Inhibition of transforming growth factor β receptor I kinase blocks hepatocellular carcinoma growth through neo-angiogenesis regulation†
Potential conflict of interest: Nothing to report.
Abstract
Curative therapies for patients with hepatocellular carcinoma (HCC) are mainly invasive, and with the exception of sorafenib, no medical treatments are available for advanced or metastatic stages of HCC. We investigated the antitumoral effect of blocking the transforming growth factor β (TGF-β) signaling pathway in HCC with LY2109761, a kinase inhibitor of TGF-β receptor I kinase. The antitumor activity of LY2109761 was associated with inhibition of molecular pathways involved in neo-angiogenesis and tumor growth of HCC. This anti-angiogenic effect is more effective than that of bevacizumab, which specifically targets vascular endothelial growth factor (VEGF). We found that the paracrine cross-talk between HCC and endothelial cells is blocked by LY210976, inhibiting blood vessel formation. This effect was mediated by SMAD2/3 and affected the secretion of VEGF. Finally, LY2109761 does not show significant effects on phsyiological angiogenetic development. Conclusion: These data support the rationale for targeting TGF-β signaling in patients with HCC. (HEPATOLOGY 2009.)
Therapeutic options for patients with hepatocellular carcinoma (HCC) are still limited. Curative approaches, including surgical resection and liver transplantation, are attempted in only 30% of patients, and even in these cases the rate of cancer recurrence within 5 years is approximately 60% to 70%.1 The molecular mechanisms regulating tumor progression of HCC are still unclear. However, with the advent of sorafenib, a multi–tyrosine kinase inhibitor, as an approved systemic therapy for advanced cases of HCC, overall survival has been improved.2 This development has paved the way for exploring new medical treatments.
HCC is characterized by a strong angiogenic activity that can be visualized with computed tomography. Based on the radiographic appearance of this disease, it is possible to differentiate neoplastic from regenerative or dysplastic HCC nodules.3 The vascular endothelial growth factor (VEGF) is a strong angiogenic factor in HCC and is mainly responsible for neovascularization.4, 5 Several studies have reported that increased levels of VEGF in the serum and tissue of patients with HCC correlate with disease progression.6, 7 Furthermore, this angiogenic activity leads to a higher risk of vascular invasion, which is in turn a negative prognostic factor in surgically treated patients.8, 9 Hence, it was not unexpected that bevacizumab should show a clinical antitumor activity in phase II studies of patients with unresectable HCC.10, 11
Recently, we have shown that LY2109761 displays an antimetastatic activity in HCC in vivo models by increasing the expression of E-cadherin on the HCC cellular surface, thus blocking the invasion into the surrounding stroma.12 Furthermore, it also inhibits the spread of HCC through blood vessels, dephosphorylating the intracytoplasmic tail of β1 integrin, the main receptor responsible for fibrinogen and fibronectin-dependent migration. Because these two extracellular matrix proteins are the major internal and external constituents of the blood vessel wall, LY2109761 blocks HCC cells intravasation by way of a a selective inhibition of the transforming growth factor β1 (TGF-β1) SMAD-dependent pathway.13
TGF-β1 can be considered as a hallmark of HCC because it is increased in the serum, tissue, and urine of patients, and its increased levels correlate with tumor progression and survival.14-16 Taken together, studies by other authors and our own investigation support the hypothesis that targeting the TGF-β1 pathway offers a new approach to medical treatment of HCC. Because of the dual functions of TGF-β1 in HCC as a metastatic promoter and cell proliferation suppressor, the aim of this study is to investigate the effectiveness of LY2109761 on HCC growth.
Abbreviations
CAM, chorioallantoic membrane; EGFR, endothelial growth factor receptor; ELISA, enzyme-linked immunosorbent assay; HCC, hepatocellular carcinoma; HUVEC, human umbilical vein endothelial cell; MAPK, mitogen-activated protein kinase; mRNA, messenger RNA; SEM, standard error of the mean; siRNA, small interfering RNA; TGF-β, transforming growth factor β; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
Materials and Methods
Cells, Antibodies, and Reagents.
