 27 We calculated the 10% (P10) and 30% (P30) accuracies according to the KDOQI guidelines.28 P10 and P30 are defined as the proportions of the estimates falling within the interval mGFR ± 10% and the interval mGFR ± 30%, respectively.
The progression of the mean absolute bias according to the mGFR value was modeled using a linear regression for each eGFR. Bland-Altman graphs29 were built to show the progression of the mean bias and of the limits of agreement (LOA) according to the mGFR value, which is the gold standard method (inulin clearance).30 The predicted values of the mean bias and of the LOA were calculated for four values of mGFR: 110, 75, and 40 mL/min/1.73 m2.
The ability of the formulas to predict a GFR <60 and <90 mL/min/1.73 m2 was assessed using receiver-operator characteristic (ROC) curves and area under the ROC curves (AUC).
The comparisons between any two formula results were carried out using the 95% confidence interval of the difference between two CCCs as obtained by a bootstrap method. A McNemar's test was performed to compare P10s and P30s. The comparisons according to the ascites severity and KDOQI classification (Table 2) used Fisher's exact test.
| Characteristics | All Population N= 202 | No Ascites N= 81 | Mild to Moderate Ascites N= 85 | Refractory Ascites N= 36 | Pa |
|---|---|---|---|---|---|
| Males/females | 145/57 | 58/23 | 61/24 | 26/10 | NS |
| Age (yr) | 55.7 [18.9-71.5] | 55.1 [18.9-67.3] | 55.7 [21.2-70.9] | 56.7 [24.9-71.5] | NS |
| Weight (kg) | 75.0 [39-137] | 74.5 [39-122] | 76.0 [49-137] | 76.5 [48-123] | NS |
| Height (cm) | 170 [145-193] | 170 [145-193] | 168 [150-190] | 170 [149-185] | NS |
| BSA (m2) | 1.86 [1.33-2.45] | 1.86 [1.33-2.35] | 1.86 [1.45-2.45] | 1.87 [1.43-2.44] | NS |
| BMI (kg/m) | 25.8 [15.8-45.2] | 25.2 [15.8-45.2] | 25.8 [18.7-45.2] | 26.7 [18.4-38.5] | NS |
| Systolic blood pressure (mm Hg) | 118 [79-170] | 120 [88-170] | 117 [91-159] | 111 [79-156] | NS |
| Diastolic blood pressure (mm Hg) | 70 [46-120] | 75 [46-120] | 68 [49-104] | 64 [46-96] | NS |
| Hypertension | 23 (11%) | 13 (16 %) | 5 (6 %) | 5 (14 %) | NS |
| Associated hepatocellular carcinoma | 70 (35%) | 32 (40%) | 31 (36%) | 7 (19%) | NS |
| Previous liver transplantation | 32 (16%) | 12 (15%) | 12 (14%) | 8 (22%) | NS |
| MELD score | 10.5 [6.4-22.6] | 9.4 [6.4-10.9] | 10.9 [6.4-22.6] | 11.6 [6.4-22.6] | NS |
| MELD (≤ 15/>15) | 169/33 | 73/8 | 69/16 | 27/9 | |
| Child-Pugh class | |||||
| A | 55 (27%) | 51 (63%) | 4 (5%) | 0 (0%) | NS |
| B | 82 (41 %) | 24 (30 %) | 43 (51 %) | 15 (42 %) | NS |
| C | 65 (32 %) | 6 (7 %) | 38 (45 %) | 21 (58 %) | NS |
| INR | 1.4 [1.0-4.1] | 1.3 [1.0-2.7] | 1.4 [1.0-4.1] | 1.5 [1.0-3.7] | NS |
| Prothrombin index | 58 [3-126] | 64 [3-126] | 56 [16-115] | 52 [21-103] | <0.05 |
| Bilirubin (µmol/L) | 35 [4-409] | 26 [4-365] | 40 [6-282] | 41 [8-409] | <0.05 |
| Serum Albumin (g/L) | 31 [9-50] | 34 [9-48] | 31 [14-50] | 29 [21-38] | <0.05 |
| Pcr (µmol/L) | 62 [29-279] | 61 [36-248] | 61 [29-279] | 70 [46-136] | NS |
| CystC (mg/L) | 1.0 [0.6-3.8] | 0.99 [0.6-2.7] | 0.99 [0.6-3.8] | 1.1 [0.6-2.8] | NS |
| mGFR (mL/min/1.73 m2) | 83 [6-167] | 86 [16-167] | 84 [6-165] | 59 [15-116] | <0.05 |
| KDOQI classification, n (%) | |||||
| I | 75 (37%) | 32 (39%) | 36 (42%) | 7 (19%) | <0.05 |
| II | 75 (37%) | 36 (44%) | 28 (33%) | 11 (31%) | <0.05 |
| III | 42 (21%) | 10 (12%) | 17 (20%) | 15 (42%) | <0.05 |
| IV – V | 10 (5%) | 3 (4%) | 4 (5%) | 3 (8%) | <0.05 |
- Values are median [interquartile range] or n (%).
