Abstract
We previously observed that Gadd45β/MyD118, a member of the Gadd45 family of inducible factors, showed the strongest immediate-early induction common to two distinctive proliferation responses of the liver: (1) regeneration induced by surgical partial hepatectomy and (2) hyperplasia induced by the primary mitogen TCPOBOP, a ligand of the constitutive androstane receptor (CAR). Gadd45β is known to be stimulated by nuclear factor (NF) κB, which is activated by tumor necrosis factor alpha (TNFα) in the early response to partial hepatectomy. We therefore investigated whether TNFα and NFκB also stimulated Gadd45β as part of the response to CAR ligands, or whether activation occurred by an alternative pathway. TCPOBOP effects were characterized in three mouse genotypes: wild-type, TNFR1−/−, and TNFR1−/−TNFR2−/−. The results showed that TCPOBOP did not activate NFκB in any of the mice, but a strong induction of Gadd45β messenger RNA was observed in all three genotypes, where TCPOBOP also induced CyP2b10, a classical target gene of activated CAR, and cyclin D1, a proliferation linked gene. Thus, the absence of TNFR signaling and induction of NFκB did not impair CAR-mediated gene induction. Moreover, hepatocyte proliferation was strongly induced, and at significantly higher levels than wild type, in both TNFR1−/− and TNFR1−/−TNFR2−/− mice. Further studies evaluated TCPOBOP-induced gene expression in CAR−/− mice, by microarray expression profiling and Northern blot. The induced changes in gene expression, including the stimulation of Gadd45β, were almost completely abolished—hence all were mediated via CAR activation. In conclusion, in the liver, Gadd45β can be induced by a distinctive pathway that requires CAR and is independent of TNFα-NFκB. The greater induction of proliferation in TNFR-null mice suggests negative cross-talk between the CAR and TNFα-NFκB controls that regulate proliferation. (HEPATOLOGY 2005.)
In recent years, the tools of molecular biology have revealed important mechanisms controlling liver regeneration, including critical growth factors, transcription factors, and signal transduction regulators.1-4 Within minutes after partial hepatectomy (PH), hepatocytes in the remnant liver undergo a transition from the quiescent G0 to the G1 phase of an active cell cycle. Although the precise mechanisms responsible for triggering this transition are not known, enhanced expression of immediate-early genes, occurring 15 minutes to 2 hours after PH, is believed to be critical.5 Immediate-early gene induction does not require protein synthesis and is activated by transcription factors that preexist in a latent form. In particular, increased binding of nuclear factor (NF)κB, AP1, and C/EBP occurs within minutes after PH,6-8 whereas STAT3 activates shortly thereafter. Activation of AP1 and NFκB, as well as DNA synthesis, can be inhibited by pretreatment of the animals with antibodies against tumor necrosis factor alpha (TNFα),9 suggesting that this cytokine plays a central role in the initiation of liver regeneration, by activating transcription factors. Several lines of evidence establish a critical role for TNFα and its rapidly activated downstream target, interleukin 6 (IL-6), in the G0-G1 transition of hepatocytes: (1) germ-free rats exhibit delayed liver regeneration after PH10; (2) liver regeneration is severely impaired in mice lacking either the TNFα receptor 1 (TNFR1−/−) or IL-6 (IL-6−/−),11, 12 (3) both defects are corrected after injection of IL-6,11, 12 indicating that regulation of IL-6 by TNFα is critical. On the basis of these findings, it appears that liver damage activates the following early sequence of events to initiate liver regeneration: (1) TNFα induces IL-6 and NFκB; (2) IL-6 induces STAT3; (3) these transcription factors activate cyclins D and E, with subsequent progression into S phase. Other growth factors, such as hepatocyte growth factor and transforming growth factor alpha, act later in the regenerative process (reviewed in ref. 3).
Liver regeneration is a response to compensatory injury, in which proliferation is essential to restore hepatic function. In contrast, numerous primary mitogens induce hepatocyte proliferation without causing liver injury. Opposite to liver regeneration after cell loss/injury, the proliferative process induced by primary mitogens, direct hyperplasia, results in an increase of hepatic DNA and liver mass.13 The potency of the mitogenic stimulus and the timing of S phase vary according to the nature of agents. Primary mitogens include a broad spectrum of chemicals with little structural similarity, such as peroxisome proliferators (PPs), halogenated hydrocarbons, retinoic acids, thyroid hormone (T3), lead nitrate, and ethylene dibromide.14 Among these agents, it is remarkable that PPs, retinoic acids, T3, and the halogenated hydrocarbon 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene (TCPOBOP) are all ligands of different receptors in the steroid/thyroid superfamily, respectively, the PP-activated receptor α, the retinoic acid receptor, the thyroid hormone receptor and the constitutive androstane receptor (CAR). As heterodimers with RXRα, these receptors all function as ligand-activated transcription factors. They directly regulate genes involved in lipid metabolism, adipogenesis, xenobiotic detoxification, and differentiation.15-17 TCPOBOP, which acts as a selective ligand for CAR, produces a particularly strong and rapid proliferative response in mouse livers that has been the main focus of our research.