Human HCC cell lines HLE and HLF were cultured as described.17 To generate HCC cells lines ubiquitously expressing green fluorescent protein, cultured cells were infected by retroviral vector pLXSN-GFP (BD Clontech). Human umbilical vein endothelial cells (HUVECs) were obtained from American Type Culture Collection (Rockville, MD). Bevacizumab (Avastin, 100 mg/4 mL) was purchased from Roche Pharmaceutical (Welwyn Garden City, UK). TGF-β1 receptor kinase inhibitor LY2109761 was kindly provided by Eli Lilly (Indianapolis, IN). The p38MAPK inhibitor SB203580 and c-Jun N-terminal kinase inhibitor SP600125 were purchased from Calbiochem (La Jolla, CA). The enzyme-linked immunosorbent assay (ELISA) kit for VEGF was purchased from R&D Systems Inc. (Minneapolis, MN). Recombinant human TGF-β1 was purchased from Calbiochem (La Jolla, CA). Recombinant human VEGF165 was obtained from R&D Systems, Inc. Rat anti-CD31 monoclonal antibody (Clone MEC 7.46) was purchased from Abcam (Cambridge, MA). Antibodies directed against total and phospho-Smad2 and phospho-Smad3 were purchased from Cell Signaling Technology, Inc. (Danvers, MA). Anti–phospho-38 mitogen-activated protein kinase (MAPK) and anti-phospho–c-Jun N-terminal kinase antibodies were purchased from New England Biolabs (Beverly, MA). After receiving informed consent, HCC tissue specimens were collected immediately after resection and snap-frozen in liquid nitrogen. The use of these tissues was approved by an internal review board.
Chorioallantoic Membrane Tumor Formation Assay.
Fertilized white Leghorn chicken eggs were incubated in a forced-air incubator. We used embryos after 9 days of incubation unless otherwise noted. To determine the size of resulting chorioallantoic membrane (CAM) tumors, the embryos were opened, and tumor diameter was determined with the aid of a dissecting microscope. Tumors were then carefully cut away from normal CAM tissue, and the wet weight of the tumor was determined. In some experiments, recombinant human VEGF (5 μg/kg, ≈500 ng/egg) was administered daily for 4 days by way of a single peritumoral injection.
Preparation of CAM for Histological Examination.
CAMs inoculated with HLE and HLF cells were dissected at different times after inoculation and fixed in 4% buffered formalin. Control CAMs were obtained from embryos inoculated with phosphate-buffered saline, without tumor cells. The tissues were cut into 5-μm-thick sections, frozen, and stained with hematoxylin-eosin.
Immuno-histochemical Staining of CD31 and Quantification of Tumor Microvessel Density.
For CD31 staining, frozen tissue sections (5 μm thick) were fixed in acetone, blocked hydrogen peroxide, and incubated with peroxidase-conjugated anti-rat immunoglobulin G. Next, slides were rinsed, incubated with diaminobenzidine (Research Genetics), and counterstained with Mayer's hematoxylin. A CD31-positive reaction was indicated by a reddish-brown precipitate in the cytoplasm. For quantification of tumor microvessel density, vessels on each section were counted in five high-power fields (magnification ×200 [×20 objective, ×10 ocular]).
ELISA for VEGF.
Conditioned medium was collected from transfected cells after incubation with or without TGF-β1 (5 ng/mL). Cell extracts were centrifuged at 15,000 rpm for 15 minutes, and the protein concentration was determined by colorimetric Bradford protein assay. VEGF was assessed by way of ELISA (Quantikine; R&D Systems), and VEGF tissue content was measured using an ELISA kit from RayBiotech, Inc. (Norcross, GA).
Isolation of Poly(A)+ RNA and Northern Blot Analysis.
RNA was isolated with Qiagen Rneasy according to the manufacturer's instructions. The VEGF complementary DNA probe used was a 0.65-kb EcoRI insert of the human full-length VEGF complementary DNA cloned in pGEMT (Promega) and verified by sequencing. The blots were exposed to Kodak X-AR film. Similar results were obtained from three independent experiments, and representative blots are shown.
Real-Time Polymerase Chain Reaction Analysis.
To quantify messenger RNA (mRNA) expression, real-time time polymerase chain reaction was performed. Reaction specificity was confirmed by electrophoretic analysis of products prior to real-time, reverse-transcription polymerase chain reaction, and bands of expected size were detected. Gene expression was normalized to the expression of the glyceraldehyde 3-phosphate dehydrogenase mRNA, yielding the relative gene expression value.
In Vivo Imaging of Chick Embryo CAM.
For imaging experiments, eggs were prepared according to the shell-less (ex ovo) chick embryo assay procedure as described.13 Digital images were acquired using AxioVision imaging software (Zeiss, Jena, Germany) and further processed using Photoshop (Adobe, San Jose, CA). For quantification of angiogenesis in untreated and treated tumors, imaging was performed once per day for several days after tumor implantation. Average vessel number, diameter, and other parameters were acquired from transmitted light images using a software developed in-house combining automatic and manual vessel identification and counting.
Pharmacologic Inhibition of the TGF-β1 Receptor.