- a P between ascites score groups; NS = statistically nonsignificant. MELD: Model for Endstage Liver Disease. INR: international normalized ratio. BSA: body surface area. BMI: body mass index. To convert plasma creatinine from µmol/L to mg/dL divide by 88.4. To convert bilirubin from µmol/L to mg/dL divide by 17.1.
All the analyses were performed using R for Windows, v. 2.15. A value of P < 0.05 was considered for statistical significance.
Results
Patients
The characteristics of the 202 patients (72% males) are shown in Table 2. Only three patients were black. The main causes of cirrhosis were alcoholic consumption (55%) and viral hepatitis (21%). The other etiologies were autoimmune pathologies (4%), cryptogenetic or nonalcoholic steatohepatitis (5%), primary biliary cirrhosis or primary sclerosing cholangitis (7%), and other causes (7%). An association with hepatocellular carcinoma was present in 35% of the patients. Sixteen percent of the patients were undergoing assessment of renal function before a second OLT. The overall mean mGFR was 80 ± 31 mL/min/1.73 m2, 74% of the patients had a GFR ≥60 mL/min/1.73 m2 (no or mild renal dysfunction) and 26% a GFR <60 mL/min/1.73 m2 (moderate or severe renal dysfunction). Thirty-two percent of the patients had a Child-Pugh score C and 18% had refractory ascites. The mean MELD score was 11 ± 4 and 84% of the patients had a MELD score ≤15. Only hepatic (prothrombin index, serum bilirubin), and renal (mGFR and KDOQI stages) function parameters presented statistically significant differences between the three ascites-severity groups. The mean GFR was significantly lower in the patients with refractory ascites (64 ± 28 mL/min/1.73 m2) versus patients with no or moderate severity (87 ± 31 and 81 ± 30 mL/min/1.73 m2, respectively). No difference in mGFR was observed between the two MELD groups.
Performance of the GFR-Estimating Equations and Ascites Severity
The performances of CystC- and Pcr-CystC-based equations (CCC, P10, and P30) were similar in the whole population and in each of the three ascites groups (Table 3). The same performances were also observed in the two MELD groups (≤or >15) (Table 4) and in the three Child-Pugh groups (results not shown). However, the Pcr-based equations (MDRD-4, MDRD-6, and CKD-EPI-Pcr) had a lower performance in the whole population and that performance (especially P10 and P30) decreased with ascites severity. Finally, the performances of CKD-EPI-CystC and the Hoek formulas were the best whatever the ascites severity: the P30s were higher and the mean biases were significantly lower compared to those of other equations (Table 3).
