Glomerular filtration rate equations for liver-kidney transplantation in patients with cirrhosis: Validation of current recommendations
See Editorial on Page 1242
Potential conflict of interest: Nothing to report.
Abstract
Simultaneous liver and kidney transplantation (SLKT) remains the procedure of choice for patients with both endstage liver disease and kidney failure. Stringent guidelines are needed to avoid unnecessary kidney transplantation. A recent consensus meeting proposed criteria based on the Modified Diet in Renal Disease (MDRD)-6 equation to estimate glomerular filtration rate (GFR). The aims of this study were to compare GFR equations to true GFR in candidates for liver transplantation (LT) and to determine the impact of inaccuracies on the current guidelines for SLKT. Three hundred stable cirrhosis patients evaluated for LT were studied. All patients had iohexol clearance to measure GFR at evaluation under stable conditions. Measured GFR (mGFR) was compared to MDRD-4, MDRD-6, and Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equations. MDRD-6 was the most accurate equation to predict GFR. In the 290 patients with mGFR >30 mL/min/1.73 m2, 15 patients (7%) had estimated GFR (eGFR) ≤40 mL/min/1.73 m2 based on the MDRD-6 equation, defining “discordant” patients. Among them, two underwent SLKT and 13 underwent LT alone. None of those who survived more than 1 year after LT alone (n = 8) developed renal dysfunction thereafter. In multivariate analysis, discordant patients were older (P = 0.03) and had lower sodium level (P = 0.02). Conclusion: The MDRD-6 equation was superior to other equations at identifying cirrhosis patients with true GFR <30 mL/min/1.73 m2. However, the MDRD-6 equation also tended to underestimate renal function in a subgroup of patients with true GFR >30 mL/min/1.73 m2, with a potential risk of unnecessary kidney transplantation if applying current U.S. recommendations for SLKT. (Hepatology 2014;59:1514-1521)
Abbreviations
-
- CKD
-
- chronic kidney disease
-
- CKD-EPI; Chronic Kidney Disease Epidemiology Collaboration; eGFR
-
- estimated glomerular filtration rate
-
- GFR
-
- glomerular filtration rate
-
- HCC
-
- hepatocellular carcinoma
-
- KDOQI
-
- Kidney Disease Outcomes Quality Initiative
-
- MDRD
-
- Modified Diet in Renal Disease
-
- MELD
-
- Model for Endstage Liver Disease
-
- mGFR
-
- measured glomerular filtration rate
-
- POD
-
- postoperative day
-
- Scr
-
- serum creatinine
-
- SLKT
-
- simultaneous liver and kidney transplantation.
Renal dysfunction is common in patients with endstage cirrhosis.1-4 Several studies have shown that impaired renal function has an independent prognostic impact on patients with cirrhosis.5 As a result, serum creatinine (Scr) has been incorporated into the Model for Endstage Liver Disease (MELD) score.5 Due to a high accuracy to predict short-term mortality in patients with cirrhosis, MELD score-based allocation policies have been widely adopted in liver transplantation (LT). Since Scr weighs heavily on the MELD score, patients with impaired renal function are prioritized with these policies. In parallel, a significant increase in simultaneous liver and kidney transplantation (SLKT) has been observed in countries where a MELD score-based allocation policy has been adopted.6
SLKT remains the procedure of choice for patients with both advanced liver and kidney failure. However, predicting the potential for posttransplant renal recovery based on pretransplant status still represents a challenging issue. For instance, type 1 hepatorenal syndrome, which is functional in nature, is likely to improve enough with LT alone so that kidney transplantation is not necessary.7, 8
