Improving transitional care after acute myocardial infarction: A scoping review
Abstract
Cardiovascular disease remains the leading cause of morbidity and mortality, posing a significant challenge to healthcare systems worldwide. Transitional care interventions, which ensure coordination and continuity of care as patients move between different levels of healthcare, have been shown to reduce unnecessary healthcare utilization and improve patient outcomes. While much attention has been given to transitional care in heart failure, this review aims to map the interventions implemented for patients following an acute myocardial infarction (AMI). A scoping review was conducted following the Joanna Briggs Institute (JBI) methodology, with literature searches performed in the Cochrane, CINAHL, MEDLINE, JBI, and SciELO databases, focusing on publications from 2013 onwards in both Portuguese and English. Seventy-five studies were included, with most combining multiple interventions that contributed to improved cardiovascular health outcomes, including increased adherence to healthy lifestyle behaviors, enhanced medication compliance, and better healthcare self-management. These interventions were effective in reducing cardiovascular-related Emergency Department visits, unplanned 30-day readmissions, and mortality following a first-time myocardial infarction. Key strategies identified included discharge planning, digital health solutions, outpatient care, and healthcare coordination. The findings of this review underscore the need for developing methodologies that enhance the transition of care from hospital to primary care following an AMI. There is an urgent need to design and implement new healthcare programs that integrate discharge interventions, digital health, outpatient care, and healthcare coordination to ensure continuity of care and optimize patient outcomes post-discharge.
Abbreviations
-
- AMI
-
- acute myocardial infarction
-
- CR
-
- cardiac rehabilitation
-
- CVD
-
- cardiovascular disease
-
- JBI
-
- Joanna Briggs Institute
-
- OSF
-
- Open Science Framework
-
- PHC
-
- Primary Healthcare Centers
-
- PRISMA-ScR
-
- Preferred Reporting Items for Systematic Reviews and Meta-analyses for Scoping Reviews
-
- RCAAP
-
- Repositórios Científicos de Acesso Aberto de Portugal
-
- RCT
-
- Randomized Controlled Trials
1 INTRODUCTION
Cardiovascular disease (CVD) stands as the leading cause of mortality and a significant contributor to disability, posing a substantial challenge to already overburdened healthcare systems [1]. Seamless care transitions are, therefore, imperative [2-4], a necessity underscored by the COVID-19 pandemic, which highlighted the potential of digital resources to enhance access, equity, and the overall quality of healthcare [2]. Following a cardiovascular event, secondary prevention programs become essential, offering cost-effective measures that can save as many lives as acute-phase treatments [5]. Consequently, these interventions should be initiated as soon as possible after a cardiovascular event.
Transitional care aims to ensure the coordination and continuity of care for patients transitioning between different services or levels of complexity, making it particularly crucial for those with complex care needs [6].
Patients recovering from an acute myocardial infarction (AMI) require complex care management, including adherence to a complex therapeutic regimen, and the implementation of lasting lifestyle changes. Given this complexity, adequate monitoring during the transition of care is crucial [2].
However, the transition from inpatient to outpatient care is often fraught with challenges such as miscommunication, medication errors, noncompliance, and inadequate follow-up, all of which can lead to premature hospital readmissions [7].
To address these issues, healthcare systems must adopt sustainable care transition models that foster collaboration among hospitals, Primary Healthcare Centers (PHCs), patients, families, and caregivers. Transitional Care encompasses a suite of measures aimed at ensuring coordinated and continuous healthcare across different care settings. These measures include pre and post-discharge interventions such as patient education, effective communication, medication reconciliation, post-discharge follow-up to assess self-management, and coordination among healthcare professionals, all of which are especially crucial for individuals with complex care needs [6].
To enhance the quality of CVD management, it is essential to synthesize scientific evidence on transitional care interventions [8]. While there has been an increasing focus on transitional care interventions for heart failure patients [9-18], less attention has been given to other CVD-related conditions, such as AMI [2].
Similar to heart failure, patients recovering from AMI require close monitoring to ensure adherence to therapy and lifestyle changes, which are essential for minimizing disease progression and improving quality of life. In this regard, insights from studies on transitional care in heart failure may apply to patients following an AMI.
Although the literature on transitional care for AMI patients is abundant, there remains a need to synthesize information regarding the characteristics and contexts of these applications. A preliminary search of research databases identified only one systematic review on transitional care following hospitalization for acute stroke or myocardial infarction [19]. However, this review focused on studies published between 2000 and 2012, included only a subset relevant to AMI patients and was limited by the quality of the research designs. Given the time that has elapsed since then, it is reasonable to expect a more extensive body of research with improved methodological rigour to have emerged.