HLE and HLF cells were seeded on day 0 in 100-mm Petri dishes and pretreated in culture with the TGF-βRI/II kinase inhibitor LY2109761 (0.1 μM), or dimethyl sulfoxide vehicle (control) on days 1 and 2. Animals were randomized into four groups (n = 10 in each group), with the developing tumors treated as follows: (1) control (vehicle: dimethyl sulfoxide); (2) 5 mg/kg bevacizumab; (3) 50 mg/kg LY2109761 alone; and (4) 50 mg/kg LY2109761 in combination with 50 μg bevacizumab. After treatment, tumors were excised from the CAM and collected for histology or other purposes. In some experiments, tumors were imaged in vivo as described above.
Gene Silencing in HCC Cell Lines.
At 24 hours after plating, cells were transfected with 10 nM of a pool of three target-specific 20- to 25-nt small interfering RNAs (siRNAs) designed to knock down Smad2, Smad3, JUN (Santa Cruz Biotechnology, Inc.), or p38MAPK (SignalSilence siRNA Kit, Cell Signaling) gene expression or nonsilencing control (control-siRNA) in TransIT-TKO siRNA Transfection Reagent (Mirus Bio Corporation, Madison, WI). Seventy-two hours after transfection, supernatants were harvested and VEGF was measured by way of ELISA as described above.
Western Blot Analysis.
Cells were lysed in proper buffer, supplemented with a protease inhibitor cocktail (Sigma, St. Louis, MO), for 30 minutes on ice. After determination of the protein concentration using the bicinchoninic acid method (Pierce Chemical Co., Rockford, IL), proteins were separated in a sodium dodecyl sulfate–polyacrylamide gel electrophoresis gel.
HUVEC Growth Inhibition Assay.
HUVEC proliferation was estimated by means of the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. The inhibitory effect of the various conditions was calculated as the percentage inhibition relative to the control group.
HUVEC Migration Assay.
HUVEC migration was assayed using modified Boyden chambers as described.17 Cells that migrated to the bottom of the filters were visualized under a microscope (magnification ×100) and counted in five randomly chosen fields. The results are expressed as the mean ± standard error of the mean (SEM) of four independent experiments.
CAM Angiogenesis Assay.
CAM assays were performed as follows. Fertilized white Leghorn chick eggs (SPAFAS, Inc., Norwich, CT) were incubated at 38°C under constant high humidity. On the third day of incubation, a square window was opened in the shell after removal of 2 to 3 mL of albumen, so as to detach the developing CAM from the shell. On day 9, 25 μL of conditioned media from HLE, treated with TGF-β1 alone or in combination with LY2109761, were directly implanted on the tops of the growing CAMs under sterile conditions. Recombinant VEGF and serum-free/insulin-free were used as positive and negative control, respectively. On day 10, the CAMs were examined and photographed in ovo under a Nikon stereomicroscope. Angiogenesis was estimated on the basis of the angiogenic index (mean number of tertiary branch points seen under the experimental conditions minus the mean number of branch points seen in buffer controls with no angiogenic stimulator).
Reverse-Phase Protein Microarray Construction.
A detailed description of the technology and associated methodology has been published previously.18, 19 All antibodies used in this study were validated for specificity by immunoblotting prior to their use on the arrays. Total protein values were assessed by staining the slides with Sypro Ruby Blot Stain (Molecular Probes, Eugene, OR). Stained slides were scanned with Axon GenePix (Molecular Devices, Sunnyvale, CA), and spot intensities were quantitated and normalized to total protein.
Statistical Analysis.
All data were described as the mean ± SEM. For statistical analysis, one-way analysis of variance or two-tailed Student t test were performed using Instat V3.05 statistical software (GraphPad Inc., La Jolla, CA) and a P value of <0.05 was considered statistically significant. All experiments were repeated on at least three separate occasions.
Results
LY2109761 and Bevacizumab Block Tumor Growth in HCC.
We have previously demonstrated that LY2109761 blocks invasion of the HCC cells into the tissue microenvironment and blood vessels.12, 13 We now investigated the effectiveness of LY2109761 in blocking HCC tumor growth using the xenograft chick embryo model.13 HLE and HLF cells, which are well-characterized invasive HCC cell lines,17 were implanted on the chick embryo CAM and allowed to form visible tumors over a period of 1 week (Fig. 1A,F). Macroscopically, these tumors appear hypervascular (Fig. 1B,G), and the microscopic examination shows a poorly differentiated tumor similar to the one seen in humans. The tumor has a diffuse and macrotrabecular pattern and a well-developed blood vessel network (Fig. 1C, H).