In response to the increase in SLKT following the MELD era, a consensus meeting was organized in 2007 with the purpose of standardizing the selection of candidates for SLKT and improving patient and renal graft survival following SLKT while limiting unnecessary kidney transplants9 (Supporting Material Appendix 1). These guidelines were revisited and revised recently in 2012.10 Several studies have shown that Scr, creatinine clearance, and standard equations for the calculation of glomerular filtration rate (GFR), namely, Cockcroft-Gault, Modified Diet in Renal Disease (MDRD), and Chronic Kidney Disease Epidemiology Collaboratioan (CKD-EPI), tend to overestimate renal function in patients with cirrhosis when compared to true GFR.11-16 Mesured GFR (mGFR) based on the clearance of exogenous markers including inulin, iothalamate, or iohexol is still considered the gold standard. Unfortunately, these techniques, which are demanding and costly, are not used in routine practice. As a result, in the most recent published SLK guidelines in 2012,10 MDRD-6 equation was recommended for GFR determination because previous studies have suggested that this equation was less likely to overestimate GFR in cirrhosis patients in comparison to the MDRD-4 or Cockcroft-Gault equations.12 However, a higher GFR threshold with MDRD-6 was used compared to iothalamate GFR to account for the ∼30%-40% overestimation that has been described when compared to iothalamate in patients with cirrhosis.10
The aims of this study were to compare current equations used for GFR estimation (MDRD and CKD-EPI) to true GFR measured by iohexol in a large population of cirrhosis patients evaluated for LT and to determine the potential impact of inaccuracies on the current guidelines for SLKT.
Patients and Methods
Study Population
We retrospectively analyzed 300 patients with cirrhosis who were evaluated for a first LT at a single institution between March 2004 and March 2012. At the time of evaluation, in addition to standard workup, all patients underwent direct measurement of GFR using plasma clearance of iohexol as described.17 All measurements were performed in stable conditions. None of the patients had direct measurement of GFR following an acute deterioration of renal function or any other acute precipitating event. Demographic, biological, and anthropometrical variables required for the calculation of GFR according to the MDRD-4, MDRD-6, and CKD-EPI equations were collected simultaneously. The equations corresponding to MDRD-4, MDRD-6, and CKD-EPI are shown in Supporting Materials Appendix 2.
The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the local Ethics Committee.
Criteria for SLKT
The decision for SLKT at our institution is made on an individual basis with a multidisciplinary approach including hepatologists, transplant surgeons, pathologists, and nephrologists. According to our institutional policy, SLKT was considered in patients with endstage liver disease and (1) chronic kidney disease (CKD) with mGFR <30 mL/min/1.73 m2 for at least 3 months or (2) CKD with mGFR between 30 and 40 mL/min/1.73 m2 and biopsy showing severe kidney parenchymal lesions including interstitial fibrosis, glomerulosclerosis, and vascular arterial lesions. Placement on renal replacement therapy immediately prior to transplantation was not necessarily considered an indication for SLKT. Based on this approach, only four patients in this cohort (1%) received SLKT.
Immunosuppression
During the study period, the immunosuppressive regimen was based on tacrolimus, mycophenolate mofetil (2 g per day), and steroids. Steroids were progressively tapered and eventually discontinued at 4 months. Target tacrolimus trough levels were 8-10 ng/mL from postoperative day (POD) 0 to POD 30, 5-8 ng/mL from POD 30 to 1 year, and 3-5 ng/mL thereafter. In patients with pretransplant Scr >1.7 mg/dL or postoperative acute kidney injury with or without renal replacement therapy, immunosuppression therapy consisted of induction with basiliximab, a monoclonal interleukin (IL)−2 receptor antagonist, steroids, and mycophenolate mofetil. Tacrolimus introduction was delayed between POD 7 and 14, depending on the course of renal recovery.
Statistical Analysis
Results for continuous variables are expressed as means ± SD. Student t test, chi-squared test, Fisher's exact test, Mann-Whitney test, and logistic regression analysis were used where appropriate. GFR estimates according to MDRD-4,18 MDRD-6,19 and CKD-EPI20 equations were compared to plasma iohexol clearance by using a paired-samples t test. Pearson's correlation analysis was used for correlation between measured GFR with plasma iohexol clearance (considered the reference) and calculated GFR according to MDRD-4, MDRD-6, and CKD-EPI formulas. Agreement was assessed using Bland-Altman plots. For all tests, P < 0.05 was considered statistically significant. Analysis was performed using SPSS 20 (Chicago, IL) and SAS 9.3 (Cary, NC) software.
