Genetic and environmental influence on resting state networks in young male and female adults: a cartographer mapping study
Arman P. Kulkarni and Gyujoon Hwang contributed equally to this study.
Abstract
We propose a unique, minimal assumption, approach based on variance analyses (compared with standard approaches) to investigate genetic influence on individual differences on the functional connectivity of the brain using 65 monozygotic and 65 dizygotic healthy young adult twin pairs' low-frequency oscillation resting state functional Magnetic Resonance Imaging (fMRI) data from the Human Connectome Project. Overall, we found high number of genetically-influenced functional (GIF) connections involving posterior to posterior brain regions (occipital/temporal/parietal) implicated in low-level processes such as vision, perception, motion, categorization, dorsal/ventral stream visuospatial, and long-term memory processes, as well as high number across midline brain regions (cingulate) implicated in attentional processes, and emotional responses to pain. We found low number of GIF connections involving anterior to anterior/posterior brain regions (frontofrontal > frontoparietal, frontotemporal, frontooccipital) implicated in high-level processes such as working memory, reasoning, emotional judgment, language, and action planning. We found very low number of GIF connections involving subcortical/noncortical networks such as basal ganglia, thalamus, brainstem, and cerebellum. In terms of sex-specific individual differences, individual differences in males were more genetically influenced while individual differences in females were more environmentally influenced in terms of the interplay of interactions of Task positive networks (brain regions involved in various task-oriented processes and attending to and interacting with environment), extended Default Mode Network (a central brain hub for various processes such as internal monitoring, rumination, and evaluation of self and others), primary sensorimotor systems (vision, audition, somatosensory, and motor systems), and subcortical/noncortical networks. There were >8.5-19.1 times more GIF connections in males than females. These preliminary (young adult cohort-specific) findings suggest that individual differences in the resting state brain may be more genetically influenced in males and more environmentally influenced in females; furthermore, standard approaches may suggest that it is more substantially nonadditive genetics, rather than additive genetics, which contribute to the differences in sex-specific individual differences based on this young adult (male and female) specific cohort. Finally, considering the preliminary cohort-specific results, based on standard approaches, environmental influences on individual differences may be substantially greater than that of genetics, for either sex, frontally and brain-wide. [Correction added on 10 May 2023, after first online publication: added: functional Magnetic Resonance Imaging. Added: individual differences in, twice. Added statement between furthermore … based on standard approaches.]
1 INTRODUCTION
Genetics and environmental factors are two key components in characterizing every individual in terms of a given phenotype. Quantifying these two components meaningfully is of great importance in understanding their influence on healthy individuals as well as in a variety of diseases and disorders. Studies have shown that normal and aberrant brain functional connectivity has been found to have strong genetic dependence and linkage in normal development and aging (Gao et al., 2017; Hoff et al., 2013; Yang et al., 2016) as well as with a variety of disease states and disorders (Glahn et al., 2010; MacNamara et al., 2016). To this end, it has been noted that resting state networks (RSNs) may be endophenotypes (Glahn et al., 2010), and more recently, the advent of Magnetic Resonance (MR) fingerprinting suggests that patterns of static resting state functional connectivity (rsFC) may be used to identify specific individuals (Finn et al., 2015), further giving validation of it as a potential marker as an endophenotype. In order to be classified as an endophenotype, however, a phenotype must satisfy a set of criteria, one of which necessitates heritability (Elliott et al., 2019; Glahn et al., 2010). [Correction added on 13 March 2023, after first online publication: added: static.]