| Performance Criteria | HOEK | MDRD-4 | MDRD-6 | CKD-EPI-Pcr | CKD-EPI-CystC | CKD-EPI-Pcr-CystC |
|---|---|---|---|---|---|---|
| All measurements (n=202) mGFR = 80.3 ± 31.1 | ||||||
| EGFR | 76.0± 23.0 | 109.4 ± 42.0a | 98.3 ± 36.5a | 98.7 ± 24.9a | 75.9 ± 26.4 | 86.7 ± 25.7 |
| P30 | 78.7b | 42.6 | 58.4 | 56.4 | 83.2b | 78.2b |
| P10 | 36.6b | 13.7 | 24.3 | 21.8 | 35.6b | 38.6b |
| CCC (95% CI) | 0.75 (0.69, 080) | 0.56 (0.48, 0.62) | 0.68 (0.61, 0.74) | 0.62 (0.55, 0.69) | 0.80 (0.74, 0.84)c | 0.82 (0.77, 0.86)c |
| No ascites (n =81) mGFR = 87.2 ± 30.7 | ||||||
| eGFR | 70.9 ± 22.6 | 112.5 ± 40.1a | 103.4 ± 36.5a | 101.6 ± 22.4a | 80.2 ± 25.4 | 90.5 ± 23.9 |
| P30 | 85.2b | 58.0 | 66.7 | 64.2 | 88.9b | 81.5b |
| P10 | 40.7b | 18.5 | 25.9 | 27.2 | 37.0b | 43.2b |
| CCC (95% CI) | 0.75 (0.66 - 0.82) | 0.59 (0.47 - 0.69) | 0.68 (0.56 - 0.77) | 0.63 (0.51 - 0.73) | 0.78 (0.69 - 0.85) | 0.82 (0.75 - 0.88)d |
| Mild to moderate ascites (n=85) mGFR = 80.6 ± 30.3 | ||||||
| eGFR | 76.0 ± 22.7 | 112.3 ± 44.5a | 99.3 ± 36.6a | 99.2 ± 26.5a | 75.8 ± 26.1 | 86.9 ± 26.4 |
| P30 | 77.6b | 38.8 | 61.2 | 58.8 | 84.7b | 81.2b |
| P10 | 32.9 | 14.1 | 25.8 | 23.5 | 36.5 | 41.2 |
| CCC (95% CI) | 0.75 (0.65, 0.82) | 0.54 (0.42 - 0.63) | 0.68 (0.57 - 0.77) | 0.67 (0.56 - 0.75) | 0.81 (0.72 - 0.87)c | 0.85 (0.79 - 0.9)c |
| Refractory ascites (n=36) mGFR = 64.4 ± 28.3 | ||||||
| eGFR | 67.2 ± 23.1 | 95.8 ± 38.2a | 84.7 ± 33.7a | 91.0 ± 25.1a | 66.5 ± 27.4 | 77.4 ± 26.3a |
| P30 | 66.7b | 16.7 | 33.3 | 33.3 | 66.7b | 63.9b |
| P10 | 36.1 | 2.8 | 16.7 | 5.6 | 30.6 | 22.2 |
| CCC (95% CI) | 0.69 (0.49 - 0.83) | 0.45 (0.26 - 0.61) | 0.58 (0.37 - 0.73) | 0.45 (0.26 - 0.61) | 0.75 (0.57 - 0.87) | 0.68 (0.49 - 0.81) |
- mGFR and eGFR are expressed as mean ± standard deviation in mL/min/1.73 m2.
- a Statistically significant differences between eGFR and mGFR.
- b P < 0.05 between Cystatin C based formulas (Hoek, CKD-EPI-CystC, CKD-EPI-Pcr-CystC) and other equations, favoring Cystatin C based formulas.
- c P < 0.05 between CKD-EPI-CystC formula and other equations, favoring CKD-EPI-CystC formula, but without significant difference between CKD-EPI-CystC and CKD-EPI-Pcr-CystC formula.
