Changes in aspartate metabolism in the medial-prefrontal cortex of nicotine addicts based on J-edited magnetic resonance spectroscopy
Abstract
This study aims to explore the changes of the aspartate (Asp) level in the medial-prefrontal cortex (mPFC) of subjects with nicotine addiction (nicotine addicts [NAs]) using the J-edited 1H MR spectroscopy (MRS), which may provide a positive imaging evidence for intervention of NA. From March to August 2022, 45 males aged 40–60 years old were recruited from Henan Province, including 21 in NA and 24 in nonsmoker groups. All subjects underwent routine magnetic resonance imaging (MRI) and J-edited MRS scans on a 3.0 T MRI scanner. The Asp level in mPFC was quantified with reference to the total creatine (Asp/Cr) and water (Aspwater-corr, with correction of the brain tissue composition) signals, respectively. Two-tailed independent samples t-test was used to analyze the differences in levels of Asp and other coquantified metabolites (including total N-acetylaspartate [tNAA], total cholinine [tCho], total creatine [tCr], and myo-Inositol [mI]) between the two groups. Finally, the correlations of the Asp level with clinical characteristic assessment scales were performed using the Spearman criteria. Compared with the control group (n = 22), NAs (n = 18) had higher levels of Asp (Asp/Cr: p = .005; Aspwater-corr: p = .004) in the mPFC, and the level of Asp was positively correlated with the daily smoking amount (Asp/Cr: p < .001; Aspwater-corr: p = .004). No significant correlation was found between the level of Asp and the years of nicotine use, Fagerstrom Nicotine Dependence (FTND), Russell Reason for Smoking Questionnaire (RRSQ), or Barratt Impulsivity Scale (BIS-11) score. The elevated Asp level was observed in mPFC of NAs in contrast to nonsmokers, and the Asp level was positively correlated with the amount of daily smoking, which suggests that nicotine addiction may result in elevated Asp metabolism in the human brain.
1 INTRODUCTION
In the world as a whole currently, there are still more over 1 billion smokers (Reitsma et al., 2021). Long-term smoking not only causes cardiovascular and respiratory diseases, but also damages the cognitive and nervous systems (Castro et al., 2023; Swan & Lessov-Schlaggar, 2007). Nicotine, a harmful substance in tobacco, is addictive, entering the body with exhaled smoke and quickly invading the brain for a few seconds, the specific pharmacological properties of nicotine will produce a sober and refreshing effect on smokers. Addicted smokers are hard to quit even though they clearly know that smoking will cause serious damage to their body, and the relapse rate of smokers who try to quit smoking is still very high (Zhu et al., 2012). The World Health Organization (WHO) has defined tobacco addiction as a mental illness, and treatment and intervention for nicotine addicts (NAs) are urgent. At present, a series of brain magnetic resonance imaging (MRI) studies (including functional MRI [fMRI], structural MRI [sMRI], and MR spectroscopy [MRS]) has been conducted on NA at home and abroad, and the understanding of the addiction mechanism of smokers is gradually deepening, but no consistent conclusion has been reached.
As known, nicotine addiction cannot only alter brain structure and function, but also cause changes in brain metabolism. Preceding MRS research on smoking has shown that nicotine can regulate biochemical metabolism in the brain, and NA is associated with changes in levels of regional metabolites containing NAA, cholinine (Cho), creatine (Cr), glutamate (Glu), and myo-Inositol (mI) (Bagga et al., 2021; Durazzo et al., 2016; Gallinat et al., 2007; Li et al., 2018; Mennecke et al., 2014; O'Neill et al., 2014). The study by Mennecke et al. (2014) showed that Glx (glutamate + glutamine) and Cho concentrations was remarkably increased in the left cingulate cortex in smokers relative to nonsmokers, and six out of seven smokers recovered normal Glx concentrations after quitting smoking, which proved that the physiological metabolism in human brain was related to nicotine addiction and withdrawal. Durazzo et al. (2016) found that smokers had lower NAA, Cr, mI, and Glu levels in dorsolateral prefrontal cortex (DLPFC), as well as lower NAA levels in lenticular nuclei; smokers also exhibited greater age-related decreases of DLPFC NAA, and anterior cingulate cortex (ACC) and DLPFC Glu concentrations. Meanwhile, they found that smokers displayed worse decision-making and greater impulsivity. Bagga et al. (2021) observed that smokers showed lower Glx/Cr and Cho/Cr ratios than nonsmokers in the PFC region. Their study also highlights that it is not one metabolite that underlies the significant differences in brain metabolism between smokers versus nonsmokers and different task conditions, but is the interplay between the metabolites (NAA and Cho, Glx and mI, and Cho and mI).