This scoping review will be guided by the methodology proposed by the Joanna Briggs Institute (JBI) and aims to map the transitional care interventions used for patients after an AMI.
-
What transitional care interventions have been implemented and evaluated for patients with AMI?
-
What are the characteristics of these interventions?
-
Who implemented the interventions?
-
In what contexts were the interventions implemented and evaluated?
-
What outcomes have been assessed?
2 METHODS
Given the exploratory nature of this review and our aim to describe, characterize, and map the literature based on the outlined questions, we opted for a scoping review methodology guided by the JBI approach. As advised by Aromataris and Munn [20], our search strategy was designed to be comprehensive, aiming to capture both published and unpublished primary sources of evidence, along with relevant reviews. This protocol has been registered on the Open Science Framework [21] since PROSPERO does not accept protocols for scoping reviews.
2.1 Search strategy
The inclusion criteria were defined using the Participants, Concept, and Context framework (Table 1). The search strategy incorporated all identified keywords and index terms, such as [“Myocardial Ischemia”[Mesh] OR ((heart attack) OR (coronary heart disease)) OR (ischemic heart disease) AND “Continuity of Patient Care”[Mesh] OR ((((((((“transitional care”) OR (“care transition”)) OR (“transitional program”)) OR (“Transition Care”)) OR (“transitional home care”)) OR (“Continuity of Patient Care”)) OR (“continuity of care”)) OR (“Care Continuity”)) OR (“Continuity Care”)], and was tailored for each information source as outlined in the review protocol [21]. Additionally, the reference lists of included publications were screened to identify further relevant studies.
| Participants | Studies that recruited participants aged 18 or over, of both genders, with acute myocardial ischemia (coronary heart disease, ischemic heart disease, acute coronary syndrome), or if results were reported separately when the study sample included other clinical groups. |
| Concept | This review considers studies exploring transitional care interventions. Transitional care interventions are established to ensure continuity and coordinated care for patients transitioning from hospital to place of usual residence. Includes pre and post-discharge interventions. They can be classified, including digital health, discharge and outpatient intervention, telephone support, home visits, and healthcare coordination. This may include concepts such as Continuity of Patient Care but is not limited to it. |
| Context | In this review, we consider various contexts. This will include hospitals, cardiac rehabilitation centers, homes, and primary health care. |
For this review, publications in Portuguese and English were included, based on evidence suggesting that excluding non-English publications from systematic reviews on clinical interventions has minimal impact on overall conclusions and can serve as a methodological shortcut [22]. Publications from 2013 to 2023 were included, as a prior systematic review on transitional care following hospitalization for acute stroke or myocardial infarction [19] covered studies published between January 2000 and March 2012.
The databases searched included Cochrane, CINAHL (EBSCOhost), MEDLINE (PubMed), JBI (Ovid), and SciELO. Additionally, unpublished studies and gray literature sources were explored, such as the Repositórios Científicos de Acesso Aberto de Portugal (RCAAP).
2.2 Study/source of evidence selection
Title and abstract screening were conducted using Rayyan by two independent reviewers to assess adherence to the inclusion criteria. The full texts of the selected citations were then meticulously assessed by two independent reviewers against the inclusion criteria. Any discrepancies between the reviewers at each stage of the selection process were resolved through discussion or, if necessary, by involving a third reviewer.
2.3 Data extraction
Data extraction was performed by two independent reviewers using a customized data extraction tool designed to capture specific study characteristics relevant to the review question. Although a critical appraisal is not mandatory in a scoping review, we conducted one using standardized JBI critical appraisal tools to gain insights into the methodological quality of the included studies.
3 RESULTS
3.1 Study characteristics
The initial electronic search yielded 5373 potential publications for review. After the screening process, 75 unique studies were identified as meeting the inclusion criteria. These results are presented in a Preferred Reporting Items for Systematic Reviews and Meta-analyses for Scoping Reviews (PRISMA-ScR) flow diagram (Figure 1).
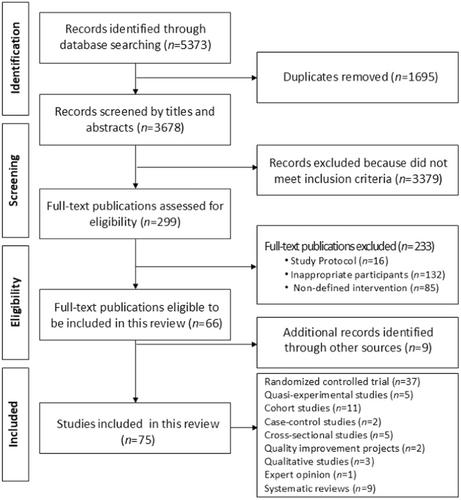
The detailed results of the quality appraisal assessment are available in the online supplement.