Results
Patient Characteristics
The baseline characteristics of the patients are summarized in Table 1. In all, 227 males (76%) and 73 females (24%) were included. The mean age was 54 years. A total of 124 patients (41%) had hepatocellular carcinoma (HCC) meeting the Milan criteria21 at the time of listing.
| Patients | Whole Population n = 300 | mGFR ≤60 mL/min/1.73m2(n = 67) | mGFR >60 mL/min/1.73m2 (n = 233) |
|---|---|---|---|
| Age (years) | 54±9 (20-69) | 57±7 (41-69) | 53±9a (20-69) |
| Male (%) | 76 | 70 | 77 |
| Ethnicity black (%) | 8% | 3% | 9% |
| Body Mass Index (kg/m−2) | 26±4.5 (15-39.2) | 26.3±4.4 (15-35) | 25.8±4.5 (16.5-39.2) |
| Cause of cirrhosis (%) | |||
| Alcohol | 43 | 58 | 40 |
| Hepatitis B virus infection | 10 | 9 | 10 |
| Hepatitis C virus infection | 29 | 16 | 32 |
| Biliary diseases | 4 | 3 | 4 |
| Other | 14 | 14 | 14 |
| Hepatocellular carcinoma (%) | 41 | 21 | 47 |
| Serum bilirubin (mg/dL) | 3.1 ± 3.6 (0.3-30.6) | 3 ± 3.9 (0.3-26.8) | 3.1 ± 3.5 (0.4-30.6) |
| International Normalized Ratio | 1.5 ± 0.6 (1-5) | 1.5 ± 0.4 (1-4) | 1.5 ±0.5 (1-5) |
| Serum creatinine (mg/dL) | 0.9 ± 0.4 (0.4-3.5) | 1.3 ± 0.6 (0.5-3.5) | 0.8 ± 0.3a (0.4-1.6) |
| Blood urea nitrogen (mg/dL) | 15.4 ± 9 (5.1-67.2) | 24.4 ± 13.7 (6.7-67.2) | 12.9 ± 4.2a (5.1-39.2) |
| Albumin (g/L) | 30 ± 6 (14-53) | 29 ± 5 (20-43) | 31 ± 7a (14-53) |
| Serum sodium (mmol/L) | 135 ± 5 (115-144) | 132 ± 5 (115-140) | 136 ± 4a (125-144) |
| Ascites (%) | 59 | 84 | 52 |
| Refractory ascites (%) | 27 | 57 | 19 |
| Child-Pugh score A/B/C (%) | 28/38/34 | 7/52/41 | 34/34/32 |
| MELD score | 15±6 (6-40) | 17±5 (8-37) | 15±5a (7-40) |
| Time to transplant (months) | 8±8 (0-25) | 7±5 (0-19) | 8±8 (0-25) |
| Waiting list mortality (%) | 11 | 21 | 8 |
- a P < 0.05; all values are mean ± standard deviation (range). mGFR: measured glomerular filtration rate; MELD: Model for Endstage Liver Disease. To obtain creatinine in μmol/L, multiply by 88.4. To obtain bilirubin in μmol/L, multiply by 17.1. To obtain urea nitrogen in mmol/L, multiply by 0.357.
During the study period, 287 patients (96%) from the 300 patients were removed from the waitlist for the following reasons: 218 patients (73%) were transplanted 8 ± 8 months after being placed on the waitlist, 32 patients (11%) died 6 ± 5 months after listing, 17 patients (6%) condition improved, 10 patients (3%) had progression of HCC, and 10 patients (3%) were removed for other reasons.