- d P < 0.05 between CKD-EPI-Pcr-CystC formula and other equations, favoring CKD-EPI-Pcr-CystC formula, but without difference between CKD-EPI-Pcr-CystC and CKD-EPI-CystC formulas.
| Performance Criteria | HOEK | MDRD-4 | MDRD-6 | CKD-EPI-Pcr | CKD-EPI-CystC | CKD-EPI-Pcr-CystC |
|---|---|---|---|---|---|---|
| MELD ≤15 (n =169) mGFR = 80.0 ± 29.4 | ||||||
| eGFR | 76.0 ± 22.2 | 106.0 ± 36.0a | 96.1 ± 33.0a | 98.0 ± 23.1a | 75.7 ± 25.6 | 86.0 ± 24.3 |
| P30 | 75.5b | 45.5 | 60.0 | 58.0 | 83.0b | 79.2b |
| P10 | 37.0b | 16.0 | 23.6 | 24.0 | 34.0b | 39.0b |
| CCC (95% CI) | 0.74 (0.67 – 0.79) | 0.55 (0.47 – 0.63) | 0.67 (0.59 – 0.74) | 0.61 (0.53 – 0.68) | 0.79 (0.73 – 0.84)c | 0.81 (0.76 – 0.86)c |
| MELD >15 (n=33) mGFR = 82.0 ± 38.9 | ||||||
| eGFR | 76.6 ± 27.4 | 127.2 ± 62.2a | 109.7 ± 50.3a | 103.6 ± 24.2a | 76.6 ± 30.5 | 89.7 ± 32.2 |
| P30 | 85.08b | 24.2 | 45.4 | 48.4 | 85.0b | 72.7b |
| P10 | 33.3b | 6.0 | 27.2 | 12.1 | 45.4b | 36.4b |
| CCC (95% CI) | 0.81 (0.68 – 0.89)b | 0.56 (0.39 – 0.69) | 0.69 (0.52 – 0.81) | 0.67 (0.49 – 0.80) | 0.82 (0.70 – 0.90)b | 0.83 (0.70 – 0.91)b |
- mGFR and eGFR are expressed as mean ± standard deviation in mL/min/1.73 m2.
- a Statistically significant differences between eGFR and mGFR.
- b P < 0.05 between Cystatin C based formulas (Hoek, CKD-EPI-CystC, CKD-EPI-Pcr-CystC) and other equations, favoring Cystatin C based formulas.
- c P < 0.05 between CKD-EPI-CystC formula and other equations, favoring CKD-EPI-CystC formula, but without significant difference between CKD-EPI-CystC and CKD-EPI-Pcr-CystC formula.
Performance of the GFR-Estimating Equations and Renal Dysfunction Severity
Bland-Altman graphs show bias changes according to inulin clearance: the overestimation of mGFR increased with the decrease of GFR with all tested equations, except MDRD-4 and MDRD-6 (Fig. 1).
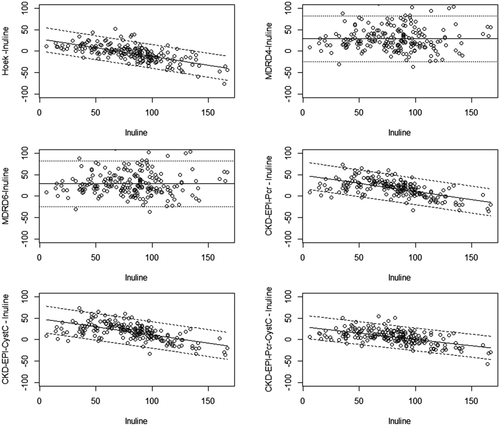
Staging renal insufficiency, as recommended by the National Kidney Foundation (KDOQI) guidelines, is a major aspect of prognosis and adequate follow-up. The present study assessed the performance of GFR-estimating equations in detecting chronic kidney disease (mGFR <60 mL/min/1.73 m2) and providing an adequate KDOQI staging. As shown by the areas under the ROC curves of GFR estimating equations (Table 5), CKD-EPI-Pcr-CystC and MDRD-6 equations had the best performance in identifying patients with mild GFR decrease (<90 mL/min/1.73 m2), whereas CKD-EPI-CystC and the Hoek equation showed the best performance in identifying patients with moderate or severe renal dysfunction (GFR <60 mL/min/1.73 m2). Finally, depending of the formula used, the GFR estimating equations led to correct KDOQI staging in only 47 to 64% of OLT candidates. However, higher percentages of good staging were observed with CKD-EPI-CystC and CKD-EPI-Pcr-CystC. Pcr-based equations tended to underestimate the severity of kidney dysfunction (indicating lower stages of KDOQI classification), whereas CystC-based equations tended to overestimate the severity of kidney dysfunction, especially at GFR values >60 mL/min/1.73 m2 (Table 6). Indeed, CKD-EPI-CystC induced a 43% misclassification (32/75) of patients with normal GFRs (i.e., ≥90 mL/min/1.73 m2) in stage 2 (mild CKD) of the KDOQI classification.