Among the included studies, there were 37 randomized controlled trials, 5 quasi-experimental studies, 11 cohort studies, 2 case-control studies, 5 cross-sectional studies, 2 quality improvement studies, 3 qualitative studies, 1 expert opinion, and 9 systematic reviews. The majority of these studies originated from the United States (n = 13), China (n = 8), Australia (n = 3), Canada (n = 4), Germany (n = 4), Italy (n = 4), New Zealand (n = 3), Portugal (n = 3), Belgium (n = 2), Brazil (n = 2), Iran (n = 2), Norway (n = 2), Sweden (n = 2), United Kingdom (n = 2), and one study each from Austria, Denmark, France, Greece, Israel, Malaysia, Poland, Qatar, Singapore, Spain, Turkey, and a collaborative study involving Germany, Spain and the UK (n = 1 each).
These studies were implemented across various settings including hospitals (n = 26), homes (n = 19), Cardiac Rehabilitation (CR) centers (n = 15), community health/PHCs (n = 3), and a combination of hospitals and PHCs (n = 2).
Interventions were implemented by a range of healthcare professionals, including hospital nurses (n = 18), CR team (n = 17), hospital team (n = 9), hospital pharmacists (n = 6), cardiologists (n = 5), CR nurses (n = 3), general practitioners (GP) (n = 2), community nurses (n = 2), hospital and community teams (n = 1), nonphysician “prevention assistants” (n = 1), primary care providers (n = 1), and psychotherapists (n = 1).
Most of the interventions were multi-component, reflecting the complexity of transitional care needs for patients following AMI. Further details regarding the characteristics of the included studies are summarized in Table 2, and additional information about the key findings of each study is provided in the online supplement. The findings are organized according to the defined categories of transitional care interventions.
| Author, year | Follow-up | Discharge int. | Digital health | Phone Support | Outpatient int. | Home visits | Healthcare coord. | Context | Healthcare professionals |
|---|---|---|---|---|---|---|---|---|---|
| Mayer-Berger et al., 2012 [23] | 3 years | × | CR centers | CR team | |||||
| Garcia et al., 2014 [24] | 1 year | × | × | × | Hospital | Hospital pharmacist | |||
| Ho et al., 2014 [25] | 1 year | × | × | × | Hospital | Hospital pharmacist | |||
| Hunger el al., 2014 [26] | 1 year | × | × | Home | Hospital nurse | ||||
| Khonsari et al., 2014 [27] | 2 months | × | Home | − | |||||
| Varnfield et al., 2014 [28] | 6 weeks | × | × | Home | CR team | ||||
| Ammenwerth et al., 2015 [29] | 1, 3 months | × | × | Hospital | Hospital team | ||||
| Budiman et al., 2015 [30] | 30 days | × | × | Hospital | Hospital pharmacist | ||||
| Fang et al., 2023 [31] | 6 months | × | × | Home | Hospital team | ||||
| Frederix et al., 2015 [32] | 6 months | × | CR centers | CR team | |||||
| Housholder-Hughes et al., 2015 [33] | 3 months | × | Hospital | Hospital nurse | |||||
| Mohammadpour et al., 2015 [83] | 45 days | × | × | Hospital | Hospital nurse | ||||
| Sherrard et al., 2015 [34] | 1 year | × | Home | Hospital team | |||||
| Bell et al., 2016 [35] | 1 month | × | × | Hospital | Hospital pharmacist | ||||
| Bhagwat et al., 2016 [36] | Scoping Review | ||||||||
| Dithmer et al., 2016 [84] | 2 weeks | × | CR centers | − | |||||
| Hamar et al., 2016 [37] | 1 month | × | × | × | Hospital | Hospital nurse | |||
| Hyun et al, 2016 [38] | 6 months | × | × | PHC/private | GP vs cardiologists | ||||
| Lau-Walker et al., 2016 [39] | 3 months | × | Hospital | Hospital nurse | |||||
| Skobel et al., 2016 [85] | 6 months | × | Home | − | |||||
| Wong et al., 2016 [4] | 1 year | × | Home | Hospital nurse | |||||
| Baky et al., 2017 [94] | At discharge | × | Hospital | Hospital team | |||||
| Bravo-Escobar et al., 2017 [40] | 2 months | × | × | CR centers | CR team | ||||
| Cao et al., 2017 [41] | 1, 3 months | × | × | × | Hospital + PHC | Hospital and Community teams | |||
| Hickson et al., 2017 [42] | 6 months | × | PHC/private | PHC team vs cardiologists | |||||