Surprisingly, the activation of proliferation by TCPOBOP lacks many early events thought to be critical in liver regeneration. Activation of latent transcription factors (AP1, NFκB, STAT3, C/EBP), increased expression of immediate-early transcription factors (c-fos, c-jun, c-myc, LRF1, egr1), and increased synthesis of growth factors (transforming growth factor alpha, hepatocyte growth factor) are all considered essential in liver regeneration, but not observed in CAR-mediated hepatocyte proliferation.18-21 These differences suggest that the signaling pathways activated by TCPOBOP via CAR are different from those activated in liver regeneration. Conversely, we recently used microarrays to characterize 59 common immediate-early genes that are regulated in both pathways.22 Thus, a critical relationship between proliferation stimulated by liver injury and mitogens cannot be ruled out, particularly because TNFα signaling is associated with a wide variety of injuries and stress.
Among the genes characterized in our microarray analysis,22 Gadd45β/MyD118 was of particular interest; it is strongly and rapidly induced by both PH and TCPOBOP treatment. This gene was originally characterized as a primary responder in myeloid differentiation induced by IL-623 and was later shown to be a homolog of Gadd45, a gene induced by growth arrest and DNA damage.24 More recent studies have shown that Gadd45β, unlike two other homologs (Gadd45α and γ), plays an anti-apoptotic role and is activated by TNFα via NFκB.25 Thus, induction of Gadd45β coincides with entry into an active cell cycle, but its action might be to protect hepatocytes from apoptosis. Regardless of how it functions, Gadd45β, as a very strong marker of the immediate-early response in both proliferation models, provides an ideal experimental system to discriminate TNFα-dependent and -independent responses in hepatocytes. To make this discrimination, we studied Gadd45β gene activation and proliferation in mice that lacked either TNFα receptors or CAR. The TNFα-receptor–null mouse has an incomplete regenerative response without cell proliferation,12 whereas the CAR-null mouse lacks the proliferative response to TCPOBOP.26 Our results show that both Gadd45β activation and cell proliferation are regulated by 2 different signaling pathways and that TNFα and NFκB are not required for liver hyperplasia.
Abbreviations
PH, partial hepatectomy; NFκB, nuclear factor κB; TNFα, tumor necrosis factor alpha; IL-6, interleukin 6; PP, peroxisome proliferators; TCPOBOP, 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene; CAR, constitutive androstane receptor; BrdU, bromodeoxyuridine; cDNA, complementary DNA.
Material and Methods
Animals.
Eight-week-old B6-129 female mice null for the TNFα receptor 1 (TNFR1−/−) gene or for TNFα receptors 1 and 2 (TNFR1,2−/−) and wild-type mice were purchased from Jackson Laboratories (Bar Harbor, ME). In additional experiments, CAR−/− mice26 were also used. The animals were fed a laboratory chow diet provided by Ditta Mucedola (Settimo Milanese, Italy) and had free access to food and water. All procedures were performed in accordance with the UFAW Handbook on the Care and Management of Laboratory Animals and the guidelines of the animal ethics committee of this university. Experiments were performed in a temperature-controlled room with alternating 12-hours dark/light cycles. Hepatocyte proliferation was induced by a single-gavage treatment with the mitogen TCPOBOP (a gift of Dr. BA Diwan, Frederick Cancer Center, MD), at a dosage of 3 mg/kg body weight, dissolved in dimethylsulfoxide–corn oil solution. Controls received an equivalent amount of the vehicle. A two-third hepatectomy was performed according to Higgins and Anderson.27
For determination of total hepatocyte proliferation, bromodeoxyuridine (BrdU, Sigma Chemical Co., St Louis, MO) was continuously given in drinking water (1 mg/mL) to TNFR1−/− and TNFR1,2−/− mice were sacrificed 2 days after treatment with TCPOBOP. In another set of experiments, TCPOBOP-treated wild-type and TNFR1−/− mice received an intraperitoneal injection of BrdU (50 mg/kg) at 22, 28, 34, and 46 hours after treatment and were sacrificed 2 hours later. Four to five mice were used per group at each time point. Liver segments were for histology or snap-frozen in liquid nitrogen and kept at −80°C until use.
Histology and Immunohistochemistry.
Liver segments were fixed in 10% buffered formalin and processed for staining with hematoxylin-eosin or immunohistochemistry. For determination of hepatocyte proliferation, a mouse monoclonal anti-BrdU antibody was obtained from Becton Dickinson (Becton Dickinson, San Jose, CA), and the peroxidase method was used to stain BrdU-positive hepatocytes, as previously described.28 Labeling index was expressed as the number of BrdU-positive nuclei/100 nuclei. Mitotic index was expressed as the number of mitotic figures/100 hepatocytes. Immunohistochemistry evaluation was done with blinding to the treatment/genotypes Results were expressed as means ± SE of four to five mice per group. At least 2,000 hepatocyte nuclei per liver were scored. Comparisons between groups were performed using Student t test.
