Socioeconomic and epidemiological milieu of maternal death due to eclampsia in West Bengal, India: A mixed methods study
Abstract
Background
West Bengal is experiencing an unanticipated risk of eclampsia among pregnant women and it persists as the leading cause of maternal mortality. This study aimed to investigate the predictors for maternal deaths due to eclampsia in West Bengal.
Methods
The study adopted retrospective mixed methods covering facility and community-based maternal death review approaches. Facility-based data were used for 317 deceased cases wherein the community-based review approach was used in 40 cases. An in-depth interview was also performed among 12 caregivers.
Results
One-third of maternal deaths occurred due to eclampsia, and this accounted for the leading cause of maternal deaths in West Bengal. A younger age, a primigravida or nulliparous status, absence of antenatal care (ANC), and residence in rural areas appeared to have the highest risk of developing eclampsia. The majority of pregnant women had an irregular antenatal check-up history, particularly during the second trimester of pregnancy. The rate of eclampsia-related maternal death was higher in women residing more than 49 km from the studied hospitals. Most of the deceased women were referred to three or more hospitals before their death. Gravidity, the number of ANC visits, the mode of delivery, and delays at different levels were significant confounders of death due to eclampsia. The risk of death due to eclampsia was two times higher among women without ANC and those who had a cesarean section than that in their counterparts.
Conclusions
Women in West Bengal have a high risk of preeclampsia and eclampsia resulting in maternal mortality and morbidity. Gravidity, the number of ANC visits, the mode of delivery, and delays in recognition of eclampsia contribute to the risk of maternal deaths. The establishment of separate eclampsia units, enhanced screening, and preventive and treatment procedures may optimize managing eclampsia.
Abbreviations
-
- ANC
-
- antenatal care
-
- ANM
-
- auxiliary nurse midwife
-
- AOR
-
- adjusted odds ratio
-
- ASHA
-
- accredited social health activist
-
- CBMDR
-
- community-based maternal death review
-
- CI
-
- confidence interval
-
- FBMDRf
-
- facility-based maternal death review
-
- IFA
-
- iron and folic acid
-
- KMC&H
-
- medical college and hospital, Kolkata
-
- MMC&H
-
- Malda medical college and hospital
-
- MMR
-
- maternal mortality ratio
-
- TT
-
- tetanus toxoid
1 BACKGROUND
Eclampsia adversely affects maternal and perinatal health, particularly in low resource countries, which contributes to substantial fetal and maternal mortality [1, 2]. In 2015, approximately 12%−16% of all maternal deaths were attributed to complications of pregnancy-induced hypertension [3]. A disproportionate high rate of maternal death due to eclampsia has been reported in low and middle-income countries, with 76,000 annual maternal deaths [2, 4]. Preeclampsia and eclampsia are prevailing causes of maternal mortality in India, and 9% of all maternal mortality is caused by eclampsia [5, 6].
Unsurprisingly, the incidence of preeclampsia and eclampsia is declining in developed nations. Nonetheless, the incidence of this life-threatening morbidity has remained constant over the last few decades in low and middle-income countries [1, 7]. The incidence of eclampsia in developed nations is approximately 1 in 2000 to 1/3448 deliveries [8], while in India, the incidence of eclampsia ranges from 1.56% to 3.75% [6, 9]. The occurrence of eclampsia and its preceding condition, preeclampsia, is observed in varying degrees in Indian States [10, 11].
West Bengal is located in the eastern part of India, and has a high risk of eclampsia in pregnant women. The incidence rate of eclampsia is >3%, with an 8%−19% fatality rate in West Bengal [12, 13]. West Bengal is currently experiencing a 10−30 times higher incidence and case fatality rate of eclampsia than developed nations [14, 15]. A recent estimate has shown that eclampsia accounts for 43.4% of total maternal deaths, with a case fatality rate of 8.061% [12]. Therefore, West Bengal has the highest rate of maternal death due to eclampsia in India [16]. The disproportionate high rate of maternal death due to eclampsia in West Bengal was the indication of lowest percentage (19.86%) of declining maternal mortality ratio among the various states of India [17].
The etiology of eclampsia remains unknown and its pathophysiology is still poorly understood [2]. Various studies have focused on identifying maternal death due to eclampsia [6]. However, studies strictly highlighting patients with eclampsia and factors affecting eclampsia-related maternal death are rare [10]. In addition, most studies related to eclampsia were based only on one approach of facility-based or community-based. However, no studies have applied both of these approaches in a single study. Furthermore, inadequate health statistics and a lack of addition of some significant confounders are notable deficiencies that have limited previous Indian studies. Therefore, this study aimed to investigate the main predictors for maternal death due to eclampsia and compare characteristics between pregnant women who died from eclampsia and those who died from other causes.