| Widmer et al., 2017 [43] | 3 months | × | CR centers | CR team | |||||
| Zhang et al., 2017 [44] | 7 months | × | × | Hospital | Hospital and PHC nurses | ||||
| Duan et al., 2018 [45] | 2 months | × | CR centers | CR team | |||||
| Fernandes et al., 2018 [86] | 1 month | × | × | CR centers | CR team | ||||
| Marbach et al., 2018 [7] | 1 month | × | × | × | Hospital | Hospital team | |||
| Murphy et al., 2018 [46] | 1 month | × | × | × | Hospital | Hospital team | |||
| Ögmundsdottir et al., 2018 [47] | 1 year | × | CR centers | Cardiologist vs CR nurse | |||||
| Paoli et al., 2018 [48] | 2 and 5 years | × | × | × | Hospital + PHC | Hospital and PHC nurse | |||
| Pristipino et al., 2018 [49] | 5 years | × | CR centers | Psychotherapist | |||||
| Reveles et al., 2018 [50] | 6 months | × | Home | Hospital nurse | |||||
| Berardo et al., 2019 [51] | 3 months | × | Hospital | Hospital nurse | |||||
| Brørs et al., 2019 [52] | Systematic review | ||||||||
| Crawshaw et al., 2019 [53] | 6 week | × | Hospital | Hospital pharmacist | |||||
| Dorje et al., 2019 [54] | 2, 6, 12 months | × | Home | CR team | |||||
| Gomes et al., 2019 [55] | 1 month | × | × | Hospital | Hospital nurse | ||||
| Jin et al., 2019 [56] | Meta-analysis | ||||||||
| Sankaran et al., 2019 [57] | 4 months | × | CR centers | CR team | |||||
| Stamm-Balderjahn et al., 2019 [87] | − | × | CR centers | CR team | |||||
| Wallert et al., 2019 [58] | 1, 2, 3, 6 months | × | CR centers | CR team | |||||
| Wu et al., 2019 [59] | 3 months | × | × | × | Hospital | Hospital nurse | |||
| Agher et al., 2020 [88] | Scoping review | ||||||||
| Drigny et al., 2020 [93] | 3 months | × | CR centers | CR team | |||||
| Ivers et al., 2020 [60] | 11 months | × | Home | Hospital team | |||||
| Nabutovsky et al., 2020 [89] | 6 months | × | Home | CR team | |||||
| Ruschel et al., 2020 [61] | 8 months | × | PHC | General practitioner | |||||
| Subedi et al., 2020 [62] | Systematic review | ||||||||
| Zhou et al. 2020 [63] | 1 year | × | × | Home | Hospital nurse | ||||
| Aggarwal et al., 2021 [64] | Expert opinion | ||||||||
| Everett et al., 2021 [65] | 6 week | × | CR centers | CR nurse | |||||
| Foccardi et al., 2021 [66] | 3 months | × | Home | CR team | |||||
| Hamilton et al., 2021 [95] | 14 days | × | × | × | Hospital | Hospital nurse | |||
| Kaldal et al., 2021 [67] | 5 years | × | × | Hospital | Hospital nurse | ||||
| Maddison et al., 2021 [68] | 6, 12 months | × | Home | − | |||||
| Marvel et al., 2021 [69] | 1 month | × | × | × | Home | Hospital team | |||
| Osteresch et al., 2021 [70] | 1, 2 years | × | × | × | CR centers | CR nonphysician | |||
| Paruchuri et al., 2021 [71] | 3 months | × | Home | CR team | |||||
| Ramachandran et al., 2021 [72] | Meta-analysis | ||||||||
| Ross et al., 2021 [73] | 2 months | × | Hospital | ||||||
| Su et al., 2021 [74] | 6 and 12 weeks | × | × | Home | CR nurse | ||||
| Tuna et al., 2021 [75] | 4 months | × | × | Hospital | Hospital nurse | ||||
| Yudi et al., 2021 [76] | 2 months | × | × | × | Home | CR team | |||
| Beauchamp et al., 2022 [77] | Scoping review | ||||||||
| McLachlan et al., 2022 [78] | 6–12 weeks, and 1 year | × | Hospital | Cardiologists hospital nurses | |||||
| Passaglia et al., 2022 [79] | 6 months | × | Hospital | ||||||
| Sefidi et al., 2022 [80] | 3 months | × | × | Hospital | Hospital nurse | ||||
| El et al. 2023 [90] | 6 months | × | × | Hospital | Hospital pharmacist | ||||
| Guo et al., 2023 [81] | Meta-analysis | ||||||||
| Krzowski et al., 2023 [91] | 1 month | × | Home | CR team | |||||
| Weeda et al., 2023 [82] | Systematic review | ||||||||
| Zafeiropoulos et al., 2023 [92] | 1 year | × | × | × | Hospital | Cardiologist | |||
3.2 Discharge intervention
According to Coleman's Care Transitions Model, discharge interventions involve educating patients and their families about health conditions and care plans during hospitalization to encourage self-management [6].