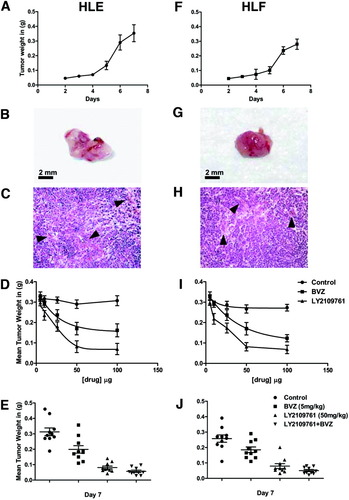
Antitumor activity of the TGF-βR1 kinase inhibitor on HCC growth in xenograft model. (A,F) Kinetics of tumor growth of (A) HLE and (F) HLF in chick embryo CAM. (B,G) Macroscopic analysis of (B) HLE and (G) HLF tumors growing on chick embryo CAM. (C,H) Histological sections of (C) HLE and (H) HLF tumors growing on chick embryo CAM. (D,I) The effect of LY2109761 and bevacizumab on HCC growth was determined by the log concentration response (IC50) on (D) HLE and (I) HLF tumors. (E) Antitumor effect of LY2109761 and bevacizumab in xenograft models of human HCC. The results shown represent the mean ± SEM of four separate experiments.
We took advantage of this high-grade vascularization to assess the inhibition HCC tumor growth achieved with two targeted drugs. Dose–response experiments showed that bevacizumab had a strong antitumoral effect on tumor growth at standard doses (5 mg/kg), reaching a plateau at higher doses. Interestingly, LY2109761 showed a similar effect on tumor growth at a dose of 50 mg/kg (Fig. 1D,I).
After a week, bevacizumab strongly (P < 0.01) inhibited the growth of HCC (Fig. 1E-J). LY2109761 treatment led to an enhanced inhibition of tumor growth (P < 0.001) compared with bevacizumab-treated animals (P < 0.01), and the combined treatment of bevacizumab and LY2109761 had the strongest (P < 0.001) antitumor growth effect (Fig. 1E,J). This synergistic effect is explained by the fact that administration of VEGF restores tumor growth in LY2109761-treated animals, although not entirely (Supporting Fig. 2A). Consistently, in vitro the addition of VEGF to HUVEC cells incubated with conditioned medium from LY2109761-HCC treated cells partially restores cell proliferation (Supporting Fig. 2B). These data suggest that the inhibition of VEGF remains the main mechanism responsible for HCC growth, but other pathways, as could be expected, also have a role.
LY2109761 Shows a Distinct Anti-angiogenic Activity Compared with Bevacizumab.
To better characterize how LY2109761 inhibits the growth of HCC, we investigated its effects on tumor angiogenesis. After 7 days of treatment with bevacizumab, HCC tumors showed a macroscopic reduction in size, with a decreased vascularization compared with the control. However, LY2109761 had a greater antivascular effect, which was not further enhanced by bevacizumab. The addition of LY2109761 to bevacizumab showed equal potency to LY2109761 alone (Fig. 2A). We also assessed blood vessel formation by staining for CD31 expression. Angiogenesis in HCC was reduced in bevacizumab-treated tumors, but the effect was even stronger in tumors treated with LY2109761 (Fig. 2B). Quantification of microvessel density showed a significant (P < 0.05) reduction in the blood vessel numbers in tumors treated with bevacizumab. A further decrease (P < 0.001) in the number of blood vessels was evident in tumors treated with LY2109761. Combination of LY2109761 and bevacizumab showed the greatest reduction in microvessel density (P < 0.001) (Fig. 2C). Intravital microscopy confirmed the histological results (Supporting Fig. 1). Further analysis of intravital imaging of neo-angiogenesis revealed that bevacizumab and LY2109761 significantly (P < 0.001, and P < 0.001, respectively) reduced the number of blood vessels (Fig. 3A,B). The diameter of tumor blood vessels was not affected by any of the treatments (Fig. 3C), whereas the surface and tortuosity of blood vessels were both significantly reduced (P < 0.001 and P < 0.05, respectively) by bevacizumab and LY2109761. Combined treatment of bevacizumab and LY2109761 did not enhance the activity of LY2109761 alone (Fig. 3D,E).
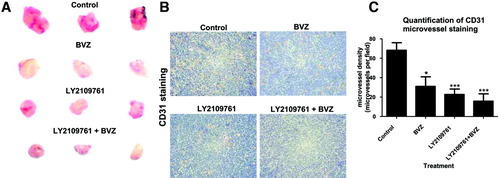
Anti-angiogenic activity of the TGF-βRI kinase inhibitor on HCC neoangiogenesis in a xenograft model. (A) Representative tumor sizes from each group of animals. (B) Representative photos show that LY2109761 treatment significantly decreased the CD31-positive cells (brown areas). (C) Quantification of CD31 microvessel staining. The density of microvessels was significantly lower after LY2109761 administration, compared with the untreated control groups. Data are expressed as the mean ± SEM. *P < 0.05, ***P < 0.001 versus control.
