Formulation of a CITE metric for evaluating the clinical implications of medical studies and their originating hospitals in China
Abstract
Background
The number of medical research publications by Chinese clinical investigators has risen substantially, contributing to 14.63% of the global total in 2019; however, their tangible impact on clinical decision-making remains limited. Various evaluation methods have been developed to measure hospital research competence in China, such as Fudan University's China hospital ranking and Science and Technology Evaluation Metrics (STEM) ranking, which predominantly focuses on factors such as academic reputation, volume of publications and patents, and research resources. However, composite indices may not fully capture the actual clinical value generated by medical research. To address this gap, we introduced the “Clinical Influence and Timeliness Evaluation (CITE)” metric to assess both the clinical importance of a given medical research study and the clinical influence of the hospital where it originated. The methodology used relies on the premise that influential medical research would be referenced in clinical guidelines, which serve as critical resources for clinicians.
Methods
The CITE metric was applied for 78,636 medical studies concerning chronic obstructive pulmonary disease (COPD) published between 2000 and 2020 and referenced in both Chinese and international clinical guidelines for COPD. Specific indexes and formulas were derived to quantify the clinical weight of a medical research study (W) and its timeliness (T), enabling a dynamic assessment of the clinical value of each study and the overall contribution of a particular hospital.
Results
In this analysis, we incorporated 499 hospitals in China and quantitatively identified their dynamic clinical influence in COPD from 2000 to 2020. Our findings offer objective and targeted evaluation metrics by focusing on clinical relevance and recognizing the collaborative nature of medical research.
Conclusion
The CITE metric provides an innovative method to gauge the true impact of medical research in China, with potential applications across different medical specialties. CITE can serve as a useful tool for understanding the relationship between research input and practical clinical outcomes, ultimately promoting more clinically relevant research endeavors.
Abbreviations
-
- CIAA
-
- clinical influence of author's affiliation
-
- CIMS
-
- clinical importance of a medical study
-
- CITE
-
- clinical influence and timeliness evaluation
-
- COPD
-
- chronic obstructive pulmonary disease
-
- CVAA
-
- contribution value of authors' affiliation
-
- STEM
-
- science and technology evaluation metrics
1 INTRODUCTION
In recent years, publications on medical research by clinical investigators in China have been steadily increasing, amounting to 14.63% of the global total in 2019 [1]. Nonetheless, China's medical research has had a limited impact on clinical decision-making [2, 3], with a notable lack of high-quality clinical studies [4, 5]. A fundamental issue lies in dynamic appraisal of the clinical relevance of medical research that cements the measure of a hospital's research competence. China has developed several evaluative methods concerning hospital research capability, such as Fudan University's China hospital ranking, the Science and Technology Evaluation Metrics (STEM) ranking of China's hospitals released by the Chinese Academy of Medical Sciences, and Innovation and Transformation ranking of China's hospitals, among others [6]. Generally, these measures quantify hospital research capacities based on various factors encompassing academic reputation, quantity of patents or papers, talent pool, academic appointments, and available research resources [7]. Although composite indices are valuable in terms of providing a comprehensive estimation of a hospital's research competence and prospective capabilities, they inadequately tackle the essential question of how much clinical value medical research actually generates.
As an applied science rooted in natural sciences, the aim of medical research should ideally be to discover new patterns of disease and to establish novel diagnostic and therapeutic techniques, all while improving patients' quality of life; these are key indicators of the caliber and usefulness of such research [8]. The inclusion of medical research outcomes as evidence in clinical guidelines released by authoritative associations and reflect its academic and application value because these guidelines summarizing the latest advances in disease diagnosis and therapy are pivotal references for clinicians in daily practice [9, 10]. Hence, we postulated that valuable medical research would be directly or indirectly incorporated into clinical guidelines [11]. We developed an accessible and objective evaluation metric, termed the “Clinical Influence and Timeliness Evaluation” or (CITE), to dynamically appraise the clinical importance of a medical research study and the clinical influence of the hospital from which it originated. Using chronic obstructive pulmonary disease (COPD) as an example, we applied the CITE metric to evaluate the clinical relevance of medical research carried out by hospitals in China and verify the applicability of this metric.