RNA Analysis.
Total RNA was prepared from TNFR1−/−, CAR−/−, and wild-type mouse livers by using TriPure isolation reagent (Boehringer, Mannheim, Germany). Microarrays analysis was carried out as previously described, using a 9,000-mouse-gene complementary DNA (cDNA) microarray produced in the Microarray Facility of Albert Einstein College of Medicine. Briefly, RNA was further purified by the guanidium isothiocyanate method followed by LiCl precipitation. Fluorescent cDNA was synthesized by using green Cy3- or red Cy5-dUTP. Hybridization and washing were carried out with institutional protocols (http://microarray1k.aecom.yu.edu/). Hybridized arrays were imaged by confocal laser scanning and quantified GenePix software (Axon Instruments Inc., Union City, CA). Hybridization data were further normalized using locally weighted linear regression (LOWESS) analysis (http://microarray1k.aecom.yu.edu/bioinfo_tools/normalization.html).
Northern blots were carried out using 20 to 30 μg heat-denatured total RNA per lane as previously described.21 The following 32P-labeled probes were used for hybridization: Cyp2b10—a 1.8-kb EcoRI fragment excised from a cDNA cloned in pUC13 (a gift from Dr. Negishi); Gadd45β—a 0.9-kb EcoRI–NotI fragment from cDNA W64388 cloned in pT7T3D-Pac10; and cyclin D1—a 1.3-kb EcoRI fragment from pcBZ054.
Electrophoretic Mobility Shift Assay.
Nuclear extracts were prepared from 200 mg liver tissue according to Schreiber et al.29 in the presence of 10 μg/mL leupeptin, 5 μg/mL antipain and pepstatin, and 1 mmol/L phenylmethyl sulfonyl fluoride (Sigma Chemical Co.). Protein concentration in the nuclear extracts was determined using the Bradford method.30 Ten micrograms nuclear extract was incubated at room temperature for 30 minutes with (2-5 × 104) of the (32P)-labeled double-stranded oligonucleotide containing the consensus NFκB DNA binding site from the IL-6 gene promoter (5′-GATCATGTGGGATTTTCCCATGT) or the STAT3 DNA binding site from the c-fos promoter (5′-GTCGACATTTCCCGTAAATCG) in 15-μL reaction mixtures containing 20 mmol/L HEPES, pH 7.9, 50 mmol/L KCl, 10% glycerol, 0.5 mmol/L dithiothreitol, 0.1 mmol/L EDTA, 2 μg poly(dI-dC), 1 μg salmon sperm DNA. Products were fractionated on nondenaturing 5% polyacrylamide gels in TBE 0.5× buffer.
Results
CAR Is Required for the Early Response to TCPOBOP.
Initial studies determined whether the entire immediate-early response that we previously characterized22 was mediated by activation of CAR, or whether some of the genes were stimulated by another mechanism. Microarray expression profiles were determined for the CAR-null mouse,26 with and without TCPOBOP treatment, using the same microarray platform as our previous study.21 Although Gadd45β is elevated by 1 hour, a 3-hour treatment interval was studied because it produced a larger set of responsive genes. Untreated and treated CAR-null livers were separately compared with normal mouse liver (Table 1).
| GenBank | Description | CAR+/+ | CAR −/− | ||
|---|---|---|---|---|---|
| +TCPOBOP* | Untreated† | +TCPOBOP‡ | Ratio§ | ||
| Stimulated by TCPOBOP in +/+ | |||||
| Regulated by TCPOBOP and PH | |||||
| AA261397 | TIG1/Retinoic acid receptor responder 1 | 3.8 | 1.1 | 1.0 | 0.9 |
| W64388 | Gadd45β/MyD118 | 3.5 | 1.0 | 1.3 | 1.3 |
| W15813 | Aminolevulinate synthase H (ALAS-1) | 3.4 | 1.3 | 1.1 | 0.8 |