2 METHODS
2.1 Study design and settings
This was a retrospective mixed methods study, which used facility- and community-based approaches to examine the deaths that occurred during pregnancy, childbirth, and puerperium. The study was conducted between November 2013 and October 2015 at Malda Medical College (MMC&H) and Hospital and Medical College and Hospital, Kolkata (KMC&H), which are situated in the northern and southern part of West Bengal, India, respectively. These two hospitals are the major tertiary-level healthcare units in eastern India, and they conduct more than 27,000 deliveries annually (https://www.wbhealth.gov.in/). The majority of pregnant women with complicated and irreversible problems from neighboring rural and urban areas, and also from other parts of India, visit these hospitals to use basic and comprehensive emergency health facilities. Therefore, these hospitals are a representative sample to help understand the contribution of eclampsia to maternal mortality in the community as a whole.
2.2 Data sources
2.2.1 Secondary sources
In this study, hospital-based records were used as secondary data of 317 women who died owing to pregnancy- and child-birth-related causes in the Department of Obstetrics and Gynecology of the selected hospitals. The details of all 317 maternal deaths were acquired using the facility-based maternal death review form. The individual case sheets, death registers, referral letters, medical records, and previous antenatal care (ANC) records were reviewed to retrieve the data related to the sociodemographic profile, the clinical profile, medical and obstetric history, health-seeking behavior, the referral status, the mode of delivery, the type of delay, causes of deaths, and the feto-maternal outcome of the deceased women.
2.2.2 Primary sources
Forty community-based maternal death reviews (20 women who died from eclampsia and 20 women who did not die from eclampsia) were performed in the community. The 40 cases were selected from the 317 maternal deaths that occurred in the studied hospitals. We used the residential address maintained in the selected hospitals to reach the respondents. We interviewed the family members and others who had witnessed any stage during the process leading to death. These people were mainly family members, neighbors, and relatives. In some cases, both sets of relatives from the parent's side and the in-law's side were also interviewed together. In primary data collection, this study adopted the maternal mortality questionnaire, which incorporated the National Rural Health Mission-validated community-based maternal death review questionnaire.
Information pertaining to the sociodemographic characteristics, health-seeking behavior, the referral history, premorbid condition, types of delays leading to maternal death, and strategy required to prevent maternal death were also obtained by using semistructured open-ended questions. This information was obtained from 12 health workers of various hierarchies who were associated with the maternal deaths or who participated in the provision of care to the deceased. In-depth interviews were performed in these 12 caregivers who comprised 2 accredited social health activists or auxiliary nurse midwives, 6 healthcare providers from first, second, and third levels healthcare facilities, and 4 were from the selected hospitals. Figure 1 shows the process of selecting interviewees. Most of the interviews were conducted in Bengali (regional language), but a few interviews were also performed in Hindi (official language of India). Written informed consent was obtained from all family members of the deceased women and also from hospital authorities.

2.3 Definitions of outcome and exposure variables
2.3.1 Outcome variables
Of the 317 pregnancy-related deaths, 105 were determined as death due to eclampsia, 209 deaths were not due to eclampsia, and the remaining 3 were unidentified. The recognized cause-specific maternal deaths were dichotomized into maternal death due to eclampsia and not due to eclampsia, and used as the main outcome variable. The death of women caused by eclampsia during pregnancy, childbirth, and puerperium was considered as maternal death due to eclampsia. The World Health Organization [18] definition and classification of causes of maternal deaths were adopted to define and classify the deaths during pregnancy and childbirth.
2.3.2 Exposure variables
The following individual characteristics were considered as predictor variables: maternal age, gestational age, locality of residence, religion, gravidity, parity, having an ANC card or the number of ANC visits, place of delivery, mode of delivery, referral status, distance from the hospital, time interval from developing complications to admission, mother's status on admission, reasons for admission, stage of pregnancy at death, and types of delays.
A delay was defined as a delay in deciding to seek appropriate care by individuals, family or both (type 1 delay), a delay in reaching an adequate healthcare facility (type 2 delay), and a delay in receiving adequate care when a facility was reached (type 3 delay).
2.4 Data analysis
Bivariate analysis with the χ 2 test was applied for the comparison between deceased cases of eclampsia and those without eclampsia in relation to various exposures. The binary logistic regression model was applied to estimate adjusted odds ratios (AORs) with their respective 95% confidence intervals (CIs) to identify the main confounders associated with death due to eclampsia. Differences were considered significant if p was <0.05. STATA version 13.0/SE was used for all statistical analyses. Personal interviews were conducted with the family members and medical staff. Most of the interviews were digitally recorded and later transcribed and translated into English. The English transcript materials were imported into QRS Nvivo, and categorized and analyzed by adopting the thematic approach.