Currently, modern analyses investigating heritability utilize either (1) the ACE/ADE (A = Additive Genetics, D = Nonadditive/Dominant Genetics, C = Shared Environment, E = Unshared Environment) Model (Neale & Cardon, 2013; Zyphur et al., 2013), or (2) Falconer's Formula (Falconer & Mackay, 1996). The ACE/ADE model primarily evaluates narrow-sense heritability due to additive genetics (h2) based on monozygotic (MZ) or dizygotic (DZ) twin differences, in which this heritability is defined as the total variation attributed to additive genetics, albeit the model can also incorporate and delineate dominant (nonadditive) genetics (d2) as well. Falconer's Formula instead evaluates broad-sense heritability, which includes all genetic influences on phenotypic variation (Falconer & Mackay, 1996). Both methods take advantage of the essential assumptions pertaining to MZ and DZ twins. That is, in general, MZ twins are known to uniquely have identical genomes (100% shared genetics) as opposed to DZ twins (on average 50% shared genetics), implying phenotypic variability between these twin zygosity groups may aid in teasing apart genetic and environmental effects (Falconer & Mackay, 1996; Neale & Cardon, 2013). While models such as the ACE model and Falconer's Formula (Falconer & Mackay, 1996; Zyphur et al., 2013) currently exist, there remains room for a more parsimonious method in the interest of characterizing individuals based on rsFC while at the same time evaluating genetic influence of the functional brain.
Furthermore and separately, there have been many studies investigating sex differences in rsFC and the brain in general (Alarcón et al., 2015; Kaczkurkin et al., 2019; Sie et al., 2019; Smith et al., 2019; Teeuw et al., 2019; Q. Wang, Hu, et al., 2021; Zhang et al., 2020), denoting rsFC attributes which may lead to prediction of males as compared with females, or the severity of differences in general between sexes (e.g., strength of functional connectivity). Moreover, it is known that certain brain characteristics, disorders, and health can be influenced by sex (Blokland et al., 2021; McCarthy, 2008; Smith et al., 2019; Q. Wang, Hu, et al., 2021), and it is understood that heritability estimates can differ by sex (Bernabeu et al., 2021). Consequently, investigation may be needed to determine heritability estimates and those related across given phenotypes of interest to tease apart any sex differences. Currently, to our knowledge, there has not been an investigation oriented toward heritability and genetic influence of sex-specific individual differences on the resting state functional brain in young adults, nor at the individual connection level, and how it constitutes region interactions. To this end, the concept of investigating across the brain in terms of what can be referred to as cartographer map (e.g., network-network heatmaps) of genetic and environmental influence, as noted by AdminNeale of OpenMx, was a large emphasis of this study.
Therefore, the purpose of this study was twofold: (1) to develop a novel approach in order to evaluate rsFC phenotypic variance differences between MZ and DZ twin pairs to elucidate (unshared) genetic influence on the low-frequency oscillation (LFO) functional brain with minimal assumptions and compare it to standard approaches in the field and (2) to determine the influence of genetics on sex differences on the functional brain utilizing the aforementioned twin pairs. This is the first study to our knowledge to assess the unshared genetic variance (unnormalized broad-sense heritability) across young healthy adults both in general and specific to sex. In order to assess these two purposes, the Human Connectome Project (HCP) (Smith et al., 2013; Van Essen et al., 2013) dataset was considered. The HCP is known for its high temporal and spatial resolution functional Magnetic Resonance Imaging (fMRI) data, large sample size and a well-characterized high-quality imaging and behavioral dataset inclusive of twins. From this dataset, 65 MZ and 65 DZ young healthy adult twin pairs were leveraged as subjects, each scanned during four sessions over two days. We demonstrate that all resting state connectivity variances of phenotypic differences of those significantly different are smaller for MZ twins as opposed to DZ twins at the population level, and that sex-wise differences in significant connections exist at the sex-specific level using the proposed approach. These results demonstrate crucial findings that verify the overall genetic contributions (those due to unshared genetic effects) that would be expected comparing monozygotic twins and dizygotic twins. Furthermore, we investigate the standard ACE/ADE model and find that these results overall corroborate in global trends, albeit with some deviation and differences in quantity and with specific focus and delineation of nonadditive and additive genetic influence, the findings in the proposed approach. These findings further elucidate which brain regions are most influenced by genetics as opposed to those which may be more influenced by the environment. This outcome encourages further implementation of this novel methodology in more aspects of functional connectivity, genetics, and characterization of individuals, or as a precursor to more complicated analyses such as those aforementioned.