| AA444946 | INSIG2 | 2.8 | 2.3 | 2.0 | 0.9 |
| AA197454 | INSIG2 | 2.7 | 2.6 | 2.7 | 1.1 |
| W82161 | Liver-expressed antimicrobial peptide 2 | 2.6 | 1.0 | 1.2 | 1.1 |
| AA064247 | Coronin family 70-kd WD-repeat tumor antigen | 2.4 | 1.0 | 0.7 | 0.7 |
| AA125030 | Hypothetical protein MGC11034 | 2.3 | 1.3 | 1.3 | 1.0 |
| W83512 | Fibrosin | 2.1 | 0.8 | 1.0 | 1.2 |
| W82737 | CAS-L enhancer of filimentation 1/Nedd9 | 2.0 | 1.1 | 1.4 | 1.2 |
| W87955 | WISP-1 (Wnt-induced secreted protein 1) | 2.0 | 0.9 | 0.9 | 1.0 |
| AA123823 | Hypothetical protein DKFZp434A1319 | 2.0 | 1.2 | 1.3 | 1.1 |
| AA177717 | Interleukin 1 receptor type I | 2.0 | 1.1 | 0.6 | 0.6 |
| AA051654 | Metallothionein 1 | 2.0 | 1.0 | 0.7 | 0.7 |
| AA259431 | Rel. Ras-binding protein SUR-8 | 1.9 | 1.7 | 1.3 | 0.8 |
| AA052712 | Retroviral LTR | 1.8 | 15.0 | 7.1 | 0.5 |
| AA144377 | Cyclic nucleotide phosphodiesterase E221245 | 1.8 | 0.8 | 0.8 | 1.0 |
| AA087673 | Murine endogenous leukemia virus | 1.7 | 9.7 | 5.2 | 0.5 |
| AA273964 | Tubulin α4 chain | 1.7 | 0.9 | 0.9 | 0.9 |
| AA255171 | Leucine-rich α2-glycoprotein | 1.7 | 1.9 | 0.9 | 0.5 |
| W99850 | Transmembrane serine protease 2 | 1.7 | 0.8 | 0.9 | 1.2 |
| AA272372 | TNFα-induced adipose protein | 1.7 | 1.3 | 0.7 | 0.5 |
| AA272876 | MafF transcription factor | 1.6 | 1.1 | 1.2 | 1.1 |
| AA387401 | cGMP-inhibited 3′,5′-cyclic phosphodiesterase B | 1.6 | 1.1 | 1.0 | 0.9 |
| W88005 | p21 (WAF/CIP1) | 1.6 | 1.5 | 1.1 | 0.7 |
| AA178549 | Intercellular adhesion molecule 1 | 1.6 | 1.1 | 0.9 | 0.8 |
| AA277474 | Src homology protein 2 | 1.5 | 1.0 | 1.0 | 0.9 |
| AA266114 | Replication protein A, 30-kDa subunit | 1.4 | 1.1 | 1.0 | 0.9 |
| AA268587 | Serum amyloid P-component | 1.4 | 2.7 | 1.5 | 0.5 |
| W45975 | TNFα receptor 2 | 1.4 | 1.2 | 1.0 | 0.8 |
| Regulated only by TCPOBOP | |||||
| AA212899 | Deiodinase, iodothyronine, type I | 4.9 | 0.8 | 1.1 | 1.4 |
| AA396132 | Rel. NEDD-4 | 4.2 | 0.7 | 0.9 | 1.2 |
| AA237173 | Liver 60-kd carboxylesterase 2 | 3.4 | 0.8 | 0.9 | 1.1 |
| W12874 | Cytochrome P450 2b10 | 3.4 | 0.5 | 0.5 | 1.2 |
| AA537672 | TNFα-induced protein 2 (TNP2. B94) | 3.3 | 0.9 | 0.8 | 0.9 |
| AA269533 | Cytochrome P450, 2b9 | 2.8 | 0.5 | 0.6 | 1.1 |
| AA275042 | Amine N-sulfotransferase | 2.3 | 1.3 | 0.9 | 0.7 |
| AA220582 | Cytochrome P450, 2f2 | 2.2 | 0.6 | 0.7 | 1.1 |
| AA212838 | Proteasome subunit, b type 7 | 2.2 | 0.9 | 1.0 | 1.1 |
| AA250415 | Rel. Yeast sporulation-induced transcript 4 | 2.0 | 1.3 | 1.0 | 0.8 |
| AA237136 | Solute carrier family 29, member 1 | 2.0 | 1.0 | 1.1 | 1.0 |
| W35954 | Insulin-like growth factor 2 receptor | 2.0 | 1.2 | 1.3 | 1.1 |
| W18851 | Rel. EIF4E BP3 | 2.0 | 2.3 | 1.9 | 0.9 |
| AA413622 | p53-responsive etoposide-induced 2.4 (EI2.4) | 1.9 | 1.1 | 0.8 | 0.7 |
| Inhibited by TCPOBOP in +/+ | |||||
| Regulated by TCPOBOP and PH | |||||
| AA050139 | Hypothetical protein C9ORF10 | 0.3 | 0.8 | 0.8 | 1.0 |
| W65054 | Growth/differentiation factor 1 (GDF-1) | 0.3 | 0.6 | 0.7 | 1.3 |
| W97245 | Rel. Chromogranin B | 0.4 | 0.9 | 1.1 | 1.2 |
| AA467267 | Rel. MinA-53 | 0.5 | 1.1 | 1.2 | 1.1 |
| AA414211 | SMURF2 | 0.5 | 0.9 | 0.9 | 1.1 |
| AA008579 | ATP-binding cassette, sub-family G, member 2 | 0.5 | 0.8 | 0.9 | 1.1 |
| AA412925 | Caspase 8 associated protein 2 | 0.5 | 1.0 | ND | ND |
| AA023221 | Inositol hexakisphosphate kinase 6 | 0.5 | 0.8 | 0.9 | 1.1 |