Normal single-voxel MRS techniques, such as Point RESolved Spectroscopy (PRESS) (Starck et al., 2009) and STimulated Echo Acquisition Mode (STEAM) (Frahm et al., 1989), can detect a variety of chemicals (including above mentioned NAA, Cr, Cho, and Glx, etc.) but the spectral signals of some important neurotransmitters, such as gamma amino butyric acid (GABA), N-acetyl aspartul glutamate (NAAG), aspartate (Asp), and so forth, is relatively low and overlaps with the peaks of other dominant metabolites (Cr, Cho, etc.). It is worth mentioning that these neurotransmitters with trace amounts in the brain can be specifically and reliably detected by the J-edited (MEGA-PRESS) technology (Menshchikov et al., 2017; Mescher et al., 1998). A recent study (Durazzo & Meyerhoff, 2021) on chronic cigarette smoking found that smokers had significantly lower GABA levels in the right DLPFC than nonsmokers, and demonstrated that GABA concentrations in ACC and DLPFC were associated with neurocognition and decision-making/impulsivity in active cigarette smokers. Previous study by Janes et al. (2013) also revealed a relationship of the heightened reactivity to drug cues with both decreasing dorsolateral ACC GABA in smoker and early withdrawal symptoms. However, Bagga et al. (2018) only showed the sex-specific increase of PFC GABA level in female smokers. In conclusion, though not entirely consistent, these scientific achievements reflect the influence of nicotine addiction on neurometabolism, which may mediate addictive behavior in smoker.
Previous studies have found that the prefrontal striatum circuit is an important circuit in the neural mechanism of nicotine addiction (Yuan et al., 2016), and the abnormality of this circuit may have some specific relationship with the reduced cognitive control ability of smokers. Multiple studies have suggested that the prefrontal cortex is mainly related to motivation and cognitive control (Kalivas & Volkow, 2005; Kringelbach, 2005), and is also a key region controlling emotion recognition (Saxe & Powell, 2006; Stone et al., 1998). Abundant sMRI studies have shown that differences in gray matter (GM) volume and cortical thickness in the frontal regions have significant implications for the study of the mechanism of addictive behavior in nicotine tolerance patients (Fritz et al., 2014; Li et al., 2015; Liao et al., 2012). As the main area of interest in brain functional imaging studies, PFC is also a part of the reward system and executive control system in the brain circuit model related to substance addiction, and has become a target for various psychiatric illnesses in clinical practice. Therefore, PFC is considered to be one of the mostly abnormal brain regions involved in the formation of nicotine-dependent behaviors. In addition, studies (Bagga et al., 2021; Durazzo et al., 2016) have shown that smoking is associated with metabolic abnormalities in the frontal lobe region of the brain.