The credibility, transferability, dependability, and confirmability of the data were taken into consideration to ensure the reliability and trustworthiness of the qualitative aspect of the study. The data were double-checked, the length of time during the interview was checked and a rapport was created with the respondents to produce valid data. The findings of this qualitative study were compared with quantitative findings. The qualitative findings were also corroborated with other similar national and global level studies. There was no controlled environment to conduct the research.
3 RESULTS
3.1 Underlying causes of death
The total number of maternal deaths in the selected health facilities was 317 over a 2-year period (Table 1). More than 70% of the maternal deaths were the result of direct obstetric causes, and 16.7% of these deaths were due to indirect obstetric causes. Eclampsia contributed to one-third of deaths, and it accounted for the leading cause of maternal death in West Bengal.
| Causes of deaths | ICD-10 code | No of women | Percent | |
|---|---|---|---|---|
| Direct causes (n = 230, 72.6) | Hemorrhage | O45.8, O46.0, 072.0, 072.1 | 75 | 23.7 |
| Infections/sepsis | O85, O86 | 31 | 9.8 | |
| Abortion | O03, O04, O05.2, O07.6, O07.7 | 9 | 2.8 | |
| Eclampsia | O14.1, O14.9, O15.0, O15.1, O15.2 | 105 | 33.1 | |
| Obstructed labor/rupture uterus | O63, O64, O65.4, O66.8 | 10 | 3.2 | |
| Other direct causes (n = 31, 9.8) | Ectopic pregnancy | O00.0, O00.1, O00.8, O00.9 | 11 | 3.5 |
| Pulmonary embolism | O22, 088, 088.2 | 17 | 5.4 | |
| IUFD with DICa | O31.2, O35.7, O36.4, O45.0, O46.0, O67.0 | 3 | 0.9 | |
| Indirect causes (n = 53, 16.7) | Jaundice | O98.4 | 11 | 3.5 |
| Heart diseases | O99.4 | 20 | 6.3 | |
| Anemia | O99.0 | 20 | 6.3 | |
| Diabetes mellitus | O24.0, O24.1, O24.2, O24.3, O24.9 | 1 | 0.3 | |
| Acute renal failure | O72.1, 090.4 | 1 | 0.3 | |
| Undetermined | 3 | 0.9 | ||
- a Intrauterine fetal death with disseminated intravascular coagulation in pregnancy (IUFD with DIC).
3.2 Sociodemographic and pregnancy-related characteristics in women with and without eclampsia-related death
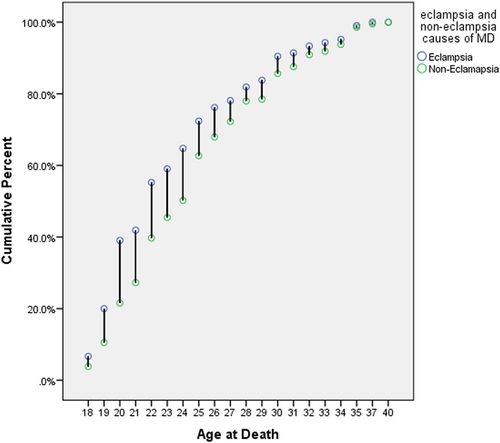
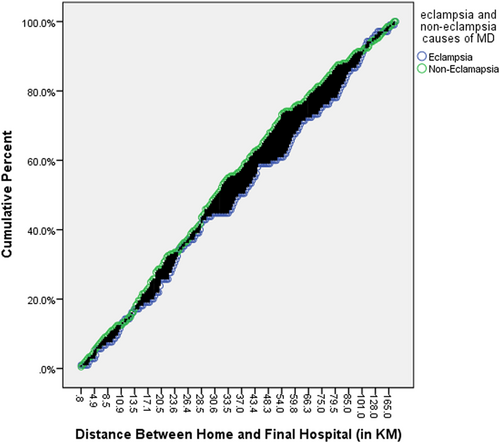
Variation in the mortality rate started to increase steadily after 18 years of age and continued up to 25 years in women with and without eclampsia who died (Figure 2). The risk of dying in women during gestation and childbirth due to eclampsia-related causes was higher in those aged 19−25 years. The majority (85%) of deceased women who had eclampsia lived in rural areas (Table 2). Primigravidas (40%) appeared to have the highest risk of developing eclampsia. The number of eclampsia-related maternal deaths decreased as the gravidity increased, while the gravidity of women was inversely related to non-eclampsia-related maternal deaths. Similar findings were also observed for parity. The number of eclampsia-related maternal deaths was higher in women residing more than 49 km from the final hospital. There was no significant difference in the number of maternal deaths due to eclampsia and non-eclampsia causes in women who lived at a relatively shorter distance (within 0−33 km) from the final hospital (Figure 3). After a distance of 33 km, the variation in maternal deaths due to eclampsia and non-eclampsia causes increased with increasing distance up to 100 km, and then eclampsia cases surpassed non-eclampsia cases.