| AA466838 | Cdc51-like protein | 0.5 | 1.4 | 0.9 | 0.6 |
| AA002947 | Papillomavirus regulatory factor PRF-1 | 0.5 | 1.1 | 0.9 | 0.9 |
| AA544321 | La antigen | 0.6 | 0.9 | 0.8 | 1.0 |
| W34824 | Rel. proline-rich protein JC4899 | 0.6 | 0.9 | 0.8 | 0.9 |
| AA061285 | Vigilin | 0.6 | 0.8 | 0.6 | 0.8 |
| AA444485 | Rel. hypothetical protein FLJ20312 | 0.6 | 1.2 | 0.9 | 0.8 |
| AA064296 | PI3-phosphate binding protein 2 | 0.6 | 0.9 | 1.0 | 1.1 |
| AA177344 | Hypothetical gene AK037004 | 0.6 | 0.7 | 0.8 | 1.1 |
| AA177920 | Rel. Peregrine | 0.6 | 0.9 | 0.8 | 0.9 |
| W10903 | Testis-expressed protein 2 (TEX2) | 0.6 | 0.7 | 0.6 | 0.9 |
| AA277451 | Interferon-inducible GTPase | 0.6 | 0.8 | 0.7 | 0.9 |
| AA467585 | EST | 0.6 | 1.2 | 1.0 | 0.8 |
| AA467571 | Hypothetical protein DKFZp434J214.1 | 0.6 | 0.8 | 1.0 | 1.3 |
| AA473938 | Rel. Ewing's tumor associated antigen 16 | 0.7 | 0.9 | 0.9 | 1.1 |
| AA060591 | Np95-like ring finger protein (ICBP90) | 0.7 | 1.0 | 1.1 | 1.1 |
| AA422752 | Rel. hypothetical protein FLJ33318 | 0.7 | 0.3 | ND | ND |
| AA119050 | Rel. Human Mcm10 homolog | 0.7 | 0.7 | 0.8 | 1.0 |
| AA253678 | CD99-like 2/XAP89 protein | 0.7 | 0.9 | 1.0 | 1.0 |
| AA461912 | Adaptin 3α | 0.7 | 1.1 | 1.0 | 0.8 |
| AA386895 | Tetratricopeptide repeat domain 3 | 0.7 | 1.1 | 1.0 | 0.9 |
| AA021816 | Adducin 3γ | 0.7 | 0.7 | 0.9 | 1.3 |
| AA064156 | Calcium-binding ADP/ATP mitochondrial carrier | 0.7 | 1.3 | 1.1 | 0.9 |
| Regulated only by TCPOBOP | |||||
| AA175786 | Hypothetical protein CGI-100 | 0.3 | 1.2 | 1.4 | 1.1 |
| AA285951 | A20-like zinc-finger protein | 0.4 | 1.0 | 1.0 | 1.0 |
| AA064156 | Solute carrier family 25, member 25 | 0.4 | 1.3 | 1.1 | 0.9 |
| AA271288 | Hypothetical protein FLJ22170 | 0.5 | 0.8 | 0.9 | 1.1 |
| AA277830 | Angiopoietin-like 3 | 0.6 | 1.1 | 1.0 | 0.9 |
| AA387586 | Aromatic-L-amino-acid decarboxylase | 0.6 | 0.7 | 0.6 | 0.9 |
| AA239277 | Peroxisomal carnitine octanoyl transferase | 0.7 | 1.1 | 0.9 | 0.9 |
- * Genes are ranked by the magnitude of TCPOBOP stimulation or inhibition in wild-type. The values are the ratios of mRNA expression (treated+/+/untreated+/+) and were previously reported in Locker et al.22
- † mRNA expression of untreated CAR−/− livers compared with untreated CAR+/+ livers as the ratio (untreated−/−/untreated+/+).
- ‡ mRNA expression in CAR−/− livers 3 hours after treatment with TCPOBOP was compared with RNA from untreated CAR+/+ livers as the ratio (treated−/−/untreated+/+).
- § The ratio of mRNA expression in TCPOBOP-treated CAR−/− livers to untreated CAR−/− livers (the ratios in the preceding 2 columns).
After treatment with TCPOBOP, stimulation of Gadd45β and virtually every gene regulation reported in our previous paper was absent in the null mouse. The immediate-early response to TCPOBOP therefore depends on CAR.
In the absence of TCPOBOP treatment, the CAR-null phenotype had limited effects on gene regulation, although a few significant changes were apparent. CyP2b9 and CyP2b10 were both down-regulated, demonstrating that endogenous or dietary ligands act through CAR to establish the basal expression levels of CAR target genes. In contrast, INSIG2 and two endogenous retroviral transcripts were upregulated. In wild-type mice, these transcripts were stimulated by TCPOBOP, so the elevated expression of these highly reactive genes in the null phenotype suggests complex compensatory mechanisms.