| GFR <60 mL/min/1.73 m2 | GFR <90 mL/min/1.73 m2 | |
|---|---|---|
| Formula | AUC (95% CI) | AUC (95% CI) |
| Hoek | 0.86 (0.80, 0.92)a | 0.67 (0.61, 0.74) |
| MDRD-4 | 0.71 (0.64, 0.78) | 0.72 (0.67, 0.77) |
| MDRD-6 | 0.79 (0.72, 0.85) | 0.77 (0.71, 0.82)b |
| CKD-EPI-Pcr | 0.68 (0.62, 0.75) | 0.67 (0.62, 0.71) |
| CKD-EPI-CystC | 0.86 (0.80, 0.92)a | 0.73 (0.66, 0.79) |
| CKD-EPI-Pcr-CystC | 0.78 (0.71, 0.85) | 0.78 (0.72, 0.83)b |
- AUC = area under ROC curves; 95% CI = 95% confidence interval.
- a P < 0.05 between CKD-EPI-CystC and other formulas, favoring CKD-EPI-CystC (without difference for Hoek).
- b P < 0.05 between CKD-EPI-Pcr-CystC and other formulas, favoring CKD-EPI-Pcr-CystC (without difference for MDRD-6).
| Error in KDOQI Staging | |||
|---|---|---|---|
| GFR Estimating Equations | Good KDOQI Staging | Underestimation of Severity of CKD | Overestimation of Severity of CKD |
| All measurements (n= 202) mGFR = 80.3 ± 31.1 [6.0 – 167.0] | |||
| Hoek | 124 (61.4%) | 30 (14.8%)a | 48 (23.8%) |
| MDRD-4 | 109 (54.0%) | 88 (43.5%) | 5 (2.5%)b |
| MDRD-6 | 124 (61.4.0%) | 71 (35.1%) | 7 (3.5%)b |
| CKD-EPI-Pcr | 95 (47.0%) | 104 (51.5%) | 3 (1.5%)b |
| CKD-EPI-CystC | 129 (63.9%) | 27 (13.3%)a | 46 (22.8%) |
| CKD-EPI-Pcr-CystC | 128 (63.4%) | 62 (30.7%) | 12 (5.9%) |
| GFR ≥ 60 mL/min/1.73 m2 (N=150) mGFR = 94.1 ± 22.5 [60.0 – 167.0] | |||
| Hoek | 89 (59.4%) | 14 (9.3%)a | 47 (31.3%) |
| MDRD-4 | 93 (62.0%) | 52 (34.7%) | 5 (3.3%)b |
| MDRD-6 | 100 (66.7%) | 43 (28.6%) | 7 (4.7%)b |
| CKD-EPI-Pcr | 81 (54.0%) | 66 (44.0%) | 3 (2.0%)b |
| CKD-EPI-CystC | 92 (61.3%) | 15 (10.0%)a | 43 (28.7%) |
| CKD-EPI-Pcr-CystC | 102 (68.0%) | 37 (24.7%) | 11 (7.3%) |
| GFR < 60 mL/min/1.73 m2 (N=52) mGFR = 40.6 ± 13.2 [6.0 – 59.0] | |||
| Hoek | 35 (67.3%) | 16 (30.8%) | 1 (1.9%) |
| MDRD-4 | 16 (30.8%) | 36 (69.2%) | 0 (0%) |
| MDRD-6 | 24 (46.2%) | 28 (53.8%) | 0 (0%) |
| CKD-EPI-Pcr | 14 (26.9%) | 38 (73.1%) | 0 (0%) |
| CKD-EPI-CystC | 37 (71.1%) | 12 (23.1%) | 3 (5.8%) |
| CKD-EPI-Pcr-CystC | 26 (50.0%) | 25 (48.1%) | 1 (1.9%) |
- a P < 0.05 between CKD-EPI-CystC and other formulas, favoring CKD-EPI-CystC (without difference with Hoek formula).