Comparison Between eGFR Equations and mGFR
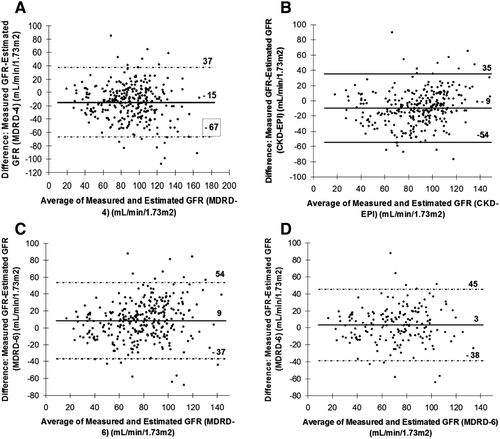
Mean values of mGFR by iohexol clearance and estimated GFR (eGFR) according to different equations are shown in Table 2. The mean interval between mGFR and transplantation in the patients who were transplanted was 8 ± 8 months. Overall, there was a statistically significant although relatively weak correlation between mGFR and eGFR, with R2 values ranging from 0.37 to 0.4 and P < 0.001 for all equations. On average, MDRD-4 and CKD-EPI equations overestimated mGFR by 15 and 9 mL/min/1.73 m2, respectively (Fig. 1A,B). In contrast, MDRD-6 equation underestimated mGFR by an average of 9 mL/min/1.73 m2 (Fig. 1C).
| Mean Value (mL/min/1.73m2) | Mean Differencea (mL/min/1.73m2) | R2 Value (P Value)b | |
|---|---|---|---|
| Whole population (n = 300) | |||
| mGFR | 82 ± 29 | — | — |
| MDRD-4 | 97 ± 32 | −15 ± 27 | 0.37 (<0.001) |
| MDRD-6 | 74 ± 25 | 9 ± 24 | 0.39 (<0.001) |
| CKD-EPI | 92 ± 25 | −9 ± 24 | 0.4 (<0.001) |
| Patients with mGFR >60 mL/min/1.73m2 (n = 233) | |||
| mGFR | 94 ± 21 | — | — |
| MDRD-4 | 106 ± 28 | −12 ± 28 | 0.14 (<0.001) |
| MDRD-6 | 81 ± 22 | 13 ± 23 | 0.15 (<0.001) |
| CKD-EPI | 100 ± 20 | −6 ± 23 | 0.14 (<0.001) |
| Patients with mGFR ≤60 mL/min/1.73m2 (n = 67) | |||
| mGFR | 42 ± 11 | — | — |
| MDRD-4 | 66 ± 24 | −23 ± 22 | 0.18 (<0.001) |
| MDRD-6 | 48 ± 20 | −6 ± 18 | 0.18 (<0.001) |
| CKD-EPI | 64 ± 24 | −22 ± 22 | 0.19 (<0.001) |
| Patients with mGFR <40 mL/min/1.73m2 (n = 26) | |||
| mGFR | 32 ± 3 | — | — |
| MDRD-4 | 53 ± 17 | −23 ± 16 | 0.05 (ns) |
| MDRD-6 | 37 ± 13 | −7 ± 14 | 0.007 (ns) |
| CKD-EPI | 52 ± 18 | −21 ± 17 | 0.04 (ns) |
| Patients without ascites (n = 124) | |||
| mGFR | 96 ± 26 | — | — |
| MDRD-4 | 104 ± 28 | −8 ± 26 | 0.5 |
| MDRD-6 | 80 ± 22 | 16 ± 24 | 0.5 |
| CKD-EPI | 98 ± 21 | −3 ± 23 | 0.5 |
| Patients with ascites (n = 176) | |||
| mGFR | 73 ± 27 | ||
| MDRD-4 | 93 ± 33 | −20 ± 25 | 0.7 |
| MDRD-6 | 70 ± 26 | 3 ± 21 | 0.7 |
| CKD-EPI | 87 ± 27 | −15 ± 22 | 0.7 |
- a Mean difference between measured GFR (iohexol clearance, mGFR) and estimated GFR according to the three equations.