| Characteristics | Eclamptic deaths (n = 105) | Non-eclamptic deaths (n = 209) | Total (n = 314) | χ 2 Value | p Value | |||
|---|---|---|---|---|---|---|---|---|
| Number | Percent | Number | Percent | Number | Percent | |||
| Age at death | ||||||||
| <24 | 68 | 64.8 | 105 | 50.2 | 173 | 55.1 | ||
| 25−29 | 20 | 19.0 | 59 | 28.2 | 79 | 25.2 | 6.03* | 0.049 |
| >29 | 17 | 16.2 | 45 | 21.5 | 62 | 19.7 | ||
| Religion | 0.84 | 0.360 | ||||||
| Hindu | 58 | 55.2 | 104 | 49.8 | 162 | 51.6 | ||
| Muslim | 47 | 44.8 | 105 | 50.2 | 152 | 48.4 | ||
| Place of residence | 0.12 | 0.732 | ||||||
| Rural | 89 | 84.8 | 174 | 83.3 | 263 | 83.8 | ||
| Urban | 16 | 15.2 | 35 | 16.7 | 51 | 16.2 | ||
| Gravida | 16.00*** | 0.000 | ||||||
| Primigravida | 42 | 40.0 | 45 | 21.5 | 87 | 27.7 | ||
| Gravida 2 | 38 | 36.2 | 73 | 34.9 | 111 | 35.4 | ||
| >2 Gravida | 25 | 23.8 | 91 | 43.5 | 116 | 36.9 | ||
| Parity | 13.12** | 0.001 | ||||||
| Nullipara | 45 | 42.9 | 51 | 24.4 | 96 | 30.6 | ||
| Primipara | 37 | 35.2 | 80 | 38.3 | 117 | 37.3 | ||
| >Primipara | 23 | 21.9 | 78 | 37.3 | 101 | 32.2 | ||
| No of living children | 10.06** | 0.007 | ||||||
| 0 | 53 | 50.5 | 68 | 32.5 | 121 | 38.5 | ||
| 1 | 31 | 29.5 | 75 | 35.9 | 106 | 33.8 | ||
| >1 | 21 | 20.0 | 66 | 31.6 | 87 | 27.7 | ||
| Distance between home and hospitals | 3.16 | 0.206 | ||||||
| <25 | 36 | 34.3 | 73 | 34.9 | 109 | 34.7 | ||
| 25−49 | 27 | 25.7 | 71 | 34.0 | 98 | 31.2 | ||
| >49 | 42 | 40.0 | 65 | 31.1 | 107 | 34.1 | ||
| Total | 105 | 100 | 209 | 100.0 | 314 | 100.0 | ||
- * p < 0.05.
- ** p < 0.01.
- *** p < 0.001.
3.3 Heath-seeking behavior and obstetric characteristics in women with and without eclampsia-related death
A total of 81% of all deceased women who had eclampsia were referred from various other lower-level healthcare units (Table 3). The majority (approximately three quarters) of eclampsia-related maternal deaths were found in those without ANC visits, whereas 50% of non-eclampsia-related maternal deaths occurred in those without ANC visits. Additionally, only 6 deceased women who had eclampsia had full antenatal check-ups (three to four times), and 35 of 209 deceased women who did not have eclampsia used ANC three to four times. Of the 105 deceased women who had eclampsia, 13% sought medical care within 5 h of developing complications. However, 36% of these women sought medical care between 6 and 12 h, and more than half of them took more than 12 h to seek admission for management of complications. Eighty-three (79.0%) and 144 (68.9%) women were unstable and 22 (2%) and 65 (31.1%) were stable on admission in women with eclampsia-related death and those with non-eclampsia-related death, respectively. The most commonly observed mode of delivery was cesarean section in women with eclampsia-related death (54.3%) and in those with non-eclampsia-related death (46.5%).