Aspartate is an excitatory neurotransmitter that has received attention for its presence in the central nervous system (CNS; Genchi, 2017). When Asp level is changed, the balance between the inhibitory and excitability of neurons is destroyed, which may be one of the important metabolic substances causing substance addiction. Asp has been confirmed to have metabolic disorders in schizophrenia patients, animal models of autism spectrum disorder and Parkinson's disease (PD), and various neurological diseases (Errico et al., 2013; Klunk et al., 1996; Nuzzo et al., 2017; Nuzzo et al., 2020; Nuzzo, Punzo, et al., 2019). For example, in the inbred BTBR mouse model of idiopathic autism, d-Aspartate (d-Asp) content was found to be significantly and stereoselectively increased in the prefrontal cortex, hippocampus, and serum, which supports the existence of d-Asp metabolism disorder in the widely used animal model of idiopathic autism spectrum disorder (Nuzzo et al., 2020). In addition, d-Asp content was found to be dramatically upregulated in the putamen of a primate model of PD (Nuzzo, Punzo, et al., 2019). Klunk et al. (1996), in perchloric acid (PCA) extracts of postmortem Alzheimer's disease (AD) brain examined by both proton (1H) and phosphorus-31 MRS, found that aspartate and L-glutamate were significantly elevated in the occipital cortex, and these changes may be responsible for the neuronal death observed in AD (associated with a reduction in NAA). Errico's team (Errico et al., 2013) discovered that d-Asp and N-Methyl D-Aspartate (NMDA) in the prefrontal cortex and striatum of the brain of schizophrenia patients continued to decrease after death. However, the metabolic changes of Asp in the medial prefrontal cortex of nicotine addiction remain unclear.
Based on previous studies, we hypothesized that the Asp metabolism in the mPFC of the brain varies in NAs. To test it, our study investigated changes in levels of Asp and other co-measured metabolites (including total NAA [tNAA], total Cho [tCho], total Cr [tCr], and mI) in the mPFC of NA using J-edited 1H MRS, and to evaluate the correlation between the Asp level and clinical features in NAs.
2 MATERIALS AND METHODS
2.1 Study subjects and inclusion criteria
From March to August 2022, a total of 45 volunteers (aged between 40 and 60 years old) were recruited in Henan Province through online platform and community publicity, including 21 NAs (smoking no <10 cigarettes per day) and 24 nonsmokers. All subjects were male and right-handed.
The inclusion criteria of the NAs group were: (1) subjects were evaluated according to the diagnostic and statistical manual of mental disorders-fifth edition (DSM-V), and all NAs met the diagnostic criteria for substance addiction; (2) smoking no <10 cigarettes a day, and the duration of smoking is more than 2 years; (3) nicotine dependence was evaluated using the Fagerstrom Nicotine Dependence (FTND) scale. The inclusion criteria of the control group included: (1) matching age, sex, and education level; (2) never smoked in their life.
The exclusion criteria of the NA and control groups were: (1) with any mental disorders or family history of mental disorders-related diseases, such as schizophrenia, epilepsy, and so forth; (2) have taken antipsychotics in the past, or have taken any drugs recently (within 1 month); (3) with other systemic diseases, such as chronic obstructive pulmonary diseases, metabolic diseases, history of intracranial tumors, history of cardiovascular and cerebrovascular diseases, or history of major brain trauma; (4) with history of any other substance dependence or behavioral addiction in addition to nicotine dependence, such as alcohol use disorder, online game addiction, and so forth; (5) with contraindications to MRI scans.
Before the MRI scan, each participant received a set of questionnaires, including basic information (age, sex, height, and weight, etc.), FTND scale, Russell Reason for Smoking Questionnaire (RRSQ) and Barratt Impulsivity Scale (BIS-11). At the same time, they were informed of the purpose of the study and the situations that may occur during the scan. All participants signed written informed consent after they agreed. This study was reviewed and approved by the local Medical Ethics Committee of the First Affiliated Hospital of Zhengzhou University.