2 DATA COLLECTION AND ANALYTICAL METHODS
2.1 Medical study data set
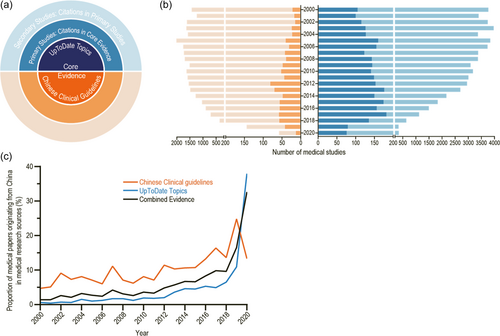
In the CITE analysis, we investigated medical studies published from 2000 to 2020 that were directly or indirectly referenced in Chinese and international clinical guidelines for COPD. The Chinese guidelines comprised 20 clinical guidelines issued before August 2021 by China's academic organizations such as the Chinese Medical Association and Chinese Physician Association. The international guidelines comprised 59 UpToDate topics updated through August 2021. The 878 articles cited in the Chinese guidelines and 2888 articles cited in the UpToDate topics constituted primary studies, and 74,870 articles cited in primary studies constituted the secondary studies (Figure 1a,b). The medical studies that corresponded to Chinese institutions accounted for 8.8% and 1.9% of Chinese and international guideline sources (Figure 1c). The Chinese medical studies investigated in this research originated from the Chinese mainland and did not include those from Chinese Hong Kong, Macao, or Taiwan.
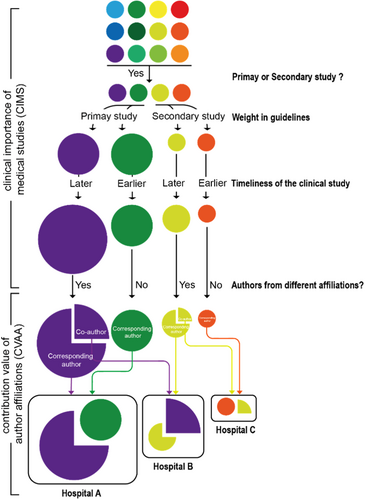
2.1.1 Calculating progressive clinical importance of medical studies
2.2 Calculating the clinical influence of hospitals
2.3 Statistical analysis
This study was a descriptive analysis. Thus, statistical inference and tests are not applicable because the data set analyzed comprises clinical studies referenced in clinical guidelines.
3 CLINICAL INFLUENCE ASSESSMENT RANKING OF HOSPITALS IN CHINA
A total of 499 hospitals in China were included in the clinical influence assessment ranking of COPD, comprising 350 hospitals in the Chinese guideline data set and 334 hospitals in the UpToDate topics data set. The sum of CIAA scores for the 20 leading hospitals from 2000 to 2020 accounted for 51.0%, 54.5%, and 50.4% of the total CIAA score in the Chinese guideline data set, the UpToDate topics data set, and the merged data set, respectively. The ranking results for the 10 leading hospitals in each data set are shown in Tables 1–3, and that of the 100 leading hospitals are shown in Table S2.