| Characteristics | Eclamptic deaths (n = 105) | Non-eclamptic deaths (n = 209) | Total (n = 314) | χ 2 Value | p Value | |||
|---|---|---|---|---|---|---|---|---|
| Number | Percent | Number | Percent | Number | Percent | |||
| Referral status | ||||||||
| Yes | 85 | 81 | 167 | 79.9 | 252 | 80.3 | 0.048 | 0.826 |
| No | 20 | 19 | 42 | 20.1 | 62 | 19.7 | ||
| ANC visit | ||||||||
| No | 74 | 73.3 | 100 | 50 | 174 | 57.8 | 16.19*** | 0.000 |
| 1−2 | 21 | 20.8 | 65 | 32.5 | 86 | 28.6 | ||
| 3−4 | 6 | 5.9 | 35 | 17.5 | 41 | 13.6 | ||
| Period of admission | ||||||||
| AN period | 32 | 30.5 | 69 | 33 | 101 | 32.2 | ||
| Intrapartum | 31 | 29.5 | 64 | 30.6 | 95 | 30.3 | 0.414 | 0.813 |
| Postpartum/natal | 42 | 40 | 76 | 36.4 | 118 | 37.6 | ||
| Duration of onset of complication to admission (h) | ||||||||
| <6 | 13 | 12.4 | 31 | 14.8 | 44 | 14 | 0.35 | 0.839 |
| 6−12 | 38 | 36.2 | 74 | 35.4 | 112 | 35.7 | ||
| >12 | 54 | 51.4 | 104 | 49.8 | 158 | 50.3 | ||
| Condition on admission | ||||||||
| Stable | 22 | 21 | 65 | 31.1 | 87 | 27.7 | 3.59* | 0.048 |
| Unstable | 83 | 79 | 144 | 68.9 | 227 | 72.3 | ||
| Labor pain | ||||||||
| Yes | 77 | 73.3 | 141 | 76.2 | 218 | 75.2 | 0.30 | 0.585 |
| No | 28 | 26.7 | 44 | 23.8 | 72 | 24.8 | ||
| Duration of labor (h) | ||||||||
| <6 | 21 | 26.9 | 45 | 31.3 | 66 | 29.7 | 1.71 | 0.426 |
| 6−12 | 36 | 46.2 | 71 | 49.3 | 107 | 48.2 | ||
| >12 | 21 | 26.9 | 28 | 19.4 | 49 | 22.1 | ||
| Mode of delivery | ||||||||
| Undelivered | 20 | 19 | 31 | 16.8 | 51 | 17.6 | 5.18 | 0.159 |
| Spontaneous vaginal | 23 | 21.9 | 63 | 34.1 | 86 | 29.7 | ||
| Vacuum/forceps | 5 | 4.8 | 5 | 2.7 | 10 | 3.4 | ||
| Cesarean section | 57 | 54.3 | 86 | 46.5 | 143 | 49.3 | ||
| Period of death | ||||||||
| AN period | 14 | 13.3 | 44 | 21.1 | 58 | 18.5 | 2.83 | 0.243 |
| Intrapartum | 9 | 8.6 | 18 | 8.6 | 27 | 8.6 | ||
| Postpartum/natal | 82 | 78.1 | 147 | 70.3 | 229 | 72.9 | ||
- Abbreviation: ANC, antenatal care.
- * p < 0.05.
- *** p < 0.001.
3.4 Distribution and association of women with and without eclampsia-related death in relation to the type of delay
A total of 80% of women with eclampsia-related death and 56.9% of those with non-eclampsia-related death had any type of delay (Table 4). More than two thirds of women with eclampsia had delay in seeking care (type 1 delay), 46% had a delay in reaching a first level health facility (type 2 delay) and approximately one quarter had a delay in receiving adequate care in a facility (type 3 delay). Type 1 delay contributed to two thirds of death due to eclampsia, but contributed to only 39% of death due to non-eclampsia causes (Table 4).
| Eclamptic deaths (n = 105) | Non-eclamptic deaths (n = 209) | Total (n = 314) | ||||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | Number | Percent | Number | Percent | Number | Percent | χ 2 Value | p Value |
| Delay in seeking care | ||||||||
| Yes | 70 | 66.7 | 81 | 38.8 | 151 | 48.1 | 21.81*** | 0.000 |
| No | 35 | 33.3 | 128 | 61.2 | 163 | 51.9 | ||
| Delay in reaching first level health facility | ||||||||
| Yes | 48 | 45.7 | 59 | 28.2 | 107 | 34.1 | 9.51** | 0.002 |
| No | 57 | 54.3 | 150 | 71.8 | 207 | 65.9 | ||
| Delay in receiving adequate care in facility | ||||||||
| Yes | 27 | 25.7 | 33 | 15.8 | 60 | 19.1 | 4.45* | 0.035 |
| No | 78 | 74.3 | 176 | 84.2 | 254 | 80.9 | ||
| Any delay | ||||||||
| Yes | 84 | 80.0 | 119 | 56.9 | 203 | 64.6 | 16.27*** | 0.000 |
| No | 21 | 20.0 | 90 | 43.1 | 111 | 35.4 | ||
- * p < 0.05.