2.2 MRI examination equipment and sequence parameters
All subjects were scanned on a 3.0 T MRI scanner (Ingenia CX, Philips Healthcare, Best, The Netherlands) in the Magnetic Resonance Department of the First Affiliated Hospital of Zhengzhou University, including routine MRI and J-edited MRS scans. During the scan, subjects should stay awake, and spongy pads were placed near their ears on both sides to stabilize their heads. In order to eliminate the interference of other organic brain lesions, we first collected some routine sequences for clinical diagnosis: including T1-weighted imaging (T1WI), T2-weighted imaging (T2WI), fluid-attenuated inversion recovery, and diffusion-weighted imaging. For anatomical reference, a high-resolution 3D T1WI scan was carried out using the gradient echo sequence with the following parameters: number of sagittal slices = 200, field of view = 256 mm2, repetition time (TR) = 9 ms, echo time (TE) = 4 ms, slice thickness = 0.9 mm, and acquisition time = 3 min 26 s. The MEGA-PRESS sequence was used for the J-edited Asp spectrum acquisition, and the parameters were implemented as follows: TR = 2000 ms; TE = 90 ms; number of signal averages = 192 (both ON and OFF spectra were repeated by 96 times, totally 192 spectra); ON/OFF frequencies = 3.89/5.21 ppm; scan time = 6 min 30 s. Unsuppressed water signals were also recorded with 32 signal averages as an internal concentration reference. The spectral data were obtained in a volume of 30 × 30 × 30 mm3 located in the frontal lobe of the brain (shown in Figure 1). The VAPOR scheme was used for water suppression (Tkác et al., 1999).
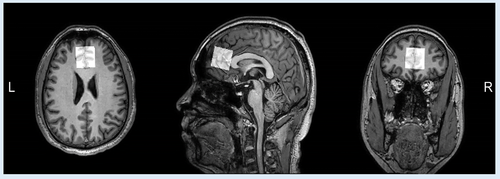
2.3 Image processing and quantification
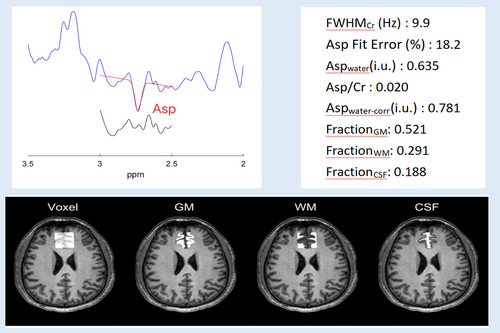
The quantification of metabolites including tCr, tNAA, tCho, and mI, from the OFF-averaged spectrum was performed using the Tarquin software (https://tarquin.sourceforge.net/) (Wilson et al., 2011).
2.4 Data analysis (statistical analysis)
The SPSS 22.0 software was used for statistical analysis. Normal test for each dataset was conducted before further analyses. If variables did not meet normal distribution criteria, the nonparametric test was used, else the independent samples t-test was used. The clinical characteristic variables (age, height, weight, and length of education), the levels of mPFC Asp (Asp/Cr and Aspwater-corr) and other metabolites (including tNAA, tCr, tCho, and mI), and brain tissue fractions for GM, WM, and CSF within voxels were compared between the NA and control groups. Quantitative data were expressed as mean ± standard deviation (X ± SD), and p < .05 was considered statistically significant. The Spearman standard was used to analyze the correlation between the Asp level and clinical characteristics assessment scales (including daily smoking amount, years of nicotine use, FTND, RRSQ, and BIS-11 score).
3 RESULTS
3.1 Clinical and demographic information
Eighteen NAs and 22 age- and sex-matched nonsmokers were eventually included after excluding subjects with underlying conditions (including 1 NA and 1 nonsmoker), alcohol dependence (including 1 NA), and poor data quality (including 1 NA and 1 nonsmoker). The demographic characteristics of the control and NA subjects are given in Table 1. There was no significant difference in age (p = .527), height (p = .443), weight (p = .229), and length of education (p = .063) between the two groups in our study. The FTND score showed that the smokers in our study were moderately nicotine-dependent smokers (FTND score: 4.61 ± 2.59).
| NAs (n = 18) | Nonsmokers (n = 22) | p-Value | |
|---|---|---|---|
| Age (years) | 48.83 ± 6.37 | 47.77 ± 6.39 | .527 |
| Height (cm) | 170.78 ± 4.19 | 171.73 ± 5.83 | .443 |
| Weight (kg) | 69.89 ± 11.83 | 74.27 ± 9.06 | .229 |
| Length of education (years) | 10.89 ± 3.62 | 12.23 ± 4.45 | .063 |
| Initial age of smoking | 19.67 ± 3.43 | — | — |
| Years of nicotine use | 28.78 ± 6.33 | — | — |
| Daily smoking amounts | 18.39 ± 7.68 | — | — |
| FTND score | 4.61 ± 2.59 | — | — |
| RRSQ score | 25.89 ± 14.84 | — | — |
| BIS-11 score | 56.17 ± 7.67 | — | — |
- Abbreviations: BIS-11, Barratt Impulsivity Scale; FTND, Fagerstrom Nicotine Dependence; NAs, nicotine addicts; RRSQ, Russell Reason for Smoking Questionnaire.