- b P < 0.05 between Pcr-based formulas (MDRD-4, MDRD-6 and CKD-EPI-Pcr) and other formulas, favoring Pcr-based formulas.
Liver dysfunction, as assessed by various tests (INR, prothrombin index, serum albumin, serum bilirubin, and MELD) was similar in the three groups of renal function (data not shown). However, 50% of patients with refractory ascites had a mGFR <60 mL/min/1.73 m2 compared to, respectively, 16 and 25% in the groups of no and moderate ascites severity. CKD-EPI-Pcr-CystC showed the best performance at normal GFR values (mean Pcr 56.3 ± 15.5 μmol/L) giving significantly higher P10 and P30 and lower bias in comparison with other equations (Table 7). When GFR decreased, the performance of GFR estimating equations decreased too: they had higher biases (i.e., increase of overestimation of GFR) with all tested equations and lower P30s and P10s. However, CKD-EPI-CystC had the best performance when GFR was <60 mL/min/1.73 m2 with significantly higher P10 and P30 and a lower mean bias, whereas Pcr-based formulas had the worst performance when GFR was <90 mL/min/1.73 m2. In stages ≥3, the Hoek formula had a poor performance, although it is based only on CystC measurement.
| Performance Criteria | HOEK | MDRD-4 | MDRD-6 | CKD-EPI-Pcr | CKD-EPI-CystC | CKD-EPI-Pcr-CystC |
|---|---|---|---|---|---|---|
| GFR ≥ 90 (N=75) mGFR= 110.9± 19.0 | ||||||
| eGFR | 91.7± 17.2a | 139.3± 37.3a | 124.7 ± 30.5 | 114.8± 15.6 | 95.2± 18.0a | 105.8± 16.3 |
| P30 | 85.3 | 60.0 | 76.0 | 93.3 | 88.0 | 98.7 |
| P10 | 28.0 | 17.3 | 28.0 | 45.3 | 32.0 | 54.7 |
| Bias at 110 mL/min/1.73 m2 | −16.5± 14.1 | 30.0± 27.6 | 15.7± 23.0 | 7.2± 15.8 | −13.5± 15.4 | 3.5± 13.6 |
| LOA | −44.1, 11.2 | −24.1, 84.0 | −29.3, 60.6 | −23.8, 38.2 | −43.6, 16.6 | −23.0, 30.0 |
| 60 ≤ GFR < 90 m2 (N=75) mGFR= 77.3± 9.0 | ||||||
| eGFR | 78.2± 15.7 | 108.6± 26.2a | 99.2± 22.8 | 102.4± 12.3 | 77.9± 18.0 | 89.3± 13.9 |
| P30 | 89.3b | 41.3 | 56.0 | 46.7 | 85.3b | 85.3b |
| P10 | 57.3b | 14.7 | 25.3 | 8.0 | 42.7b | 41.3b |
| Bias at 75 mL/min/1.73 m2 | −2.1± 14.1 | 28.9± 27.6 | 18.4± 23.0 | 20.4± 15.8 | −2.8 ± 15.4 | 15.2± 13.6 |
| LOA | −29.8, 25.5 | −25.0, 83.0 | −26.4, 63.2 | −10.5, 51.4 | −32.8, 27.3 | −11.3, 41.8 |
| GFR < 60 (N=52) mGFR= 40.6± 13.2 | ||||||
| eGFR | 50.3± 16.3a | 67.6± 28.9a | 59.1± 23.7a | 70.2 ± 25.2a | 45.2± 16.9 | 55.2± 19.6a |
| P30 | 53.8 | 19.2 | 36.5 | 17.3 | 73.1c | 38.5 |
| P10 | 19.2 | 7.7 | 17.3 | 7.7 | 30.8c | 11.5 |
| Bias at 40 mL/min/1.73 m2 | 12.2± 14.1 | 27.9± 27.6 | 21.1± 23.0 | 33.6± 15.8 | 7.9± 15.4 | 21.1± 13.6 |
| LOA | −15.6, 39.6 | −26.2, 82.1 | −23.8, 66.2 | 2.5, 64.7 | −22.2, 38.1 | −5.5, 47.8 |
- mGFR and eGFR are expressed as mean ± standard deviation in mL/min/1.73 m2; LOA: limits of agreement.