- ** p < 0.01.
- *** p < 0.001.
3.5 Factors associated with maternal death related to eclampsia
The binary logistic regression showed a robust association between gravidity (AOR: 0.59; 95% CI: 0.53–0.96), ANC (AOR: 2.13; 95% CI: 1.19–3.12), mode of delivery (AOR: 2.41; 95% CI: 1.27–3.23), and any delay (AOR: 0.60; 95% CI: 0.27–0.89) for eclampsia-related maternal death (Table 5). The odds ratio of eclampsia-related maternal death was two times higher in women without ANC than in women who received ANC.
| Characteristics | Odds ratio | 95% CI |
|---|---|---|
| Age at death | ||
| 18−22(R) | ||
| 23−27 | 2.44 | 0.89−6.66 |
| 28−32 | 1.40 | 0.41−4.77 |
| ≥33 | 0.68 | 0.10−4.33 |
| Place of residence | ||
| Rural(R) | 1.32 | 0.495−3.54 |
| Urban | ||
| Religion | ||
| Hindu(R) | 1.11 | 0.52−2.38 |
| Muslim | ||
| Gravida | ||
| Primigravida(R) | ||
| 2nd | 0.59** | 0.53−0.96 |
| 3rd | 0.04 | 0.01−4.19 |
| ≥4th | 0.09 | 0.05−6.41 |
| Parity | ||
| Nullipara(R) | ||
| Primiparous | 0.39 | 0.03−4.34 |
| 2nd parous | 4.06 | 0.10−7.58 |
| ≥3 | 2.35 | 0.02−5.41 |
| ANC care | ||
| Full(R) | 2.13*** | 1.19−3.12 |
| No | ||
| Mode of delivery | ||
| Vaginal(R) | 2.41** | 1.27−3.23 |
| Cesarean | ||
| Referral status | ||
| Yes(R) | 1.66 | 0.63−4.34 |
| No | ||
| Condition on admission | ||
| Stable(R) | 1.05 | 0.36−5.04 |
| Unstable | ||
| Delay | ||
| Yes® | 0.60** | 0.27−0.89 |
| No | ||
- Abbreviations: ANC, antenatal care; CI, confidence intervals; R, reference category.
- ** p < 0.01.
- *** p < 0.001.
4 RESULTS FROM THE COMMUNITY-BASED STUDY
4.1 Antenatal history, complications during pregnancy and awareness
The community-based study showed a mixed reaction towards ANC check-ups. Some respondents replied favorably regarding ANC check-ups and some replied against it. All of the patients had a history of access to ANC. Many of them even had access to three or more ANC visits. However, many of the deceased women's relatives never took the women to more than one ANC session. Some of them visited two thirds of 1 km from a health subcenter for antenatal check-ups. Some women also visited private hospitals and some even consulted homeopathic doctors. The antenatal and complication history is discussed in the context of patients with eclampsia and those without eclampsia for better understanding the circumstances leading to death.
An important finding from the interviews is that women who had suffered from eclampsia were deprived of ANC at the second trimester. One of the respondents reported “Our ‘Boudi’ was admitted to Ramharipur PHC for a few days in her first trimester, but after discharge, she did not have any problems. Therefore, we did not take her there any more. We took her to the hospital once more when she developed a severe headache 1 to 1½ months before her expected day of delivery” (deceased 22-year woman's sister-in-law).
However, the majority of deceased women without eclampsia had a history of using ANC in all three trimesters. When describing ANC events of a deceased woman without eclampsia, her husband noted that “We wanted our daughter-in-law to have a healthy and safe delivery. Therefore, we took her every time for a check-up” (narrated by the woman's mother-in-law).
In this study, most relatives of deceased women with eclampsia were not acquainted with symptoms of high blood pressure, headache or swelling. One respondent reported that the pregnant woman complained of a headache, and swelling of the face and limbs as follows: “Our daughter-in-law was sitting in the courtyard and suddenly complained of uneasiness and blurring of vision. We took her to a private doctor. The doctor diagnosed her with high blood pressure. We did not know before this diagnosis that the complication was due to a high blood pressure” (father-in-law of the deceased woman).
4.2 Analysis of the referral history
Community-based in-depth interviews showed that majority of the deceased women had been referred from level 1, 2, or 3 healthcare units and reached the studied hospitals in a critical condition. This referral chain was categorized into two schematic overviews of eclampsia and non-eclampsia for better understating.