| Rank | Hospital | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | First Affiliated Hospital of Guangzhou Medical University (Guangzhou Respiratory Diseases Research Institute) | 0.00 | 0.00 | 18.81 | 17.79 | 14.89 | 12.75 | 13.75 | 19.06 | 19.04 | 21.97 | 23.78 | 23.83 | 24.47 | 24.75 | 24.85 | 20.14 | 16.91 | 16.92 | 13.18 | 11.29 | 9.61 | 15.75 |
| 2 | Peking University Third Hospital | 0.47 | 6.79 | 4.38 | 3.05 | 2.50 | 5.64 | 2.64 | 3.67 | 4.35 | 4.31 | 4.53 | 4.31 | 3.95 | 3.23 | 2.81 | 2.13 | 4.99 | 4.83 | 5.66 | 6.64 | 5.70 | 4.74 |
| 3 | Capital Medical University Affiliated Beijing Chaoyang Hospital | 48.82 | 24.98 | 16.00 | 11.02 | 8.92 | 7.29 | 6.77 | 5.78 | 5.92 | 5.58 | 6.40 | 6.16 | 5.68 | 4.69 | 3.78 | 2.79 | 1.75 | 2.20 | 2.31 | 2.69 | 2.20 | 4.35 |
| 4 | First Affiliated Hospital of China Medical University | 0.00 | 0.00 | 0.00 | 3.71 | 3.14 | 2.70 | 1.26 | 2.93 | 4.69 | 4.70 | 3.92 | 3.73 | 3.31 | 2.64 | 2.31 | 1.72 | 1.17 | 1.80 | 1.91 | 1.74 | 3.00 | 2.89 |
| 5 | Xinqiao Hospital of Army Medical University | 15.80 | 5.95 | 3.80 | 6.42 | 5.43 | 4.60 | 5.69 | 5.39 | 4.56 | 4.27 | 4.45 | 4.21 | 3.76 | 2.94 | 2.31 | 1.62 | 0.90 | 0.77 | 0.51 | 0.34 | 0.59 | 2.36 |
| 6 | Fudan University Affiliated Zhongshan Hospital | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.03 | 3.62 | 2.03 | 2.98 | 2.75 | 2.21 | 2.24 | 2.00 | 1.70 | 1.70 | 1.47 | 1.10 | 1.33 | 1.63 | 2.27 | 2.67 | 2.18 |
| 7 | West China Hospital of Sichuan University | 15.80 | 13.15 | 8.45 | 5.84 | 8.24 | 6.94 | 3.15 | 1.66 | 1.41 | 1.12 | 0.70 | 0.52 | 0.52 | 0.53 | 1.99 | 1.86 | 1.64 | 1.50 | 1.84 | 1.80 | 2.20 | 2.11 |
| 8 | China-Japan Friendship Hospital | 0.00 | 0.20 | 0.13 | 0.09 | 0.28 | 0.24 | 0.11 | 1.23 | 1.03 | 0.95 | 0.76 | 0.72 | 0.65 | 0.52 | 0.42 | 0.33 | 0.22 | 0.08 | 5.09 | 4.42 | 3.49 | 1.96 |
| 9 | Shanghai Jiao Tong University Affiliated Ruijin Hospital | 0.00 | 0.00 | 0.00 | 0.02 | 0.33 | 0.29 | 0.14 | 2.42 | 2.21 | 2.41 | 3.10 | 3.03 | 2.82 | 2.45 | 2.23 | 3.05 | 2.29 | 1.60 | 1.13 | 0.83 | 0.91 | 1.61 |
| 10 | General Hospital of Northern Theater Command of the Chinese People's Liberation Army | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.03 | 3.62 | 4.33 | 3.79 | 3.87 | 3.24 | 3.08 | 2.75 | 2.18 | 1.85 | 1.25 | 0.63 | 0.28 | 0.17 | 0.10 | 0.70 | 1.60 |
| Rank | Hospital | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | First Affiliated Hospital of Guangzhou Medical University (Guangzhou Respiratory Diseases Research Institute) | 0.00 | 0.00 | 0.00 | 0.81 | 0.72 | 0.60 | 1.35 | 8.47 | 10.37 | 11.63 | 14.60 | 12.72 | 12.72 | 11.85 | 14.47 | 14.91 | 11.24 | 9.13 | 7.88 | 7.89 | 9.33 | 16.40 |
| 2 | Fudan University Affiliated Zhongshan Hospital | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.13 | 0.12 | 0.10 | 0.67 | 0.54 | 0.43 | 0.51 | 0.41 | 0.91 | 2.92 | 2.66 | 2.03 | 3.24 | 3.03 | 2.87 | 3.85 | 3.90 |
| 3 | Capital Medical University Affiliated Beijing Chaoyang Hospital | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 4.48 | 3.98 | 3.17 | 3.52 | 3.25 | 5.21 | 4.44 | 3.69 | 3.01 | 2.17 | 1.75 | 1.22 | 1.11 | 2.35 | 1.99 | 1.61 | 3.82 |
| 4 | Peking Union Medical College Peking Union Hospital | 0.00 | 0.00 | 0.00 | 0.00 | 0.14 | 1.06 | 0.94 | 0.75 | 3.80 | 3.23 | 2.58 | 4.51 | 3.61 | 2.87 | 2.20 | 1.87 | 2.76 | 2.40 | 1.93 | 1.62 | 1.37 | 3.33 |