3.2 Comparison of metabolite levels between the two groups
Compared with the control group, Asp/Cr (p = .005) and Aspwater-corr (p = .004) levels in mPFC were significantly higher in NAs. There was no statistically significant difference between the two groups in fractions of GM (p = .088), WM (p = .093), and CSF (p = .752) within the MRS acquisition voxel. There was also no statistically significant difference between the two groups in tNAA (p = .365), tCr (p = .265), tCho (p = .673), and mI (p = .327) levels in the mPFC (Table 2).
| NAs (n = 18) | Nonsmokers (n = 22) | p-Value | |
|---|---|---|---|
| Asp/Cr | 0.019 ± 0.004 | 0.015 ± 0.003 | .005 |
| Aspwater-corr (i.u.) | 0.68 ± 0.16 | 0.54 ± 0.12 | .004 |
| FWHMCr (HZ) | 9.728 ± 1.752 | 9.905 ± 1.141 | — |
| Asp Fit Error (%) | 15.839 ± 2.687 | 16.689 ± 4.214 | — |
| FractionGM | 0.489 ± 0.045 | 0.513 ± 0.040 | .088 |
| FractionWM | 0.345 ± 0.036 | 0.325 ± 0.037 | .093 |
| FractionCSF | 0.166 ± 0.029 | 0.162 ± 0.037 | .752 |
| tNAA (mM) | 9.308 ± 2.145 | 8.789 ± 1.344 | .365 |
| tCr (mM) | 7.712 ± 1.142 | 7.316 ± 1.037 | .265 |
| tCho (mM) | 2.421 ± 0.467 | 2.472 ± 0.263 | .673 |
| mI (mM) | 6.641 ± 0.801 | 6.929 ± 0.985 | .327 |
- Abbreviations: Asp, aspartate; i.u., institutional units; FWHM, full width at half maximum; GM, gray matter; WM, white matter; CSF, cerebrospinal fluid; tNAA, total N-acetylaspartate; tCr, total creatine; tCho, total cholinine; mI, myo-Inositol; mPFC, medial-prefrontal cortex; NA, nicotine addict.
3.3 Correlations of Asp levels with daily smoking amounts, years of nicotine use, FTND, RRSQ, and BIS-11 score
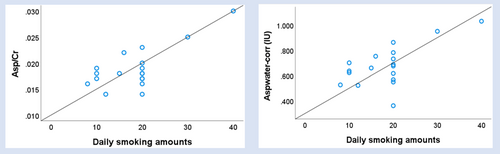
Asp/Cr (r = .730, p < .001) and Aspwater-corr (r = .642, p = .004) were positively associated with daily smoking amounts. However, no significant correlation was found between Asp levels (Asp/Cr and Aspwater-corr) and the years of nicotine use, the FIND score, the BIS-11 score, or the RRSQ score (Table 3 and Figure 3).
| Asp/Cr | Aspwater-corr | |||
|---|---|---|---|---|
| r-Value | p-Value | r-Value | p-Value | |
| FTND score | .416 | .086 | .499 | .076 |
| RRSQ score | .199 | .429 | .213 | .397 |
| BIS-11 score | .126 | .618 | .239 | .339 |
| Daily smoking amounts | .730 | <.001 | .642 | .004 |
| Years of nicotine use | −.174 | .49 | −.172 | .495 |
- Abbreviations: Asp, aspartate; NA, nicotine addict; FTND, Fagerstrom Nicotine Dependence; RRSQ, Russell Reason for Smoking Questionnaire; BIS-11, Barratt Impulsivity Scale.