2 MATERIALS AND METHODS
2.1 Demographics
Sixty-five MZ (age = 29.1 ± 3.6 years, 35 female twin pairs) and 65 DZ (age = 29.1 ± 3.6 years, all same-sex twins, 37 female twin pairs) healthy twin pairs (zygosity genotyped [ZygosityGT]) from the HCP were analyzed. Sex was recorded as a self-report measure of sex at birth. Demographics are summarized in Table 1. There were no significant differences in age, sex ratio, education (SSAGA_Educ), average (raw post-ICA-FIX signal) DVARS (average of each scan's DVARS measures independently [four in total]), employment status (SSAGA_Employ), household income (SSAGA_Income) as well as in education score differences (all Wilcoxon Rank Sum Test aside from chi-squared test for sex ratio, and two-sample t-test/Wilcoxon Rank Sum Test for DVARS) between twin pairs across any group (p > .15, MZ versus DZ, Male MZ [MMZ] vs. Male DZ [MDZ], Female MZ [FMZ] vs. Female DZ [FDZ] [Missing one female DZ twin measure [associated pair dropped] for SSAGA_Employ, SSAGA_Educ, and SSAGA_Income]). Education score differences (albeit, SSAGA_Educ is ordinal, and the difference should be viewed with caution in that sense) were computed in the same way as the differences in Section S8, with the absolute value of the within-pair difference for consistency. For variable matching across zygosity (and sex), α = .05, but p < .15 denotes practical significance unrelated to α. (Note: DVARS is a measure of motion, defined as the spatial standard deviation of successive difference images.) Finally, equality of phenotypic variances of these variables which are matched and or lend to similar environments were tested using Levene's test and are reported, for the sake of information, in Appendix Table SVF. [Correction added on 13 March 2023, after first online publication: DVARS note added and table citation updated to SVF.]
| Twin pair | Groups | All | Male | Female |
|---|---|---|---|---|
| MZ | # Pairs (N) | 65 | 30 | 35 |
| Age (years) | 29.1 ± 3.6 | 27.3 ± 3.6 | 30.7 ± 2.8 | |
| Education (years) | 14.9 ± 1.8 | 14.9 ± 1.8 | 15.0 ± 1.8 | |
| Difference in education (years) | 0.9 (0 to 5) | 0.9 (0 to 4) | 0.9 (0 to 5) | |
| Employment status | 1.48 ± 0.8 | 1.55 ± 0.7 | 1.41 ± 0.9 | |
| Income level | 5.4 ± 2.2 | 5.29 ± 2.2 | 5.61 ± 2.1 | |
| Average DVARS | 399.0 ± 47.2 | 423.6 ± 41.5 | 378.0 ± 41.4 | |
| DZ | # Pairs (N) | 65 | 28 | 37 |
| Age (years) | 29.1 ± 3.6 | 26.9 ± 3.3 | 30.7 ± 2.8 | |
| Education (years) | 15.2 ± 1.7 | 15.3 ± 1.6 | 15.2 ± 1.8 | |
| Difference in education (years) | 1.1 (0 to 5) | 1.1 (0 to 5) | 1.0 (0 to 3) | |
| Employment status | 1.53 ± 0.7 | 1.71 ± 0.6 | 1.40 ± 0.8 | |
| Income level | 5.19 ± 2.1 | 4.88 ± 2.1 | 5.42 ± 2.1 | |
| Average DVARS | 396.9 ± 46.6 | 428.3 ± 28.6 | 373.2 ± 43.6 |
2.2 Data collection
Sixty minutes of eyes fixation resting state fMRI (rsfMRI) data which had been acquired over four scanning sessions across two days as part of the HCP and had been preprocessed were used (Glasser et al., 2013). The processed images are publicly available on the ConnectomeDB web database (Hodge et al., 2016). Permission was obtained from the HCP Connectome Coordination Facility to access restricted demographic and behavioral data. MR images were acquired using a 3T Connectome scanner adapted from Siemens Skyra at Washington University, in St. Louis using a 32-channel head coil with simultaneous multi-slice imaging (SMS, 8 bands, 72 slices, TR = 720 ms, TE = 33.1 ms, 2.0 mm isotropic voxels). The preprocessing included the HCP minimal preprocessing pipelines (Glasser et al., 2013) and