Eclampsia schematic overview: Most of the women who died due to eclampsia had been taken to more than three health facilities to reach Kolkata or Malda hospital (Figure 4a). A mother-in-law of a 20-year-old deceased woman reported the following: “Our daughter-in-law complained of blurring of vision on Sunday at 4.00 p.m. In the evening, we took her to a private doctor's clinic, and he gave her medicine. He said that she would be alright, but she developed complications again late in the night. In the morning, we took her to the primary health center, which was 2 km away, and she was admitted there. She was referred to the secondary level health setup again at 5 p.m. when her condition deteriorated. We reached the local hospital at approximately 8 p.m. the second day. She was kept there for 2 days. When she was in a critical condition, she was referred to a higher-level hospital, which was a subdivisional hospital. A male neonate was delivered by cesarean section at the subdivisional hospital. Five hours after the delivery, the doctors told us that the patient was having convulsions and that she needed to be taken to Malda. Three hours after admission to Malda Medical College and Hospital, the patient died.”
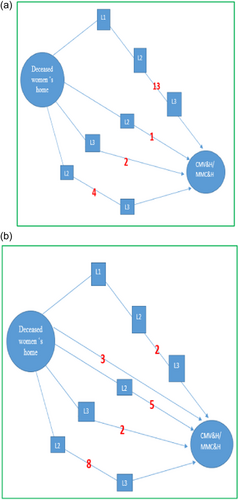
Non-eclampsia schematic overview: Most of the deceased women who had eclampsia were referred to three or more hospitals before their deaths. However, non-eclampsia cases were referred to one or two hospitals (Figure 4b).
4.3 Reasons for the referrals
The main reasons for referring the patient were hemorrhage, eclampsia, and preeclampsia. Severe anemia, jaundice, and heart problems in pregnancy were also recorded in the admission documents. A poor socioeconomic status, early age of marriage, and lack of proper food intake aggravated the situation.
The irreversible condition of a patient is due to poor management in peripheral health institutions. In this study, the patients were transferred to the final hospitals without examining their blood pressure or other medical conditions. Sometimes, patients with eclampsia were only administered 5−6 g of magnesium sulfate instead of a 14 g dose. Such a situation was clearly stated by a medical practitioner in one of the final hospitals: “We do not doubt patients requiring cesarean section are not examined properly, and for managing f eclampsia, no proper doses are provided. These are the reasons that accelerate mortality.”
5 DISCUSSION
Preeclampsia and eclampsia constitute a major public health concern in West Bengal and affect reproductive-aged women and their offspring. Furthermore, preeclampsia and eclampsia increase the risk of the double burden of disease (risk of cardiovascular and neurological disease) in later life [7]. The etiology of eclampsia remains unknown. Therefore, this study attempted to determine the overall circumstances contributing to maternal death by using facility and community-based maternal death review approaches in a region with a high number of eclampsia cases in India.
The present study showed that there were notable differences in background maternal characteristics of the studied patients with and without eclampsia-related death. Death due to eclampsia was significantly more common in the younger age group (<24 years), primigravidas, nulliparous women, women without ANC, and women who lived in rural areas than in their counterparts [12, 19]. Primigravida women and a younger age group appeared to have the highest risk of developing complications, which are similar results to those found in other studies [20, 21]. Social customs, a low socioeconomic status and illiteracy are thought to be responsible for the high rate of eclampsia-related death in younger and primigravida mothers [17].
Notably, eclampsia-related maternal death was more common than non-eclampsia-related maternal death in women who resided in distant areas from the studied hospitals. This variation in the rate of death between these two groups of women raises an important issue concerning delays in referral of patients with eclampsia, the availability of magnesium sulfate and good nursing care in referral healthcare units [22]. Therefore, the heterogeneity between regions relating to eclampsia- and non-eclampsia-related deaths in this study might be a reflection of how distant factors play an instrumental role [1, 23]. The disparity of the prevalence of preeclampsia/eclampsia is not only a reflection of the variability in maternal risk factor distribution, but may also be associated with variations in the facility and regional characteristics, such as diagnostic capacities or the accessibility of services [2].
The irregular and lack of ANC showed a strong relationship with eclampsia-related maternal death. This study showed that the likelihood of having eclampsia-related maternal death was two times higher in women without ANC than in those who received full ANC. There was an inverse association between eclampsia-related maternal death and increased ANC coverage, which is consistent with previous studies [24, 25]. The late second and early third trimesters are the most critical stages for developing eclampsia, and a low reporting rate of prenatal care during these gestational ages are responsible for the failure of detecting preeclampsia [2, 17]. The community-based in-depth interviews in this study showed that the majority of pregnant women had an irregular antenatal check-up history, particularly during the second trimester of pregnancy. There was a twofold increase in the risk of maternal death in patients with eclampsia, mostly due to a delay in seeking care, a delay in transportation, a delay in referral, poor management, and a shortage of an intensive care unit for eclampsia [23]. Illiteracy and unawareness of life-threatening signs, a low and late appearance of symptomatic patients during the antenatal period at healthcare units and poor administration of magnesium sulfate in patients with eclampsia at the referral time led to the progression of complications of eclampsia. This in turn could result in maternal death [23, 26]. The community-based in-depth interviews showed that most of the deceased women who had eclampsia were referred to three or more hospitals before their death compared with deceased women without eclampsia who were referred to one or two hospitals. The in-depth-interviews with the healthcare providers suggested that the critical condition of a patient was due to poor management in peripheral health institutions. These patients are being transferred to the final hospitals without examining blood pressure or other medical conditions [27].