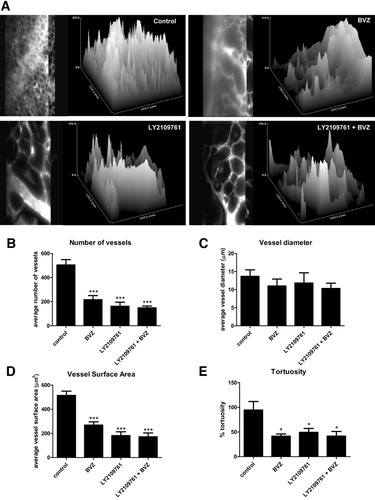
Intravital quantification of the TGF-βRI kinase inhibitor effectiveness on HCC blood vessel formation. (A) Three-dimensional image reconstruction of representative vasculature of HLE tumors. Images show all vascular traces projected onto the two-dimensional x–y plane and show the original volume-rendered data of vascular networks following vascular tracing and diameter measurements. As compared with control, treatment with bevacizumab, LY2109761, or LY2109761 + bevacizumab decreased the number (B), surface (D), and tortuosity (E) but not the diameter (C) of the vessels. Data are expressed as the mean ± SEM from three independent experiments. *P < 0.05, ***P < 0.001 versus control.
Paracrine and Autocrine Regulation of VEGF by TGF-β1.
To further investigate the mechanism by which LY2109761 blocks neo-angiogenesis in HCC, we investigated the paracrine and autocrine inhibition of VEGF production. TGF-β1 stimulates HLE cells to secrete VEGF in the conditioned medium. This paracrine stimulation is time-dependent, reaching a peak after 72 hours, and is completely inhibited by LY2109761 (Fig. 4A). LY2109761, at a concentration of 1 μM, completely inhibited the secretion of VEGF induced by stimulation of cells with 5 ng/mL of TGF-β1 for 72 hours (Fig. 4B). Remarkably, LY2109761 completely inhibited the secretion of VEGF induced by the presence of an endogenous TGF-β1 autocrine loop after 48 hours, whereas a TGF-β1 neutralizing antibody showed only a 30% reduction (Fig. 4C). The inhibition of VEGF secretion is also regulated at a transcriptional level, because LY2109761 abolished the synthesis of VEGF mRNA after 24 hours (Fig. 4D). After 8 hours, Northern blot experiments showed that TGF-β1–stimulated VEGF production was completely inhibited by LY2109761 (Fig. 4E). Finally, inhibition of VEGF production was dose-responsive starting at concentrations of 0.1 μM of LY2109761 (Fig. 4F). The cellular expression of VEGF in HLE- and HLF-forming HCC was also measured by way of ELISA. We found that in bevacizumab-treated tumors, VEGF levels are significantly (P < 0.001) reduced as compared with controls. Surprisingly, VEGF tissue levels were also significantly (P < 0.001) reduced in LY2109761-treated tumors. This effect was not enhanced by combining bevacizumab with LY2109761 (Fig. 4G).
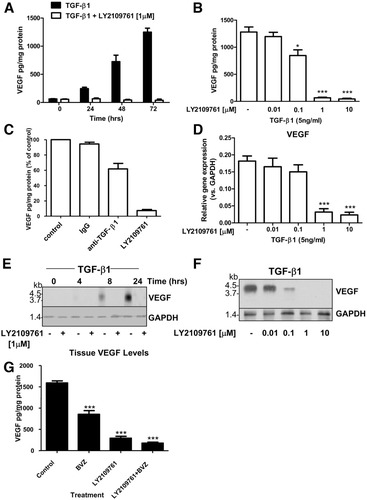
VEGF gene expression is abrogated by inhibition of TGF-βRI. (A) Detection of VEGF in human HLE. The mean ± SEM of four separate experiments, each performed in triplicate, are shown. (B) Inhibition of VEGF production in HLE following incubation with increasing doses of LY2109761. The mean ± SEM of four separate experiments, each performed in triplicate, are shown. *P < 0.05, ***P < 0.001. (C) Serum-starved HLE cells were incubated with 50 ng/mL neutralizing TGF-β1 antibody or with a control antibody for 72 hours, and the VEGF concentration in supernatants was determined using ELISA. The results shown represent the mean ± SEM of four separate experiments, each conducted in triplicate. (D) Effects of TGF-β1 on VEGF gene expression. Values are expressed as the fold induction and represent the mean ± SEM of three independent experiments. ***P < 0.001. (E) Analysis of VEGF mRNA in HLE cells stimulated with TGF-β1 and treated with LY2109761, or for 6 hours with the indicated concentrations of LY2109761 (F). (G) Tissues were collected after treatment and VEGF was measured using ELISA. Data are expressed as the mean ± SEM. *** P < 0.001 versus control.