Gadd45β/MYD118 Is a Strong Target of Two Different Early Response Pathways.
Our previous study demonstrated the early activation of Gadd45β expression in liver after TCPOBOP, and De Smaele et al.25 and Jin et al.31 have shown that Gadd45β is induced by TNFα via activation of NFκB. The data in Table 1 indicate that CAR regulates Gadd45β but do not rule out an intermediate role for NFκB. Thus, we used the strong regulation of Gadd45β to determine whether PH and TCPOBOP act through independent or overlapping signaling mechanisms.
Gadd45β messenger RNA (mRNA) was analyzed in the livers of TNFR1−/− mice. Analysis by Northern blot of RNA indicated that Gadd45β mRNA was present at low levels in the livers of wild-type and TNFR1−/− mice and was strongly induced by TCPOBOP in both strains (Fig. 1). CyP2b10, a well-known CAR target gene, and cyclin D1, an early marker of TCPOBOP-induced proliferation, were also strongly induced in both wild-type and TNFR1−/− mice. Hence, TNFα-signaling is not required for TCPOBOP-mediated induction of Gadd45β or other CAR target genes. Indeed, the induction of all 3 genes, and the basal level of CyP2b10, was somewhat greater in the null mouse, suggesting that TNFα has a negative effect on these gene regulations in the wild-type.
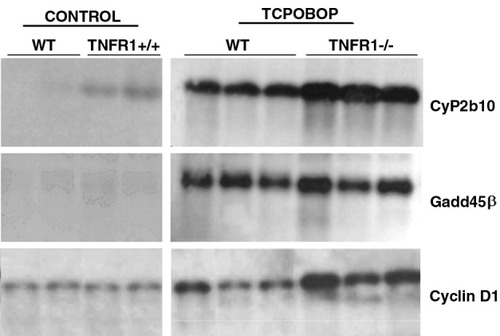
Induction of MyD118/Gadd45β, Cyp2b10, and cyclin D1 mRNA levels in TNFR1−/− and wild-type mice (WT) mice treated with TCPOBOP. Total RNA was prepared from mouse liver 3 hours after treatment with TCPOBOP (3 mg/kg) and subjected to analysis by Northern blot as described in Materials and Methods. Note that the panels showing the four control specimens represent a longer film exposure, to visualize weak basal gene expression. Each lane represents an individual sample. TNFR1−/−, tumor necrosis factor alpha 1 receptor; TCPOBOP, 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene.
Many stimuli other than TNFα can activate NFκB,32, 33 so further experiments determined whether induction of Gadd45β could occur through a TNFα-independent NFκB-dependent pathway. Because STAT3 signaling can be activated by TNFα via IL-6 and by several other pathways, we also included a study of this factor. Wild-type and TNFR1−/− mice were treated with TCPOBOP and sacrificed 30 minutes later. Gel shift analysis (electrophoretic mobility shift assay) studies (Fig. 2) indicated that no NFκB activation occurred in the livers of either wild-type or mutant mice after TCPOBOP administration. Conversely, in agreement with previous studies,7, 12 activation of NFκB and STAT3 occurred in the liver of wild-type mice 30 minutes after PH.
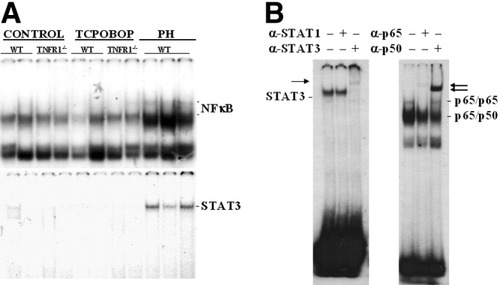
(A) DNA binding activity of NFκB (above) and STAT3 (below) in the livers of mice treated with TCPOBOP or subjected to PH. Arrows mark the specific gel shift bands. (B) Supershift studies with specific antibodies were carried out on representative specimens. The specificity of the bands was also demonstrated by competition with a 100-fold excess of specific unlabeled oligonucleotide (data not shown). Nuclear extracts from livers of mice sacrificed 30 minutes after TCPOBOP treatment or PH were incubated with 32P-labeled double-stranded oligonucleotide containing a consensus NFκB or STAT3 binding site. NFκB, nuclear factor kappaB; TCPOBOP, 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene; PH, partial hepatectomy.
To directly test whether induction of Gadd45β by TCPOBOP occurred through an alternative pathway mediated by ligand-activated CAR, we measured the hepatic levels of Gadd45β mRNA in the livers of CAR−/− mice (Fig. 3). Treatment of CAR−/− mice with TCPOBOP did not induce Gadd45β mRNA, confirming that CAR was required for this treatment response. As expected, CAR knockout mice also showed no induction of a classical CAR-target gene, CyP2b10 or cyclin D1, an early marker of TCPOBOP-induced proliferation. These analyses also confirmed the microarray analysis of Table 1.