Among the 75 studies analyzed, 28 reported on discharge interventions. Most commonly, these interventions were combined with telemonitoring [69, 74, 76], telephone support [30, 35, 44, 55, 75, 80, 83] or outpatient interventions [46, 67, 78, 86, 90, 95].
Before discharge, patients' needs were assessed using pre-set surveys or measures. These assessments were used to tailor interventions, set goals, and develop action plans to address behavioral risk factors based on individual patient needs. Studies that incorporated telemonitoring during discharge often included training for patients on how to use digital health platforms [69, 74, 76].
The roles of different healthcare professionals during discharge interventions were outlined: physicians provided education on diagnosis, treatment plans, medications, and associated risks and benefits [41, 46, 92]; hospital nurses focused on educating patients about identified risk factors and promoting self-care [6, 37, 39, 41, 44, 46, 48, 51, 55, 67, 74, 75, 80, 83, 86, 95]; hospital pharmacists addressed therapeutic reconciliation and educated patients about medication side effects [6, 24, 25, 30, 35, 46, 53, 90, 94]; and dietitians provided personalized dietary advice [46].
Several studies developed instruments to support discharge preparation, such as personalized patient educational protocols [39], educational programs [55], multidimensional nurse forms [48], relational tools to support emotional recovery [51], patient passports [87], handbooks [44, 59, 75], leaflets [25, 41, 55, 92], illustrated medication schedules, and pillboxes [35], or written and individualized discharge plans [41]. Additionally, discharge protocols were implemented to enhance medication reconciliation processes, improve the quality of written documentation [95], increase patient engagement [39], and support older patients in their daily activities [80].
Discharge interventions were primarily provided by hospital nurses [37, 39, 51, 55, 59, 67, 75, 80, 83, 95], hospital pharmacists [24, 25, 30, 35, 53, 90], hospital teams [6, 41, 46, 69, 94], CR teams [74, 76, 86, 87], or cardiologists [92], to improve cardiovascular health outcomes such as better adherence to healthy lifestyle behaviors [44, 59, 74-76, 86], enhanced medication adherence [24, 25, 30, 55, 59, 75, 92], and promoted healthcare self-management [39, 51, 59, 69, 83], ultimately leading to a reduction of unplanned 30-day readmissions [7, 30, 35, 37, 41, 46, 59, 67, 90, 94, 95].
3.3 Digital health
With technological advancements, digital health solutions have emerged as valuable tools for controlling cardiovascular risk factors, ensuring therapy adherence, and facilitating lifestyle modifications [2]. These solutions encompass a range of interventions, including telemedicine [54, 61, 63, 69, 74, 89, 92], telemonitoring [28, 29, 40, 43, 45, 54, 57, 63, 69-71, 74, 76, 85, 89, 91], telephone support [4, 23, 34], and text messaging [27, 28, 31, 32, 60, 66, 68, 69, 71, 73, 76, 79, 91], all aimed at ensuring continuity and coordinated care following hospital discharge.
A key component of Coleman's Care Transitions Model is conducting follow-up telephone calls to address progress, review personalized care plans, including medication management, and ensure effective communication and coordination among healthcare providers [6].
Telephone support has proven to be a crucial post-discharge for addressing patient and family needs, identifying medication-related issues, reinforcing health self-management behaviors, and coordinating care or appointment reminders. Often, telephone support is combined with hospital discharge interventions, typically provided by the hospital team [7, 46]. In some studies, telephone support was integrated as a complement to other digital health interventions [28, 70, 76, 92], enhancing its effectiveness.
The effectiveness of telephone support depends on the strategies employed and the healthcare professionals delivering the support. Telenursing interventions were associated with increased activities of daily living, patient independence [80], and higher levels of self-care knowledge, motivation, and skills [83]. Additionally, nurse-led telephone-based care coordination programs were linked to higher rates of right-siting to PHCs [5].
Pharmacist involvement in medication education, when coupled with discharge interventions and telephone support, significantly enhanced medication adherence and literacy [25, 30].
Furthermore, combining hospital team discharge interventions with telephone support from PHC teams demonstrated effectiveness in reducing hospital readmission rates, improving the quality of care transitions, enhancing treatment compliance, and increasing chronic disease self-efficacy [41, 48].
Telemedicine interventions were often combined with telemonitoring [54, 63, 69, 74, 89] and/or telephone support [92], facilitating continuous monitoring and support for patients.
Notably, one study demonstrated the safety of telemedicine use in PHC settings by a General Practitioner (GP) [61].