- a Statistically significant differences between eGFR and mGFR.
- b P < 0.05 between Cystatin C based formulas (Hoek, CKD-EPI-CystC, CKD-EPI-Pcr-CystC) and other equations, favouring Cystatin C based formulas.
- c P < 0.05 between CKD-EPI-CystC formula and other equations, favoring CKD-EPI-CystC formula.
Discussion
In patients with cirrhosis, renal function has a significant impact on early pretransplant mortality, posttransplant mortality, and posttransplant renal function.4, 31 Whereas MELD score is the criterion to prioritize patients for liver allocation, the presence of ascites (and its severity) is a well-known determinant of GFR due to hemodynamic mechanisms. In practice, a MELD score of 15 is now considered as the limit above which a liver transplantation should be considered (except when hepatocarcinoma is present).32, 33 Therefore, methods of GFR evaluation in pre-OLT should be evaluated according to the MELD score and to the presence of ascites.
Given the limitations of the GFR-estimating equations, the question of which parameter (Pcr or CystC) and which equation should be used in pre-OLT-patients to estimate GFR is still challenging. Therefore, we undertook a comparison of the performance of the recently published equations with the classical ones (i.e., MDRD-4, MDRD-6, and Hoek)21, 22 in a population of cirrhosis patients referred for renal evaluation by a reference method before OLT. In addition, we compared the performance of various equations according to ascites severity and to MELD score.
The present study patients were very similar to those of other studies especially in terms of etiology, age, sex, and severity of liver dysfunction.10, 15, 34-36 The mean GFR (about 80 mL/min/1.73 m2) was comparable to that found by other recent studies,14, 15, 34, 36 with 73% of patients with no or mild renal dysfunction according to the KDOQI classification. As CystC could be influenced by malignancies, we checked that CystC was not influenced by hepatocarcinoma. Whereas the mGFR was similar in patients with and without hepatocarcinoma, the mean CystC was not different between the two groups (results not shown).
In the whole population, CystC-based equations (Hoek, CKD-EPI-CystC, and CKD-EPI-Pcr-CystC) performed better than Pcr-based equations (MDRD-4, MDRD-6, and CKD-EPI-Pcr), with significantly lower absolute biases and significantly higher P10s and P30s. These results are in agreement with previously published studies that concluded that CystC-based equations had significantly lower biases and higher precisions than Pcr-based formulas.34, 37 Besides, MDRD-4, MDRD-6, and CKD-EPI-Pcr significantly overestimated mGFR, as previously shown by several studies.10, 14, 15, 35-37 However, these studies showed large ranges of biases (7 to 44 mL/min/1.73 m2 for MDRD and 8 to 42 mL/min/1.73 m2 for CKD-EPI-Pcr) probably because of different methods of Pcr determination (compensated Jaffé method versus enzymatic method). The diagnostic potential to detect CKD as a GFR below 60 mL/min/1.73 m2 was significantly higher with the CKD-EPI-CystC equation compared to all other tested formulas (as demonstrated by the higher AUC, Table 5). Depending on the equation used, only 47 to 64% of cirrhosis patients were properly staged according to the KDOQI classification. This emphasizes the importance of a true GFR determination in pre-OLT evaluation, whenever this is possible. Finally, the present results confirmed the bad performance of MDRD-4 and MDRD-6 in cirrhosis patients; this argues against the use of these equations in pre-OLT evaluation.