4 DISCUSSION
In this study, the Asp levels in the mPFC region of NAs were quantified with J-edited 1H-MRS and compared with those of nonsmokers. Our results demonstrated that Asp levels (no matter with reference to the creatine or water signal) in the mPFC of NAs were elevated relative to the control group, and were positively correlated with daily smoking volume. These suggest that Asp metabolism can be changed in the pathogenesis of NAs, and nicotine may be an important factor in the increase of Asp levels.
MRS provides a noninvasive method for measuring brain metabolite levels and their changes. J-edited MRS can be used to separate overlapped signals of metabolites, which allows us to directly and accurately detect some low concentration metabolites with J-coupling networks in specific brain regions (Menshchikov et al., 2017). To date, the MEGA-PRESS have been widely used in the study of brain metabolism in healthy volunteers, psychiatric disorders (e.g., schizophrenia, PD, AD), and various diseases involving the death of neurons (Elmaki et al., 2018; Menschikov et al., 2016; Menshchikov et al., 2017; Menshchikov, Semenova, et al., 2019; Shukla et al., 2021). The previous study has also shown that MEGA-PRESS can be used to measure the Asp level with high accuracy (Menshchikov, Manzhurtsev, et al., 2019). Besides, Gannet is an open-source software based on MATLAB, which is specially developed for editing spectra of metabolites such as GABA and GSH (Edden et al., 2014). In this study, the Gannet tool was further developed for fitting of the Asp signal with reliable quantification results obtained.
Asp is a central regulatory molecule in the glutaminergic system, acting as exciting neuromediator in the CNS (Cavallero et al., 2009). Changes of the Asp level can regulate the metabolic functions of brain and nerves, and further cause behavioral and cognitive changes. Neuropharmacological studies have revealed that d-Asp via interaction with glutamate site on each of the GluN2 subunits, acting as endogenous NMDA receptor agonist (Errico et al., 2011, 2015). A review study showed that the development of behavioral sensitization to psychostimulants, opioids, and nicotine is thought to be mediated by NMDA-mediated neurotransmission, and NMDA antagonists could potentially be helpful as pharmaceutical adjuncts for the therapy of addictions (Bisaga & Popik, 2000). According to research by Jain et al. (2008), modulating glutamatergic neurotransmission with NMDA receptor antagonists could assist with alleviating nicotine withdrawal symptoms and make quitting cigarettes easier. In addition, recent studies have shown that a sustained increase in d-Asp enhances the NMDA receptor-mediated response of dopamine neurons in the substantia nigra in mice (Krashia et al., 2016). Research into the biological mechanism of addictive substances and mental addiction has shown that the dopamine system in the mesencephalic limbic system, also known as the “reward system,” plays a key role in addiction (Volkow et al., 2011). Therefore, changes in Asp levels may affect dopamine metabolism, which not only regulates excitation transmission, consciousness, and emotional processing, but also regulates the neuronal activity of reward circuits associated with addiction.
Based on its pharmacological characteristics, Asp, like glutamate, stimulated nerve cell levels at low doses; at higher doses, these nerve cells can be overstimulated, leading to cell damage or death; and when it triggers a cascade of neurotransmitters, it can cause further damage to the brain. Previous studies have shown that increased Asp levels are associated with structural changes in neuron cells, and excessive Asp levels can induce severe neuroinflammatory processes and cell death (Boccella et al., 2015; Nuzzo, Feligioni, et al., 2019; Punzo et al., 2016). Our study found increased Asp levels in the mPFC of the brain of NAs, which we believe may be associated with the changes in the structure and cognitive functions of the mPFC as well as the addictive behavior (Bagga et al., 2021; Janes et al., 2016). The increased Asp level may be resulted from the nicotine addiction introduced dysfunction of brain metabolic regulation system, reflecting the inconsistence in structure and function of neuronal cells in the prefrontal brain region of NAs. Given that this study is a preliminary cross-sectional study and changes in Asp metabolism in other parts of the brain are not yet known, future longitudinal studies with a large number of participants will be necessary to further elucidate the association between changes in Asp metabolism and addictive behaviors in patients with nicotine tolerance.