Many mobile applications offer interactive educational content, allowing users to input biometric values [28, 29, 40, 43, 45, 54, 57, 63, 69-71, 74, 76, 85, 89, 91], receive feedback, and set goals [29, 45, 71, 74, 76]. These features have been associated with improved risk factor control, clinical outcomes, and cost-effectiveness. Additionally, some apps enable the scheduling and tracking of follow-up appointments, promoting care coordination [69].
Text messaging, when combined with telemonitoring [28, 69, 71, 76, 91] and telephone support [28, 31, 76], has shown potential in improving healthcare self-management, adherence, and completion of CR programs. Although text messages alone [27, 32, 48, 60, 66, 68, 73], often serving as medication or follow-up care reminders, may enhance medication adherence and completion of CR [27, 32, 60, 66] programs, their impact on cardiovascular risk factor control [68, 79] and long-term medication adherence remains less clear [60].
3.4 Outpatient intervention
Outpatient intervention refers to the support provided to patients who do not require hospitalization but still need medical care, treatment, or monitoring. This type of care can be provided by hospitals, clinics, or PHCs to manage and treat health conditions without requiring an inpatient hospital stay.
Outpatient interventions were reported in 19 studies, either exclusively [33, 38, 47, 49, 50, 58, 65, 78, 93] or in combination with discharge interventions [24, 26, 46, 59, 63, 67, 86, 90, 95]. These interventions were typically led by CR or hospital teams and, in some cases, were complemented with telemonitoring [29, 40, 63, 70].
Home visits were relatively underrepresented in the studies reviewed, mostly involving elderly patients with AMI [26, 59]. The combination of home visits and phone support led to improvements in blood lipid levels, functional status, and nutrition risk among elderly patients with AMI [26]. This intervention contributed to better medication adherence, the adoption of healthy lifestyle habits, and lower rates of cardiovascular events [59].
Nurse-led interventions were associated with earlier access to follow-up [78]. Improvements in blood pressure and LDL-cholesterol levels were notable, although the influence on smoking habits, physical activity, and medication adherence was somewhat less evident. The frequency of follow-up consultations appeared to be crucial for sustaining the intervention's effect [50, 67, 70].
Tailored, nurse-led CR programs showed promise in improving risk factor management among post-AMI patients [47, 50]. Adding short-term ontopsychological support improved clinical outcomes up to 5 years post-AMI [49]. Similarly, a brief inpatient psychoeducational intervention positively impacted knowledge about AMI, promoted risk factor control, and encouraged positive health habits, effectively enhancing CR [86].
Participation in “Heart School” in Sweden during CR was associated with a significant reduction in all-cause and cardiovascular mortality following a first-time AMI [58], underscoring the importance of structured educational programs in achieving long-term outcomes.
3.5 Healthcare coordination
Healthcare coordination is an important component of Coleman's Care Transitions Model, aimed at ensuring clear communication among the patient, their primary care provider, and all healthcare professionals involved in their care. This coordination includes transmitting discharge summaries and care plans to the outpatient care team and ensuring that follow-up appointments are scheduled and maintained [6].
Among the 75 studies analyzed, only nine specifically addressed healthcare coordination, underscoring its importance in improving patient outcomes and reducing hospital readmissions. The focus on making hospital discharge information accessible to PHCs, including discharge plan [41] and identified drug-related problems with accompanying recommendations [24, 25], highlights the importance of collaboration across multidisciplinary settings.
For instance, the ALLEPRE trial, which implemented a multidisciplinary network of PHC and hospital nurses, led to improved health outcomes, increased treatment compliance, and reduced rehospitalization rates [48]. Similarly, in China, a hospital-community partnership transitional program demonstrated effectiveness in reducing hospital readmissions, improving care transitions, enhancing medication adherence, and increasing chronic disease self-efficacy [41].
Additionally, several studies helped patients identify appropriate healthcare support [6, 37, 69], offering sustainable approaches to reducing hospital readmissions [37]. An analysis of Australian national records highlighted the importance of follow-up appointments with GPs and cardiologists for AMI survivors 6 months post-event [38]. These findings underscore the critical role of healthcare coordination in post-AMI care, emphasizing the need for collaboration among healthcare professionals across different settings.
4 DISCUSSION
This comprehensive overview of transitional care for AMI patients highlights the importance of multifaceted interventions to improve long-term cardiovascular health outcomes, including reducing unplanned readmissions and cardiovascular mortality. These interventions encompass various strategies, each playing a crucial role in ensuring effective post-AMI care.