TGF-β–Regulated VEGF Production Is SMAD-Dependent
Next, we determined whether the anti-angiogenic effect of TGF-β inhibition was specific for the canonical TGF-β pathway. Hence, we studied the role of both SMAD-dependent and SMAD-independent signaling pathways in HCC and their association with VEGF-dependent angiogenesis. In HLE cells, SMAD-2 and SMAD-3 were knocked down as measured by protein levels (Fig. 5A). TGF-β1 efficiently stimulates the production of VEGF in cells transfected with control-siRNA, whereas in SMAD-2 and SMAD-3 silenced cells, the production of VEGF was significantly reduced (Fig. 5B). The SMAD-independent pathway was investigated by silencing JUN and p38MAPK protein levels (Fig. 5C). TGF-β1–stimulated VEGF production in both control-siRNA tranfected cells and p38MAPK-silenced or JUN-silenced cells (Fig. 5D). Furthermore, LY2109761 inhibited the phosphorylation of SMAD-2 and SMAD-3 induced by TGF-β1. In contrast, no effect was observed on the SMAD-independent signaling pathways, indicating that the production of VEGF induced by TGF-β1 occurred through activation of the SMAD-dependent signaling pathway (Fig. 5E). To confirm these data, HLE cells were treated with pharmaceutical inhibitors of p38MAPK and pJUN (SB202190 and SP600125, respectively). Both drugs inhibit proper targets, though they do not display any effect on VEGF production (Supporting Fig. 3).
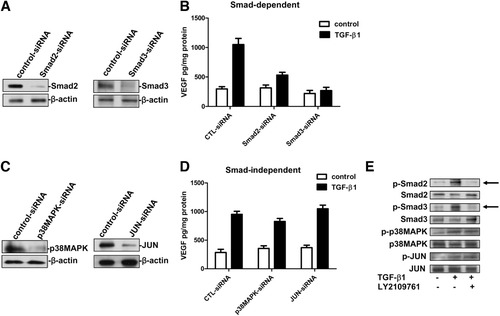
VEGF gene expression induced by TGF-β1 is Smad-dependent. (A) Western blot analysis showing the Smad2 and Smad3 protein knockdown in HLE cells after transfection with siRNAs. (B) VEGF levels in the supernatants from HLE transfected with Smad siRNAs. (C) Western blot analysis showing the p38MAPK and JUN knockdown in HLE cells after transfection with siRNAs. (D) VEGF levels in the supernatants from HLE transfected with p38MAPK and JUN siRNAs. Data are shown as means ± SEM from triplicate experiments, each of which contained triplicate cultures. (E) Representative immunoblot showing TGF-β1–induced pSmad2, pSmad3, p38MAPK, and pJUN phosphorylation in HLE cells. LY2109761 selectively blocks the phosphorylation of Smad2 and Smad3 (arrows), thus blocking the activation of the Smad-dependent TGF-β1 signaling pathway.
HCC Cells Stimulate TGF-β–Dependent Angiogenesis in Surrounding Tissue.
We also investigated the biological and physiological relevance of TGF-β–dependent angiogenesis on the tumor surrounding tissue. Conditioned media of HLE cells treated with TGF-β1 alone significantly (P < 0.001) promoted migration of HUVECs. This effect was as strong as the migration caused by recombinant human VEGF. In contrast, conditioned media from cells stimulated with TGF-β1 and then treated with bevacizumab inhibited HUVEC migration. More importantly, treatment with LY2109761 alone or in combination with bevacizumab following TGF-β1 stimulation completely inhibited migration of HUVECs (Fig. 6A). Therefore, HUVEC proliferation is significantly (P < 0.001) inhibited by the conditioned media from TGF-β1–stimulated HCC cells treated with LY2109761 (Fig. 6B).