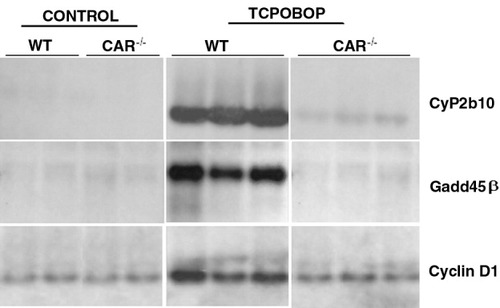
Northern blot analysis of Gadd45β, cyclin D1, and Cyp2b10 mRNA levels in CAR−/− and wild-type mice (WT) mice treated with TCPOBOP. Total RNA was prepared from mouse liver 3 hours after treatment with TCPOBOP (3 mg/kg) and subjected to analysis by Northern blot as described in Materials and Methods. Each lane represents an individual sample. CAR, constitutive androstane receptor; TCPOBOP, 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene.
TCPOBOP-Induced Proliferation in the TNFR1−/− Mouse.
Expression of Gadd45β, CyP2b10, and cyclin D1 all showed stronger responses in the TNFR1−/− mouse compared with wild type. We therefore examined whether these changes were associated with a downstream effect on cell proliferation. As an initial evaluation, TCPOBOP-treated mice were continuously labeled with BrdU and sacrificed 48 hours after treatment. TNFR1−/− mice showed significantly more proliferation than controls (Fig. 4). The higher labeling index, 69% versus 45% in wild type (P = .048; Fig. 4C) correlated with enhanced mitotic activity (2.45% vs. 1.56%) (Fig. 4D).
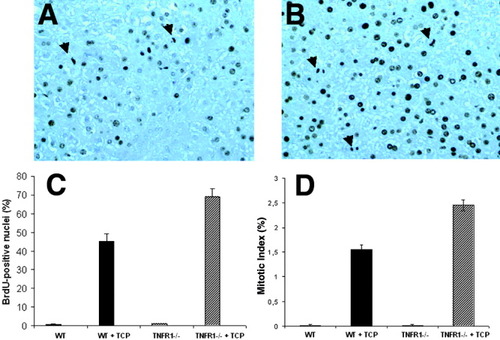
Cumulative proliferation of hepatocytes after treatment with TCPOBOP. Mice were treated with a single dose of TCPOBOP (3 mg/kg, intragastrically) and sacrificed 2 days later. All mice were given BrdU (1 mg/mL) in drinking water until the time of death. Histological sections show immunoperoxidase localization of BrdU in nuclei of wild-type (A) and TNFR1−/− (B) mice. Arrows indicate cells in mitosis. (C) Labeling index (LI): at least 2,000 hepatocyte nuclei per liver were scored. LI was expressed as number of BrdU-positive hepatocyte/100 nuclei. Results are expressed as means ± SE of four mice per group. (D) Mitotic index (MI): MI was expressed as number of hepatocytes entering mitosis/100 hepatocytes. Results are expressed as means ± SE of four mice per group. TCPOBOP, 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene; BrdU, bromodeoxyuridine.
Because TNFα also acts through a second receptor (TNFR2), similar experiments were performed with mice null for both receptors (TNFR−/− Fig. 5). As for the single-gene knockout, analysis of BrdU incorporation indicated a higher labeling index in TNFR−/− compared with wild-type mice (50% vs. 35% in wild type, P = .054). This effect could have resulted from accelerated entry into S phase or higher overall recruitment of hepatocytes into the cell cycle. To evaluate these possibilities, a kinetic analysis was carried out in the TNFR−/− mouse, using 2-hour BrdU pulse labels at various times after treatment (Fig. 6). This indicated that the increased proliferation was a prolonged effect. There were no significant differences between knockout and wild-type mice at 24 and 36 hours, but at 48 hours, the null mouse showed a 3-fold increase in the rate of proliferation observed in the wild type (9.2% vs 3.3%, P = .042).
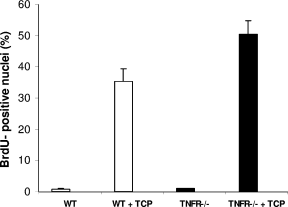
Labeling index of hepatocytes from TNFR double knockout and wild-type mice after treatment with TCPOBOP. Mice treated with a single dose of TCPOBOP (3 mg/kg, intragastrically) were sacrificed 2 days later. All mice were given BrdU (1 mg/mL) in drinking water until the time of death. For the labeling index (LI), at least 2,000 hepatocyte nuclei per liver were scored. LI was expressed as number of BrdU-positive hepatocytes/100 nuclei. Results are expressed as means ± SE of four mice per group. TNFR, tumor necrosis factor receptor; TCPOBOP, 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene; BrdU, bromodeoxyuridine.