Discharge interventions are fundamental to ensuring a smooth transition from hospital to home. Personalized education, medication reconciliation, and care coordination, provided by various healthcare professionals, are key components. Patient-centered care, tailored to individual concerns and educational needs, facilitates the creation of individualized care plans. Tools such as patient passports and discharge protocols further support patient understanding and adherence to post-discharge recommendations [96].
Digital health technologies, including telemedicine, telemonitoring, telephone support, and mobile applications, offer promising avenues for promoting care continuity and self-management after hospitalization. However, low electronic health literacy presents a significant barrier to the effective utilization of these technologies [52]. Efforts to promote electronic health literacy among patients and healthcare professionals are essential for maximizing the potential benefits of digital health interventions [88]. Notably, about half of the studies using digital health were developed by CR Centers. Despite the proven benefits, referral and participation in CR programs remains low [96], indicating that digital health solutions are accessible to only a small portion of the population.
In this review, only one app allowed for the scheduling and tracking of follow-up appointments [69], highlighting the critical need for digital health tools that promote coordination of care across different healthcare sectors. Coordination of care is crucial [96], and implementing and evaluating digital health technologies that facilitate this coordination is crucial.
Telephone support also facilitates post-discharge communication, medication management, and health self-management behaviors. Telenursing and pharmacist involvement in medication education can enhance patient outcomes. Combined telephone support with discharge interventions reduces hospital readmission rates and improves treatment compliance. The advantage of telephone support over written messages lies in its ability to facilitate dialogue and clarify doubts, although it requires more time from healthcare professionals, typically provided by hospital teams. Initiating telephone support within a hospital environment ensures continuity of patient support and follow-up after discharge [37].
Outpatient nurse-led interventions focus on maintaining pharmacotherapy, improving lifestyle behaviors, and reducing complications post-AMI. In all the analyzed studies, outpatient interventions were provided by hospital teams or CR teams, underscoring the importance of developing and evaluating studies on the maintenance of secondary prevention post-AMI in PHC settings.
Home visits, particularly when combined with telephone support, have been shown to improve medication adherence, functional status, and nutrition risk in elderly patients with AMI [26, 59].
Effective healthcare coordination between hospital and PHC teams is crucial for ensuring continuity of care and improving health outcomes. Integrated care approaches, incorporating digital health solutions and coordinated care strategies, have the potential to enhance cardiovascular health outcomes and patient satisfaction. This demands partnerships between patients and healthcare professionals, along with ongoing education, training, and support for healthcare providers to optimize care pathways and efficiency within the healthcare system [3].
The lack of effective coordination and communication between hospitals and PHCs, including inadequate follow-up, is often associated with reduced patient adherence to therapeutic regimen over time. This situation frequently leads to rehospitalisation and adverse outcomes. The adoption of personalized care plans can promote self-care management and enhance patient satisfaction. However, especially after hospital discharge, patients often feel unsettled, posing a challenge to effective self-care management. Therefore, innovative care approaches that extend across healthcare sectors and link healthcare providers is necessary [2].
Nurse-led, telephone-based care coordination programs have been associated with a higher rate of right-siting to PHCs. Right-siting refers to providing patient care in the most appropriate healthcare setting at the lowest possible cost. Shifting care from tertiary to primary care can potentially free specialized resources to address new patients and more emergent cases, thereby mitigating rising healthcare costs. Despite patients' reluctance to be referred to primary care—stemming from a belief that they may receive suboptimal care—follow-up in PHCs does not seem to result in more clinical events. This outcome is reassuring to both cardiologists and patients and encourages early right-siting [4].
Scheduling an appointment with a PHC nurse within 4 weeks post-discharge after AMI also supports a smooth transition from hospital to PHC. Early addressing of risk factors, lifestyle modifications, anxiety, and depression can be facilitated, with prompt referral to appropriate healthcare professionals as needed [97].
To improve the transition of care for patients post-AMI and achieve better monitoring outcomes, several strategies can be adopted. These include nurse-led telephone monitoring and the use of applications that allow patients with higher digital literacy to input biometric data. Such strategies enable closer monitoring of patient progress after AMI, allowing for the early detection of exacerbations and timely interventions, including therapeutic adjustments or medical consultations, to avoid the need for emergency services and potential hospitalizations.
The reviewed publications highlighted that integrated care could improve continuity of care and improve outcomes for both patients and healthcare professionals. At the patient level, establishing partnerships between patients and healthcare professionals through shared decision-making fosters person-centered care. For healthcare professionals, ongoing education, training, and support are critical for enhancing care pathway integration, early discharge planning, and care coordination efficiency. At the healthcare system level, adopting new care models that embrace digital health and upskill the workforce can reduce costs and improve care efficiency [2].
Overall, these interventions underscore the potential for digital health solutions and coordinated care strategies, as well as the importance of multidisciplinary approaches to enhance cardiovascular health outcomes and patient satisfaction.