- b Correlation between mGFR and the three estimates of GFR Modified Diet in Renal Disease (MDRD), Chronic Kidney Disease Epidemiology Collaboration.
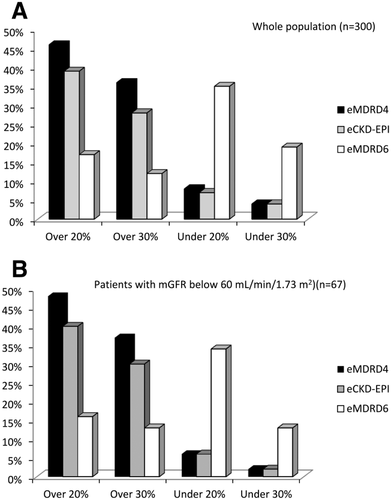
In the whole population, the proportion of patients with a 20% and 30% overestimation of mGFR was higher with MDRD-4 and CKD-EPI than with MDRD-6 (Fig. 2A). However, the proportion of patients with a 20% and 30% underestimation of mGFR was higher with MDRD-6 than with MDRD-4 and CKD-EPI. The same trends were observed in the subgroup of patients with mGFR <60 mL/min/1.73 m2. MDRD-4 and CKD-EPI tended to overestimate measured GFR. In average, the MDRD-6 equation also overestimated the mGFR (Table 2). However, a substantial proportion had an underestimation of mGFR with MDRD-6 (Fig. 2B).
In the subgroup of patients with mGFR <60 mL/min/1.73 m2 (n = 67), there was a statistically significant correlation between mGFR and eGFR (P < 0.001 for all equations) (Table 2). Both MDRD-4 and CKD-EPI overestimated mGFR, differences being higher than in the whole population (23 and 22 mL/min/1.73 m2 respectively). Even though, on average, MDRD-6 also overestimated mGFR, the difference with mGFR in this group was lower (6 mL/min/1.73 m2) as compared to other equations. Differences between mGFR and eGFR were comparable in patients with mGFR <40 mL/min/1.73 m2 with a lower difference seen with the MDRD-6 equation; however, these differences were no longer significant (Table 2). In the subgroup of patients with ascites (n = 176), the correlation between mGFR and eGFR was statistically significant for all equations (Table 2). Again, the MDRD-6 equation was more accurate than MDRD-4 and CKD-EPI at assessing true GFR, with an average overestimation of only 3 mL/min/1.73 m2 (Fig. 1D). In these patients, the MDRD-4 and CKD-EPI equations overestimated true GFR by 20 mL/min/1.73 m2 and 15 mL/min/1.73 m2, respectively.
Accuracy of MDRD-6 to Predict Severe Renal Dysfunction and Impact the Decision for SLKT
Ten patients in this cohort had severe renal dysfunction defined as mGFR <30 mL/min/1.73 m2. None of these patients had proteinuria >1g/day. The sensitivity of MDRD-6 to identify these patients was good, with a threshold value of 40 mL/min/1.73 m2 for eGFR, as recommended by the current guidelines.10, 12 Indeed, 8 out of the 10 patients (80%) with mGFR <30 mL/min/1.73 m2 had MDRD-6-based eGFR <40 mL/min/1.73 m2. From the remaining 290 patients with mGFR over 30 mL/min/1.73 m2, 23 (8%) had MDRD-6-based eGFR <40 mL/min/1.73 m2. In these 23 patients, mGFR ranged from 31 to 111 mL/min/1.73 m2. The specificity of MDRD-6 to identify patients with mGFR <30 mL/min/1.73 m2 was 0.96.