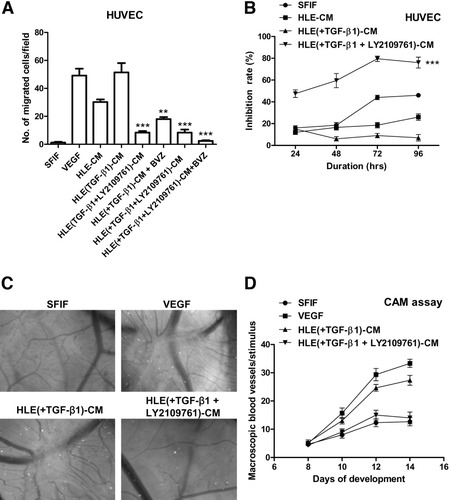
Inhibition of the cross-talk between HCC cells and surrounding endothelial cells by the TGF-βRI kinase inhibitor. (A) Effect of conditioned medium from HLE stimulated with TGF-β1 in the presence or absence of LY2109761 on the migration of HUVECs. The results are expressed as the mean ± SEM of four independent experiments. **P < 0.01, ***P < 0.001. (B) Effect of conditioned medium from HLE stimulated with TGF-β1 in the presence or absence of LY2109761 on HUVEC growth. The results are expressed as the mean ± SEM of four independent experiments. ***P < 0.001. (C) Effect of conditioned medium from HLE stimulated with TGF-β in the presence or absence of LY2109761 on the CAM angiogenesis (magnification ×25). (D) Representative CAM assay showing the effect of conditioned medium from HLE stimulated with TGF-β in the presence or absence of LY2109761 applied on top of the CAM on day 8. Data represent the mean ± SEM of three independent experiments. CM, conditioned medium.
Finally, we tested the same conditioned media in an in vivo model. VEGF promoted new blood vessel formation in the CAM assay. Also, conditioned media of HLE cells treated with TGF-β1 stimulated blood vessel formation to the same extent as VEGF. No angiogenesis was observed in the conditioned media of HLE treated with TGF-β1 and LY2109761 (Fig. 6C). Quantification of the experiment showed that VEGF and HLE conditioned medium stimulated with TGF-β1 have a similar impact on angiogenesis in HCC (Fig. 6D).
LY2109761 Does Not Affect Embryogenesis.
To evaluate the anti-angiogenic effect of LY2109761 on the normal development of tissues and organs, we used a chick embryo model. Animals developed normally in both treatment and control groups (Fig. 7A). The percentage of surviving animals after each treatment was similar among the groups (Fig. 7B). Also, the weight at the end of each experiment was unchanged (Fig. 7C). Importantly, the liver, heart, and brain developed normally (Fig. 7D). Histology of the heart and liver did not show any difference between treated and untreated animals (Fig. 7E), nor did embryos show any abnormal development (Fig. 7F). In conclusion, at the indicated doses of LY2109761, no major organ toxicity nor abnormal embryonal development were observed.

LY2109761 anti-angiogenic activity toward physiological blood vessels. (A) Morphometric analysis of chick embryos during treatment with LY2109761 shows normal development of the embryo without differences between the untreated or control-treated embryos at the same stage of development. (B) Toxicity for LY2109761, bevacizumab, or LY2109761 in combination with bevacizumab in fertilized white Leghorn chicken eggs. All experiments were terminated 3 days prior to hatching. (C) Bar graph showing no significant reduction in weights of embryos after treatment with LY2109761, bevacizumab, or LY2109761 in combination with bevacizumab. Data represent the mean ± SEM of three independent experiments. (D) Wet organ mass (in grams) during egg development following treatment with LY2109761 compared with the control group. Data represent the mean ± SEM of three independent experiments. (E) Histological sections of chicken embryo heart and liver exposed to LY2109761 or control (vehicle; original magnification ×600). No apparent differences in histology of the heart and of the liver were observed, and no abnormalities were noted in the examined sections. (F) No apparent differences in the chick embryo development were detected between LY2109761 treatment and controls.
TGF-β Signaling Has a Distinct Phosphorylation Pattern in Ex Vivo–Treated HCC Patient Samples, Which Is Blocked by LY2109761.
To further explore the relevance of inhibiting the TGF-β pathway in HCC, we cultured HCC human samples treated ex vivo with TGF-β1 and LY2109761 medium for 48 hours as described.12 Sample proteins were arrayed to examine the activation status of several key signaling molecules in a randomly selected series of four patients. The arrays were probed with phospho-specific antibodies against 14 key points associated with cancer progression and angiogenesis, including vascular endothelial growth factor receptor (VEGFR), endothelial growth factor receptor (EGFR), AKT, and extracellular signal-regulated kinase. We found that LY2109761 treatment inhibits angiogenesis by dephosphorylating VEGFR Y951 and VEGFR Y996 significantly more than TGF-β1 treatment (P < 0.05). Moreover, treatment with LY2109761 significantly inhibits the phosphorylation of EGFR and AKT and its downstream endpoints, phospho-mTOR and phospho-GSK3-αβ, which are key points for proliferation and cell survival (P < 0.05) (Fig. 8).