4.1 Strengths and limitations
This review included a rigorous methodology and a comprehensive search strategy. Although scoping reviews do not require a methodological evaluation of the included articles, this approach allows for a more detailed evaluation. While the review identified a wide range of transitional care interventions for post-AMI patients, the heterogeneity in study designs, implementation contexts, and evaluation methods may limit the generalizability of the results. Another limitation is the comprehensiveness of the available data. Many studies lack detailed information on the implementation and long-term outcomes of transitional care interventions. The absence of prolonged follow-up limits the ability to assess the enduring impact of these interventions on cardiovascular health and patients' quality of life.
Furthermore, the review identified a lack of standardized measures and outcomes across studies, complicating the comparison and synthesis of findings.
5 CONCLUSION
Healthcare systems face distinct challenges when managing patients discharged from the hospital after an AMI. This scoping review has identified key characteristics of transitional care interventions for post-AMI patients, drawing from the available literature.
During hospitalization following AMI, it is important to assess patients' concerns and educational needs to tailor interventions according to shared goals. Discharge interventions typically include personalized education, medication reconciliation, and care coordination, all of which are facilitated by various healthcare professionals and supported by discharge protocols and patient information leaflets. Upon discharge, all aspects of self-care should be reviewed using methods such as tech-back or motivational interviewing. The patient should also receive a copy of their discharge letter, which includes an individualized care plan, therapeutic targets, and follow-up care instructions.
Digital health solutions, including telemedicine, telemonitoring, telephone support, and text messaging, have shown promise in promoting care continuity after hospitalization. These interventions are associated with higher levels of patient activation for self-management and a lower risk of hospital readmissions. However, low digital health literacy remains a significant barrier. Thus, it is essential to promote digital health literacy not only among patients but also among healthcare professionals. Further studies are needed to explore the use of digital health outside the context of CR centers and to examine how digital health can improve coordination across levels of healthcare and optimize healthcare resources.
Telephone support offers an effective means of clarifying concerns post-discharge, though it requires substantial time from healthcare professionals, primarily those on hospital teams. Combining discharge intervention by the hospital team with telephone support provided by the PHC team can reduce 30- and 90-day hospital readmission rates, improve the quality of care transitions, enhance treatment compliance and medication adherence, and increase chronic disease self-efficacy.
It is essential to ensure that hospital discharge information, such as individualized care plans detailing patient's educational needs, shared goals, therapeutic targets, drug-related problems, and recommendations, as well as follow-up care, is accessible to PHCs. This emphasizes the importance of collaboration among nurses, pharmacists, and physicians across both hospital and primary care settings.
Earlier access to follow-up care and more frequent follow-ups post-discharge appear to be crucial for effective risk factor management. Home visits may also be necessary, particularly for elderly patients, to maintain functional status.
It is urgent to design new healthcare programs that integrate discharge interventions, digital health, telephone support, outpatient interventions, home visits, and healthcare coordination to facilitate continuity of care and care coordination posthospital discharge for AMI patients.
Cost-effectiveness is a critical factor in the successful integration of transitional care into clinical practice, as it directly influences the feasibility and sustainability of these interventions within healthcare systems. Effective transitional care programs not only aim to improve patient outcomes and reduce hospital readmissions but also to deliver these benefits in a financially viable manner. By demonstrating cost savings, such as through reduced emergency department visits, fewer complications, and shorter lengths of stay, these programs can justify the initial investment required for their implementation. Moreover, cost-effective transitional care can ease the financial burden on healthcare providers and payers, making it more likely for these programs to be adopted on a larger scale, thereby improving access to high-quality care for patients recovering from AMI. Thus, economic considerations are essential for the widespread adoption and long-term success of transitional care initiatives in clinical practice.
AUTHOR CONTRIBUTIONS
Filipa Homem: Conceptualization (equal); formal analysis (equal); investigation (equal); methodology (equal); writing—original draft (equal). Anaísa Reveles: Conceptualization (equal); formal analysis (equal); investigation (equal); methodology (equal); writing—original draft (equal). António Amaral: Writing—review and editing (equal). Verónica Coutinho: Writing—review and editing (equal). Lino Gonçalves: Writing—review and editing (equal).
ACKNOWLEDGMENTS
The authors extend their gratitude to the ULSC Cardiology team for their motivation and commitment to continuous improvement, to Coimbra Local Health Unit for the support and encouragement in the integration of care, and to UICISA: E/ESEnfC for supporting the development of research that demonstrates the results of nursing care.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
Not applicable.
INFORMED CONSENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
The authors confirm that the data supporting the findings of this study are available within the article and its online supplementary materials.