| 5 | West China Hospital of Sichuan University | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 | 0.29 | 0.25 | 0.20 | 0.74 | 0.68 | 0.55 | 0.53 | 2.82 | 2.96 | 2.84 | 2.63 | 2.00 | 1.59 | 1.49 | 1.51 | 2.64 | 3.20 |
| 6 | Fuwai Hospital of Chinese Academy of Medical Sciences | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.12 | 0.58 | 0.52 | 0.40 | 0.70 | 2.82 | 2.30 | 2.01 | 1.80 | 1.34 | 2.38 | 3.39 | 2.97 | 2.51 | 3.13 |
| 7 | Tongji University Affiliated Shanghai Pulmonary Hospital | 0.00 | 0.00 | 0.00 | 0.58 | 0.52 | 0.43 | 0.37 | 0.29 | 0.19 | 0.24 | 0.26 | 0.34 | 0.26 | 0.29 | 0.25 | 0.48 | 3.35 | 2.92 | 2.59 | 2.29 | 1.96 | 2.29 |
| 8 | Guangdong Provincial People's Hospital | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.03 | 0.02 | 0.02 | 2.85 | 2.46 | 2.20 | 2.16 | 1.87 | 1.88 |
| 9 | Sun Yat-sen University Affiliated Sun Yat-sen Memorial Hospital | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.07 | 0.06 | 0.05 | 0.04 | 0.03 | 0.03 | 2.85 | 2.42 | 2.15 | 1.92 | 1.65 | 1.74 |
| 10 | Second Affiliated Hospital of Zhejiang University | 0.00 | 0.00 | 0.02 | 0.04 | 0.04 | 0.03 | 0.82 | 0.70 | 0.48 | 3.43 | 2.70 | 2.51 | 1.99 | 1.56 | 1.12 | 1.01 | 0.65 | 0.45 | 0.48 | 0.51 | 0.44 | 1.54 |
| Rank | Hospital | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | First Affiliated Hospital of Guangzhou Medical University (Guangzhou Respiratory Diseases Research Institute) | 0.00 | 0.00 | 9.41 | 9.30 | 7.81 | 6.68 | 7.55 | 13.77 | 14.71 | 16.80 | 19.19 | 18.28 | 18.60 | 18.30 | 19.67 | 17.53 | 14.07 | 13.03 | 10.53 | 9.59 | 9.47 | 16.08 |
| 2 | Capital Medical University Affiliated Beijing Chaoyang Hospital | 24.41 | 12.49 | 8.00 | 5.51 | 4.46 | 5.89 | 5.38 | 4.48 | 4.72 | 4.42 | 5.81 | 5.30 | 4.69 | 3.85 | 2.98 | 2.27 | 1.49 | 1.66 | 2.33 | 2.34 | 1.91 | 4.09 |
| 3 | Fudan University Affiliated Zhongshan Hospital | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.08 | 1.87 | 1.06 | 1.83 | 1.65 | 1.32 | 1.38 | 1.21 | 1.31 | 2.31 | 2.07 | 1.57 | 2.28 | 2.33 | 2.57 | 3.26 | 3.04 |
| 4 | Peking University Third Hospital | 0.24 | 3.40 | 2.19 | 1.53 | 1.25 | 2.82 | 1.32 | 1.84 | 2.48 | 2.65 | 2.70 | 2.52 | 2.30 | 2.07 | 2.07 | 1.65 | 2.99 | 2.80 | 3.34 | 3.76 | 3.22 | 2.99 |
| 5 | West China Hospital of Sichuan University | 7.90 | 6.58 | 4.23 | 2.92 | 4.13 | 3.62 | 1.70 | 0.93 | 1.08 | 0.90 | 0.63 | 0.52 | 1.67 | 1.74 | 2.42 | 2.25 | 1.82 | 1.55 | 1.67 | 1.66 | 2.42 | 2.66 |
| 6 | Beijing Union Medical College Beijing Union Hospital | 0.00 | 0.00 | 0.00 | 0.56 | 0.55 | 2.73 | 1.52 | 0.95 | 2.37 | 2.03 | 1.71 | 3.19 | 2.65 | 2.88 | 2.35 | 1.97 | 2.21 | 1.93 | 1.48 | 1.19 | 0.95 | 2.23 |
| 7 | First Affiliated Hospital of China Medical University | 0.00 | 0.00 | 0.00 | 1.86 | 1.57 | 1.56 | 0.81 | 1.61 | 2.75 | 2.92 | 2.41 | 2.25 | 2.02 | 1.75 | 1.76 | 1.44 | 1.00 | 1.23 | 1.32 | 1.17 | 1.75 | 1.99 |
| 8 | China-Japan Friendship Hospital | 0.00 | 0.10 | 0.07 | 0.05 | 0.14 | 0.12 | 0.06 | 0.62 | 0.52 | 0.48 | 0.39 | 0.37 | 0.33 | 0.35 | 0.29 | 0.29 | 0.25 | 0.26 | 3.52 | 3.11 | 2.67 | 1.73 |
| 9 | Fuwai Hospital of China Academy of Medical Sciences | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.06 | 0.29 | 0.26 | 0.20 | 0.35 | 1.41 | 1.15 | 1.01 | 0.90 | 0.67 | 1.19 | 1.70 | 1.49 | 1.26 | 1.61 |
| 10 | Xinqiao Hospital of Army Medical University | 7.90 | 2.98 | 1.90 | 3.21 | 2.72 | 2.31 | 2.86 | 2.73 | 2.61 | 2.64 | 2.63 | 2.63 | 2.29 | 1.79 | 1.40 | 1.03 | 0.59 | 0.62 | 0.44 | 0.42 | 0.51 | 1.58 |
4 DISCUSSION AND FUTURE DIRECTIONS