We demonstrated that Pcr-based equations showed the worst performance in patients with refractory ascites and those with a MELD score >15. These results are in agreement with those of Francoz et al.,15 who reported that patients with severe ascites were more likely to have an overestimation of their true GFR with MDRD. Conversely, the performance of CystC-based equations decreased only in the group of refractory ascites and is rather the same in both MELD groups, which confirms the results of Ustundag et al.,38 who found that CystC, but not Pcr, correlates with GFR in each stage of liver failure and has a significant diagnostic advantage in detecting lower GFRs in this setting.
In the present study the performance of GFR-estimating equations decreased with CKD progression; this confirms previous studies.14, 15, 34 Even if CKD-EPI-Pcr has significantly lower bias for normal renal function (GFR ≥90 mL/min/1.73 m2), its performance dropped and became lower than that of Cyst-C-based equations when GFR decreased. These results are in agreement with those of Stevens et al.,39 who suggested that the CKD-EPI-Pcr formula provides more accurate GFR estimations in the 60-120 mL/min/1.73 m2 range of eGFR, probably because of a “correction” term for patients with low Pcr values. In opposition, CKD-EPI-CystC exhibited a good performance even when the renal function is impaired; a significantly higher P10 and P30 and a lower bias were seen when GFR was less than 60 mL/min/1.73 m2. This extended performance over all stages of CKD is a major advantage when GFR is by definition unknown.
The present results show that the use of Pcr-based equations to estimate GFR in pre-OLT patients is inaccurate. Conversely CystC-based equations (especially CKD-EPI-CystC) have better performance whatever the ascites severity or the Child-Pugh score and when a significant renal dysfunction is present (GFR <60 mL/min/1.73 m2). To our knowledge, this is the first time the superiority of CKD-EPI-CystC in pre-OLT patients was demonstrated with standardized Pcr and CystC determinations. One of the strengths of the present study is the use of the reference standard for GFR measurement (i.e., inulin clearance) and the standardization of Pcr and CystC measurements according to international laboratory recommendations. Yet one limitation is the low number of patients with very severe liver dysfunction (MELD >20). This is why these results should be interpreted with caution in patients with a MELD score >15 or with an eGFR <60 mL/min/1.73 m2 because the number of patients in these groups was low (n = 33, 16.3%, and n = 52, 25.7%, respectively). In addition, the mean MELD score in patients with MELD score <15 was very low in pre-OLT-patients (10.0 ± 2.5) as in those with MELD score >15 (17.7 ± 2.3); thus, this population might not represent the typical group of patients that are transplanted in most centers, at least in the USA. Further investigations are required to evaluate the performance of CystC-based equations in patients with more severe hepatic or renal dysfunction (MELD score >20 and GFR <60 mL/min/1.73 m2).
In conclusion, Pcr-based equations are inaccurate in estimating GFR in pre-OLT patients even when an IDMS-traceable enzymatic Pcr assay is used. Whenever a CystC-assay traceable to primary reference materials is performed and when a direct measurement of GFR by exogenous glomerular marker is not possible, CystC-based equations, especially CKD-EPI-CystC, may be recommended to evaluate the renal function and for KDOQI staging, although their results should be interpreted with caution in the patients with more severe hepatic dysfunction until a further evaluation.
Acknowledgment
The authors thank Mrs. Monique Duvareille for collecting the missing data for article revision.