The burden of eclampsia-related maternal deaths in the study population showed considerable variation regarding the mode of delivery. The rate of maternal death attributed to eclampsia was higher in women who had a cesarean section than in those who had a normal delivery [28, 29]. Studies have shown that a prior surgical cesarean section leads to an increase to the progression of preeclampsia in a subsequent pregnancy. The extended interval between pregnancies among patients who previously experienced cesarean section is a major risk factor for preeclampsia [30, 31], suggesting that the advantage of a high parity regarding the risk of preeclampsia is only transient. This evidence supports the considerably high and increasing cesarean section rate in West Bengal in India [32, 33]. This may explain the preponderance of death due to eclampsia in patients who have a cesarean section in this region. The most plausible explanation is that eclampsia in severely frail patients requires extensive administration of magnesium sulfate at referral healthcare units, and the poor administration of magnesium sulfate leads to a large number of cesarean sections in the region [12, 34].
5.1 Strengths and limitations
This mixed method study has a number of limitations. The hospital records used in this study may not have been good quality, including incomplete entries. The number of ANC visits was analyzed in the study, but information such as clinical examinations, use of tetanus toxoid immunization and iron and folic acid tablets, could not be ascertained from the case records. This lack of information could have had a major effect on differentiating between deceased women with and those without eclampsia. A community-based maternal death review also has limitations because it solely depends upon the narration provided by family members. Therefore, this review is subject to recall bias. This study relied on community reports of signs and symptoms of illness to classify the medical causes of death. In spite of these limitations, this study contributes to understanding the severity of eclampsia in relation to maternal health in our society. Furthermore, our findings could help in identifying confounding factors and deficiencies in the healthcare delivery system, and might contribute to planning strategies and interventions for curtailing pregnancy-related death due to eclampsia.
6 CONCLUSIONS
The study area has an unanticipated risk of eclampsia among pregnant women, which persists as the leading cause of maternal mortality. Eclampsia-related maternal deaths were more common than non-eclampsia-related deaths in areas that were distant from the hospitals in this study. Gravidity, the number of ANC attendance and delays at different levels play a major role in determining the preponderance of eclampsia-related maternal death. This study suggests that ensuring regular ANC (particularly during the late second or early third trimester), early referral and sharing knowledge about life-threatening signs are important to avert death due to eclampsia. The establishment of separate eclampsia units at lower level health facilities equipped with modern intensive care unit facilities is recommended in West Bengal to prevent the burden of eclampsia-related maternal death.
AUTHOR CONTRIBUTION
Md Illias Kanchan Sk: conceptualization, data curation, methodology, formal analysis, investigation, software and writing − original draft preparation, review and editing.
ACKNOWLEDGMENTS
I would like to thank the Principals and Registrars of Kolkata and Malda Medical Colleges and Hospitals for providing maternal death-related data. I am grateful to Prof. Balram Paswan and Prof. T. K. Naskar for their continuous insight and guidance during my research. I am also thankful to Mr. Sandeep Chatterjee, Mr. Samiun Mondal, and Dr. Ismile Sheikh, the assistant superintendent of the respective medical college and hospitals, for their help in completinge this study. The author received no financial support for the research, authorship, and/or publication of this article.
CONFLICT OF INTEREST
The author declares no conflict of interest.
ETHICS STATEMENT
The Institutional Ethics Committee for Human Research (IECHR) of Medical College and Hospital, Kolkata and Malda Medical College and Hospital approved the study. The whole analysis was performed anonymously. Confidentiality was maintained during data collection, storage, and analysis.
INFORMED CONSENT
Written consent to undertake the study was provided by the Ministry of Health and Family Welfare (MoHFW), Government of West Bengal. Prior written consent was also obtained from all family members to conduct the community-based in-depth interviews.
Open Research
DATA AVAILABILITY STATEMENT
The data set analyzed for this study is available with corresponding author which can be accessed on reasonable request. Anonymized data without participant's identification will be made available. The data are not publicly available due to privacy or ethical restrictions.