Clinical and laboratory studies advance medical technology and treatment and eventually improve patient care and outcomes. In recent years, the Chinese government has been actively promoting the construction of research hospitals, hoping to improve population health and the national health system by increasing investment in biomedical research [12]. In line with this, it is crucial to gauge the clinical relevance of medical research.
Clinical guidelines, which assist clinicians in delivering optimal patient care, are an ideal reference for assessing the practical value of medical research [13, 14] and have been adopted by national health commissions as essential tools for improving the quality of medical care [15]. In a prior investigation, we conducted a quantitative examination of the frequency with which Chinese clinical research was referenced in clinical guidelines. Interestingly, we discovered that the citation rate of Chinese research in clinical guidelines lagged significantly behind the burgeoning growth in the number of Chinese medical papers in various international medical articles [16]. This staggering discrepancy underscores the pressing need to clarify the clinical relevance of Chinese medical research. In a bid to systemically and progressively appraise the clinical value of medical research emerging from Chinese hospitals, we developed the CITE evaluation method, which hinges primarily on clinical guidelines as reference.
Compared with existing domestic metrics evaluating the research competence and academic impact of hospitals using composite indicators [7], the CITE metric focuses on evaluating the contribution of studies in improving clinical practice and patient care. The practicality of the CITE metric is underscored from several aspects. First, CITE zeroes in on the clinical relevance of medical research, thereby dramatically streamlining the assessment indicators compared with other methods. This sharply curtails the risk of overwhelming the analysis process with extraneous assessment indicators and compromising its initial purpose [17]. This approach strategically sets the assessment scope for medical studies as a dual-tiered citation centering on clinical guidelines. The exponential surge in medical study assessments ensures the inclusion of numerous hospitals while still giving attention to clinical relevance. Furthermore, the CITE metric is exhaustive in nature. In current medical research, collaboration across various medical centers is increasingly becoming the norm. Regrettably, existing assessment systems offer little recognition to the research input of collaborating healthcare institutions, often reserving this for the principal investigator's institution. Using various evaluation components, our metric captures clinical research outcomes that are directly or indirectly incorporated into guidelines by hospitals that are either leading or participating in the research. Moreover, CITE offers insight into the evolution of clinical value over time. It is well established that medical research, a process that often spans years, generates clinical value that also endures for several years. With this in mind, we instituted the “timeliness” concept, effectively portraying the longevity of clinical value over its “validity period.” This more scientifically reflects the sustained clinical value of medical research and prevents extreme fluctuations in the evaluation results.