In the subgroup of patients with ascites, 4 out of 10 patients with mGFR <30 mL/min/1.73 m2 had MDRD-6 >40 mL/min/1.73 m2. In addition, 19 out of 166 patients with mGFR >30 mL/min had MDRD-6 <40 mL/min/1.73 m2.
In univariate analysis, factors associated with such discordance between MDRD-6 and mGFR (namely, MDRD-6 <40 mL/min/1.73 m2 and mGFR >30 mL/min/1.73 m2) were older age, low serum sodium, and refractory ascites (Table 3). In multivariate analysis, older age (odds ratio [0R]: 1.08, 95% confidence interval [CI] (1.01-1.15), P = 0.02) and low serum sodium (0R: 0.86, 95% CI (0.79-0.93), P < 0.0001) were the only predictive factors for misclassification.
| Discordance | Concordance | ||
|---|---|---|---|
| n = 23 | n = 267 | P Value | |
| Age (years) | 58 ± 6 | 53 ± 9 | 0.009 |
| Male (%) | 83 | 75 | 0.6 |
| Black ethnicity (%) | 9 | 8 | 0.7 |
| Body mass index (Kg/m2) | 27 ± 4 | 26 ± 5 | 0.4 |
| Alcoholic cirrhosis (%) | 61 | 40 | 0.08 |
| Hepatocellular carcinoma (%) | 30 | 44 | 0.2 |
| Serum bilirubin (mg/dL) | 2.1 ± 2.2 | 3.1 ± 3.4 | 0.2 |
| International normalized ratio | 1.4 ± 0.4 | 1.5 ± 0.4 | 0.6 |
| Serum creatinine (mg/dL) | 1.9 ± 0.7 | 0.8 ± 0.2 | <0.001 |
| Blood urea nitrogen (mg/dL) | 33.9 ± 14.8 | 13.4 ± 5 | <0.001 |
| Albumin (g/L) | 28 ± 5 | 31 ± 7 | 0.09 |
| Serum sodium (mmol/L) | 131 ± 6 | 135 ± 4 | 0.005 |
| Refractory ascites (%) | 48 | 25 | 0.008 |
- The “Discordance” group corresponds to patients with eGFR <40 mL/min/1.73 m2 and mGFR >30 mL/min/1.73 m2. The “Concordance” group corresponds to patients with eGFR >40 mL/min/1.73 m2 and mGFR >30 or patients with eGFR <40 and mGFR <30 mL/min/1.73 m2. eGFR: Estimated glomerular filtration rate; mGFR: Measured glomerular filtration rate. To obtain creatinine in μmol/L, multiply by 88.4. To obtain bilirubin in μmol/L, multiply by 17.1. To obtain urea nitrogen in mmol/L, multiply by 0.357.
| Patients | Age | MELD Scorea Evaluation/Latest | GFR (mL/min/1.73m2) | MDRD6 (mL/min/1.73m2) | Outcome After Listing | Creatinine 2-Year Posttransplant (μmol/L) | Alive 3-Year Posttransplant |
|---|---|---|---|---|---|---|---|
| 1 | 67 | 14/21 | 32 | 29 | LTAb | 1.2 | yes |
| 2 | 64 | 12/12 | 42 | 27 | LTAb | 1.3 | yes |
| 3 | 61 | 17/25 | 59 | 23 | LTAb | 1.2 | yes |
| 4 | 59 | 17/17 | 34 | 38 | LTAb | 1.2 | yes |
| 5 | 65 | 14/20 | 40 | 28 | LTAb | 1.2 | yes |
| 6 | 58 | 21/13 | 43 | 27 | LTAb | 0.9 | yes |
| 7 | 53 | 11/7 | 80 | 39 | LTAb | 1.4 | yes |
| 8 | 66 | 14/13 | 45 | 27 | LTAb | — | no |
| 9 | 62 | 18/18 | 41 | 30 | LTAb | — | nof |