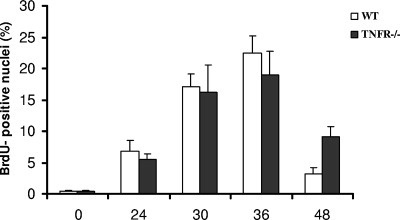
Labeling index of hepatocytes from TNFR1−/− null and wild-type mice after treatment with TCPOBOP. Animals given a single dose of TCPOBOP were sacrificed 24, 30, 36, and 48 hours later. Two hours before killing, all animals were given a single intraperitoneal injection of BrdU (50 mg/kg). At least 2,000 hepatocyte nuclei per liver were scored. LI was expressed as number of BrdU-positive hepatocyte nuclei/100 nuclei. Results are expressed as means ± S.E. of four mice per group. TNFR1, tumor necrosis factor 1 receptor; TCPOBOP, 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene; BrdU, bromodeoxyuridine; LI, labeling index.
Discussion
Gene Regulation by CAR.
Because the regenerative response is so well characterized in hepatocytes, the existence of a second mitogenic pathway has been controversial. However, although hepatocyte proliferation is often mediated by the injury/regeneration response, in other circumstances it is part of an adaptive response to stress stimuli that are not sufficient to lead to cell death (direct hyperplasia). As a substitute for injury, either TNFα or NFκB might be directly activated by CAR—the activation could be transcriptional or posttranscriptional. As yet another possibility, the experimental process, which includes handling, gavage, and anesthesia, could induce sufficient injury to activate TNFα. The Gadd45β gene provides a critical test of all of these possibilities.
The studies presented here show that CAR is required for the diverse set of early gene regulations that TCPOBOP induces in the liver. These responses, both upregulations and downregulations, fall into two classes. One class is regulated by TCPOBOP but not PH, and includes genes already known to be direct transcriptional targets of CAR, such as CyP2b10. This gene is regulated by a specific “phenobarbital-response element” that contains two strong DR4 binding sites for RXR:CAR heterodimers.34 Although it is not surprising that such transcriptional responses are abolished in the CAR-null mouse, they nevertheless provide a clear demonstration that the knockout is effective.
The second class, genes that are regulated by both PH and TCPOBOP treatment, is more problematic. As for the first gene set, virtually all responses were abolished in the CAR-null mouse, demonstrating that CAR was essential for gene activation. Among these regulated genes, Gadd45β stands out, because its strong early expression has a kinetic pattern that is virtually identical to that of CyP2b10.22 Unlike the latter gene, no obvious DR4 RXR:CAR binding sites have been characterized for Gadd45β. In contrast, the gene has strong NFκB sites.31 Moreover, a causal relationship between NFκB and transcriptional activation has been found in both embryonic fibroblasts and a T-cell hybridoma.25 The requirement for CAR does not rule out critical roles for other factors such as NFκB: (1) CAR could directly activate another factor; (2) CAR could stimulate transcription of a transcription factor that is the direct regulator of Gadd45β; (3) CAR could collaborate with a factor that binds other sites in the Gadd45β promoter; or (4) CAR and other factors could act together in a single peptide complex.
The various studies of null-mice presented here unequivocally establish that the two regulations of Gadd45β are independent. Transcriptional activation occurs in the TNFR1−/− mouse that lacks the TNFα response, and it occurs without induction of NFκB. The lack of STAT3 induction rules out an additional regulatory possibility. Because the TNFR1−/− mouse lacks a proliferative response to PH, these differences in Gadd45β regulatory stimulation also confirm that proliferation is activated by two different sets of early gene responses.
Cross-Talk.
Despite the independence of early signals, the findings do suggest an interaction between the two pathways, because CAR-mediated gene inductions and proliferative responses were stronger in the TNFR1−/− mouse. The effects were moderate but obvious and indicate a degree of negative cross-talk between the two pathways. In contrast to the transcriptional stimulation, increased proliferation was apparent only at 48 hours. The findings suggest that after proliferation is induced in an initial group of hepatocytes (typically 40%-50% of all hepatocytes), an additional group is slowly activated in the knockout. In contrast, an active TNFα response blocks recruitment of these late-proliferating cells.
In conclusion, Gadd45β is a striking marker of the immediate-early phase of hepatocyte cell proliferation. Although the timing and degree of activation are comparable in both PH and TCPOBOP treatment, the gene is activated by distinctive transcription factors in each model. Thus, the two pathways act through different mediators from their earliest stages. The striking early regulation of Gadd45β raises a possibility that this protein has a critical role in both pathways. Recent literature suggests that Gadd45β mediates an antiapoptotic response that is not directly linked to proliferation.25 If so, it is a reminder that both liver regeneration and drug-induced hyperplasia are complex responses that include not only proliferation but also cellular and metabolic adaptations. Resistance to apoptosis could be a valuable adaptive component of both responses. Nevertheless, whether Gadd45β protects hepatocytes from apoptosis is not yet established, and some published studies have indicated other roles for this protein. For example, Gadd45β is proapoptotic and antiproliferative in myeloid leukemia cells.24, 35 These contradictory effects indicate the importance resolving the function of Gadd45β in hepatocytes.