This study has several limitations. One prominent issue lies in the lack of reference data and insufficient processing capacity of manual data to determine reasonable values within this field. For example, the factor , assigned to secondary studies, is calculated as the ratio of the number of primary to secondary studies; and the factor , representing the degree of contribution from participating hospitals, is defined as the reciprocal of the median number of hospitals participating in studies in addition to the corresponding hospital. It is important to concede that the validity of these values may require future exploration to ensure their appropriateness. Similarly, it is more reasonable to assign specific values to parameters such as and for each study rather than uniform values, which can be achieved in the future through automated analysis using software algorithms. Furthermore, introduction of the value = 10 in this study was a calculated move aimed at creating a balance between sensitivity to the variations in research output and mitigating unreasonable annual volatility. However, we acknowledge that this approach may fall short in terms of accurately illustrating the lifespan of the clinical impact generated by all varieties of medical research. Therefore, this factor too invites further scrutiny and assessment so as to refine the evaluation process. Additionally, in this analysis, we did not examine the publication of studies directly; instead, we examined studies referenced in guidelines. This can lead to delays in the results (as shown in Figure 1b) and could also change past-year evaluation rankings depending on the prevailing citation in the guidelines. Therefore, a single year's hospital rankings may not be unequivocally credible, warranting a review over several years.
In our study, the practical applicability of the CITE metric was successfully established within the domain of COPD. In the future, the extension of this approach to encapsulate a wider range of disease fields holds promise. This could facilitate a thorough and dynamic analysis of the clinical value and transformation of medical investigations conducted across various specialties and hospitals, which could prove invaluable by serving as a robust reference for gauging the input–output relationship within medical research. Ultimately, this could stimulate a more problem-centric approach to medical investigations, thereby encouraging the pursuit of research endeavors that are more aligned with solving real-world clinical issues.
AUTHOR CONTRIBUTIONS
Gao Jianchao: Conceptualization (lead); data curation (lead); formal analysis (lead); funding acquisition (lead); investigation (equal); methodology (equal); project administration (lead); resources (lead); software (lead); supervision (equal); validation (equal); visualization (equal); writing—original draft (equal); writing—review and editing (equal). Chen Xiaoyuan: Conceptualization (equal); data curation (supporting); formal analysis (supporting); investigation (equal); methodology (equal); project administration (supporting); writing—original draft (equal); writing—review and editing (equal). Gao Chenyan: Conceptualization (equal); data curation (supporting); formal analysis (supporting); investigation (equal); methodology (equal); project administration (equal); supervision (equal); validation (equal); visualization (equal); writing—original draft (equal); writing—review and editing (equal).
ACKNOWLEDGMENTS
We sincerely appreciate Professor Chen Pingyan from Southern Medical University and Professor Wu Yangfeng from Peking University Clinical Research Institute for their invaluable suggestions in the data analysis for this research. Our acknowledgment extends to Professor Ma Jun from the Institute of Hematology and Oncology, Harbin First Hospital, to Professor Li Haiyan from Peking University Third Hospital, and to Professor Wang Huanling from Peking Union Medical College Hospital whose seminal contributions to prior research have greatly informed this study. The opinions, findings, and conclusions herein represent the views of the authors and do not necessarily represent the views of affiliated institutions.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
Not applicable.
INFORMED CONSENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Data are available in the article's supplementary material.