| 10 | 50 | 21/21 | 103 | 39 | LTAb | — | nof |
| 11 | 66 | 17/15 | 44 | 28 | LTAb | — | nof |
| 12 | 58 | 22/22 | 38 | 30 | LTAb | — | nof |
| 13 | 46 | 22/22 | 40 | 19 | SLKTc | 1 | yes |
| 14 | 58 | 19/19 | 33 | 38 | SLKTc | 1.1 | yes |
| 15 | 59 | 22/34 | 56 | 32 | Died on WLd | — | — |
| 16 | 56 | 30/30 | 70 | 39 | Died on WLd | — | — |
| 17 | 46 | 20/38 | 32 | 20 | Died on WLd | — | — |
| 18 | 50 | 9/24 | 35 | 16 | Died on WLd | — | — |
| 19 | 54 | 11/11 | 43 | 35 | Died on WLd | — | — |
| 20 | 59 | 17/26 | 44 | 27 | Died on WLd | — | — |
| 21 | 60 | 20/20 | 111 | 23 | WLd | — | — |
| 22 | 64 | 16/16 | 38 | 39 | WLd | — | — |
| 23 | 62 | 19/12 | 31 | 38 | Removed from WLd, e | — | — |
- a The MELD score was calculated at evaluation and thereafter at the last follow-up before transplantation, death or removal from the waiting list.
- b LTA, liver transplantation alone.
- c SLKT, simultaneous liver and kidney transplantation.
- d WL, waiting list.
- e Improved.
- f Died in the early posttransplant period in a context of multiple organ failure.
Individual outcomes of the patients with discordant results between mGFR and MDRD-6 are summarized in Table 4. Six patients died on the waiting list, one patient was removed from the waiting list due to improvement in liver function, and two patients were still on the waitlist at the end of the study period. None of these patients required renal replacement therapy. Fourteen patients were transplanted including 12 with LT alone and two with SLKT. The decision for SLKT was based on kidney biopsy showing severe glomerulosclerosis and vascular lesions. Among those with LT alone, seven (58%) were still alive at 2 years with Scr between 0.9 and 1.4 mg/dL. Five patients (42%) died within the first postoperative month due to sepsis and multiple organ failure.
Among the eight patients with mGFR <30 mL/min and MDRD-6 <40 mL/min, two had SLKT (one is alive 6 years after transplantation with normal liver and kidney functions and the other patient died 2 months after transplantation in a context of septic shock); two were removed from the waiting list due to improvement in liver and renal function (none of them required hemodialysis, with a follow-up of 3 and 4 years, respectively); one patient who was still on the waiting list at the end of the study had been placed on dialysis; one patient received LT alone and died 1 year after transplantation (the cause of death was unknown) and the remaining two patients died on the waiting list due to septic shock progressing to multiple organ failure.