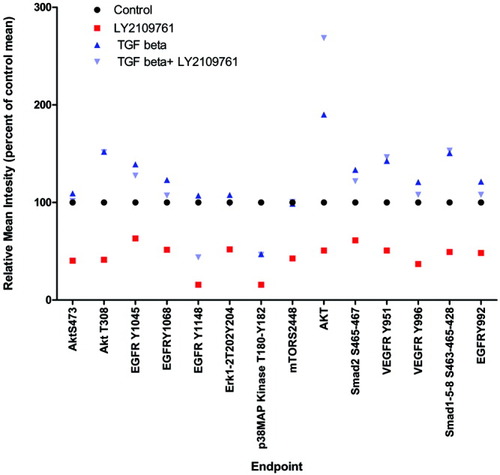
LY2109761 anti-angiogenic and antiproliferative effect on ex vivo–treated samples. Proteomic analysis of HCC human samples treated ex vivo with vehicle, TGF-β1, or LY2109761. Statistically significant differences were observed in the group treated with LY2109761 when compared with vehicle and TGF-β1 (P < 0.05). LY2109761 down-regulates the phosphorylation of the EGFR and VEGFR endpoints and downstream endpoints, indicating an anti-angiogenic and antiproliferative effect.
Discussion
Therapeutic options for HCC are still restricted to invasive approaches. Sorafenib is the only approved medical treatment for HCC, but its use is limited in advanced stages of Child-Pugh class A.2 The underlying liver disease, together with the heterogeneity of HCC, often precludes the use of chemotherapy. Hence, approval of the use of sorafenib in patients with advanced HCC has opened a new perspective on how to develop novel agents for the treatment of HCC. Based on this observation, a tailored map of therapeutically relevant targets is being considered, against which novel agents are being developed to treat HCC. TGF-β seems to represent such a target, because it is involved in several different pathological pathways relevant to HCC. In fact, we have reported previously that the inhibition of TGF-βR hampers the invasiveness of HCC in the surrounding tissue by increasing the expression of E-cadherin on cellular surface, as well as the intravasion of HCC cells by dephosphorylating the intracellular tail of β1 integrin.12, 13 Therefore, the inhibition of such a pathway should yield a domino therapeutic effect leading to tumor growth progression.
In this study, we demonstrate that selective blockade of the TGF-β receptor I by LY2109761 inhibits HCC by reducing tumor angiogenesis. This effect was mediated by at least two different mechanisms: (1) a phenomenon known as normalization, which consists of a reduction of both the number and the tortuosity of blood vessels, the two most important morphological changes responsible for increased tumor oxygenation and drug diffusion to the tumor20; and (2) blocking of the tumor/host cross-talk in the surrounding microenvironment. Interfering with the TGF-β pathway resulted in a distinct anti-angiogenic effect that was more effective than that of bevacizumab and not has not been described previously. However, if LY2109761 displays an anti-angiogenic activity similar to that of bevacizumab, why does it exert a more efficient effect on HCC tumor growth? We hypothesize that pathways other than VEGF could be involved, as suggested by analysis of the kinases performed on human HCC specimens treated with the TGF-βR inhibitor. Moreover, we cannot rule out the possibility that a higher dosage of LY2109761 leads to inhibition of a wider spectrum of kinases. The pharmacodynamics and pharmacokinetics of LY2109761 are also relevant to explain its possible synergistic effect with bevacizumab, taking into account the fact that because it is a competitive inhibitor of TGF-βRI, the concentration levels are crucial to determine its effectiveness. Finally, we cannot rule out the hypothesis that pathways other than VEGF could participate in tumor neo-angiogenesis; this is also suggested by the growing experience reporting partial success with the use of anti-angiogenic drugs in cancer treatment.
In this perspective, the effect of TGF-β inhibition on both vessels and the microenvironment could have a clinical impact: first, the micrometastasis rate should be reduced in HCC patients, and second, microvascular invasion should diminish, resulting in an overall delay of tumor progression in HCC.9, 21, 22 Consistently with this hypothesis, we have previously shown that LY2109761 inhibits the spread of HCC cells in the surrounding stroma by increasing the expression of E-cadherin and by blocking vascular intravasation through dephosphorylation of the intracytoplasmic tail of β1 integrin at residues T788-789.12, 13 Hence, LY2109761 selectively blocks one pathway and its associated down-stream signaling proteins, which leads to a reduced spread or vascular dissemination of cancer cells. This activity is different from that of multi–tyrosine kinase inhibitors, such as sorafenib or even bevacizumab.23 Therefore, LY2109761 has the advantage of being potent without inducing a large number of undesired effects. This is supported by our observation that LY2109761 has no untoward effect on physiological angiogenesis, as determined by embryogenic assays, and does not appear to pose cardiovascular complications, as described for TGF-β/SMAD-dependent organogensis.24, 25 In conclusion, our data represent the rationale for investigating the use of LY2109761 or a similar TGF-β inhibitor in clinical trials.
Acknowledgements
We thank Michael Lahn for his support and review of the manuscript.