Although there are relatively few MRS studies on Asp at present, the role of Asp metabolism in the human brain has been paid more and more attention. A 1H MRS study by Ljungberg et al. found that Asp and Cho in caudate nucleus of untreated children/adolescents with obsessive-compulsive disorder were significantly correlated with CY-BOCS obsessive-compulsive subscore (Ljungberg et al., 2017). Menshchikov et al. used J-edited 1H MRS found that concentrations of NAA and Asp in the frontal lobe of the brain significantly decreased after brain injury; and they believed that the decrease in NAA concentration might be caused by the decrease in Asp concentration (Menshchikov, Semenova, et al., 2019). Our study not only found elevated levels of Asp in the prefrontal lobe of the brain in NAs, but also found that Asp was associated with daily nicotine intake in the correlation analysis. Therefore, we think that nicotine should be clearly correlated with the level of Asp metabolism in the brain, particularly in prefrontal regions implicated in the development and maintenance of addictive disorders. Of course, further studies are needed to obtain more evidence to support and validate the specific role of Asp in nicotine tolerance patients.
In addition, the differences between the NAs and nonsmokers are not caused by one metabolite, but may also be caused by interactions between metabolites (Bagga et al., 2021; Menshchikov, Semenova, et al., 2019). Most previous studies have shown that NAA, Cr, mI, and Glu in the brain of NAs are significantly decreased, while Cho is significantly increased (Durazzo et al., 2016; Gallinat et al., 2007; Mennecke et al., 2014). However, we found no significant differences in the levels of tNAA, tCr, tCho, and mI in the prefrontal lobe of the brain of NAs and nonsmokers, which may be due to the small sample size limited to men and the choice of MRI instruments and analyzing software.
This study has some limitations. First, the subject population was limited to middle aged, healthy male smokers with moderate levels of nicotine addiction, so we cannot popularize this influence to a more general population. Considering that this study is a preliminary study, future surveys with a larger and more diverse samples may clarify these issues. In addition, our study did not analyze other metabolites (such as Glu, GABA, and GSH, etc.) in NAs to elucidate the interactions between metabolites. Finally, longitudinal studies are warranted to further elucidate the link between the development of nicotine-addictive behaviors and Asp metabolic levels changes.
5 CONCLUSION
Our study investigated the influence of nicotine addiction on brain Asp metabolism, and contributes novel information in the imaging study of nicotine addiction. Results indicate that NAs had elevated levels of Asp in the mPFC compared with the control group, and was positively correlated with the amount of daily smoking, suggesting that nicotine has a significant effect on brain metabolism, which may be an important reason for the increase of brain Asp level in NAs.
AUTHOR CONTRIBUTIONS
Miaomiao Yu and Ke Xu designed the experiment. Miaomiao Yu, Ke Xu, Man Xu, Jianxin Ren, Xiaoyu Niu, Xinyu Gao, Mengzhe Zhang, Zhengui Yang, Jinghan Dang, and Qiuying Tao performed the experiment. Miaomiao Yu, Ke Xu, and Liangjie Lin processed and analyzed the data. Miaomiao Yu and Liangjie Lin drafted the article. Shaoqiang Han, Weijian Wang, Jingliang Cheng, and Yong Zhang reviewed the article and made recommendations. All authors contributed to the article and provided approval for the final version of the article.
ACKNOWLEDGMENTS
We thank all participants for investing their time and effort in this study. Also, we thank everybody that helped with recruitment of participants.
FUNDING INFORMATION
This study was supported by the National Natural Science Foundation of China (81601467 and 81871327), the Medical Science and Technology Research Project of Henan Province (201701011), and the Scientific Research and Innovation Team of The First Affiliated Hospital of Zhengzhou University (2023007).
CONFLICT OF INTEREST STATEMENT
The authors declare that the research reported here was conducted in the absence of any commercial or financial relationships that can be construed as potential conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation. The data that support the findings of this study are available from the corresponding author upon reasonable request.