Discussion
Our series, which included 300 consecutive patients who systematically had plasma iohexol clearance for their transplant evaluation, enabled us to determine the precision of different equations to estimate GFR in patients with cirrhosis. In line with previous series, we found that the widely used MDRD-4 and CKD-EPI equations tended to overestimate true GFR with a difference of 10 to 15 mL/min/1.73 m2.15, 16, 22, 23 In addition, we found that overestimation was higher in patients with GFR <60 mL/min/1.73 m2 defining those with CKD Stage 3 according to the Kidney Disease Outcomes Quality Initiative (KDOQI) CKD classification.24
Both MDRD-4 and CKD-EPI are based on Scr, which is an inaccurate marker of renal function in patients with cirrhosis. In these patients, increased volume of fluid distribution and decreased creatine production related to both poor muscle mass and impaired liver function result in an overestimation of GFR for any given value of Scr. Based on the 2012 guidelines,10 SLKT should be considered when MDRD-6-based eGFR is <40 mL/min/1.73 m2 in patients with CKD for >3 months.10 This recommendation is based on the assumption that when MDRD-6-based eGFR is <40 mL/min/1.73 m2, true GFR is <30 mL/min/1.73 m2. Since both MDRD-4 and CKD-EPI overestimated true GFR, our data validate the conclusion that the MDRD-6 equation is superior to the MDRD-4 and CKD-EPI equations to identify stable cirrhosis patients with markedly impaired renal function, including those with ascites. It must be noted that in this series the proportion of patients in whom eGFR underestimated true GFR was superior with the MDRD-6 equation compared to the MDRD-4 and CKD-EPI equations. An important consequence of this finding is that applying the current recommendations with a threshold value of MDRD-6 eGFR <40 mL/min/1.73 m2 would have resulted in considering SLKT rather than LT alone in at least 7 out of 290 patients who had true GFR >30 mL/min/1.73 m2. This proportion could be higher in the subgroup of patients with ascites (5%). Therefore, attempts to reduce the rate of overestimation of GFR and to better identify those with true GFR <30 mL/min/1.73 m2 resulted in an increase in the rate of underestimation with a risk of performing unnecessary kidney transplantation in patients with relatively preserved renal function. This conclusion is supported by the finding that in our series, none of the patients with MDRD-6-based eGFR <40 mL/min/1.73 m2 who received LT alone and survived more than 1 year had to be placed on long-term dialysis and/or listed for kidney transplantation.
The results of this study illustrate the limitations of the existing Scr-based equations in cirrhosis. Several equations have been validated in the general population, the most recent being the CKD-EPI equation.20 Unfortunately, a number of biases, in cirrhosis patients result in substantial differences between calculated GFR and true GFR. In this study we found that advanced age and low serum sodium but not refractory ascites were significantly and independently associated with discordant results between MDRD-6 and true GFR. The reason why, in contrast to MDRD-4,16 the precision of MDRD-6 was not significantly influenced by refractory ascites could be that this latter equation takes into account serum albumin concentration. Indeed, a statistical interaction between albumin and ascites can be reasonably expected. These results suggest that other factors specific to cirrhosis should be taken into account for estimating GFR. Serum cystatin C, which is independent of muscle mass, may be superior to Scr for predicting the outcome in patients with renal dysfunction.25 However, recent studies failed to demonstrate an advantage of cystatin C over Scr at assessing renal function and predicting outcome in patients with cirrhosis.26, 27 Overall, specific equations are still needed in cirrhosis patients.
Besides the precise assessment of residual renal function, prediction of reversibility with improvement in renal function following LT alone is a major issue. In this series, only four patients, all of whom had mGFR <40 mL/min/1.73 m2 had SLKT. The decision for SLKT rather than LT alone was based on the value of mGFR in stable condition and markers of chronic kidney parenchymal changes based on transjugular renal biopsy. The results of posttransplant radionuclide renal scan, to determine native renal GFR after SLKT, have been reported recently.28 In this single-center series where the institutional criteria for SLKT were more liberal than the UNOS criteria,29 47 out of 78 patients (60%) did not meet the native renal pre-SLKT UNOS criteria after combined transplantation. On multivariate analysis, only abnormal renal imaging within 3 months pretransplantation was predictive of native renal nonrecovery.28 These data indicate that the potential for renal recovery following LT alone may be underestimated and that predictive markers of native renal recovery should be refined.
In conclusion, this study confirms that the MDRD-6 equation is more accurate than the MDRD-4 and CKD-EPI equations to identify cirrhosis patients with true GFR below 30 mL/min/1.73 m2 supporting the potential usefulness of MDRD-6 in the decision making for SLKT. However, a limitation of MDRD-6 is that it underestimates true GFR in a small proportion of cirrhosis patients, which may contribute to unnecessary kidney transplantation. Thus, we suggest that when possible a decision for SLKT ideally should be based on direct measurement of GFR or renal biopsy. Predictive factors of renal recovery after LT alone should also be taken into account. Specific equations are still needed to better assess renal function in cirrhosis.