FMRIB's Independent-component-analysis-based X-noisifier (FIX) correction method (Salimi-Khorshidi et al., 2014). There were no significant differences in relative motion RMS (root mean squared) of the pre-ICA-FIX signal, as well as no significant difference in DVARS between MZ and DZ groups for the ICA-FIX signal (regardless of any significant differences in motion parameter estimates prior to ICA-FIX, as ICA-FIX further corrects for motion) that was used for band-pass filtering (see Section S8, Table S2 details for more information, such as absolute motion). See: https://github.com/cgratton/Neurohackademy_Tutorial/tree/master for code by Dr. Caterina Gratton which was adapted for DVARS. [Correction added on 05 September 2023, after first online publication: added: RMS (root mean squared).]
Additional preprocessing steps performed post-ICA-FIX were (1) conversion from CIFTI to NIFTI space for each individual run, (2) band-pass filtering (0.01–0.1 Hz) (Analysis of Functional NeuroImages [AFNI] version #21.2.03; Cox, 1996) each individual run in NIFTI space, (3) conversion back to CIFTI space, (4) parcellation of each run, (5) demeaning each individual parcellated run, and (6) concatenation of the four parcellated 15-minute scans (2 pairs with opposite phase encoding gradients; all steps performed using wb_command version 1.5 [http://www.humanconnectome.org/software/connectome-workbench] except bandpass filtering). Specifically for parcellation, time series from 360 cortical brain parcels (or areas) defined by HCP's Glasser parcellation (Glasser et al., 2016) and 19 subcortical parcels from FreeSurfer's subcortical segmentation (Fischl et al., 2002) were extracted per subject. Raw pairwise Pearson's correlations were computed from the 379 parcels (or nodes) to generate the connectivity matrices and each rsFC connection was Fisher-Z transformed. Seven female subjects had approximately 90 to <100% complete rsfMRI scans, instead of 100% complete scans. For the HCP twin data used in this study, if there is genotype information available, it is deposited in dbGaP (database of Genotypes and Phenotypes) https://www.ncbi.nlm.nih.gov/gap/. Further information specific to the genotyping aspect of the Human Connectome Project is located at: https://www.humanconnectome.org/study/hcp-young-adult/project-protocol/genotyping
2.3 Proposed Approach (twin analysis)
An algebraic formulation to the approach is as follows for MZ twins, although the same logic applies for DZ. The subsequent formulation is made possible by relying on the assumption that for both zygosities (MZ and DZ), there are similar environments experienced for twin pairs (e.g., such that the magnitude of the unshared environmental (variance) effects are similar across zygosities).
2.4 Network categorization (cartographer mapping)
Network categorization was based on Glasser defined networks (Glasser et al., 2016). The subcortex was further expanded based on the FreeSurfer based parcellation (Desikan et al., 2006) of anatomical regions (e.g., cerebellum, thalamus, caudate, putamen, etc.). The resulting significant network–network connections were binned based on each respective network into a newly defined network connectivity matrix (e.g., also denoted as a cartographer map, using d3.js adapted from: https://gist.github.com/tommaybe/5558084) to demonstrate the network effect of the results. Connectogram visualizations of these results were generated using Circos (Krzywinski et al., 2009). [Correction added on 05 September 2023, after first online publication: added: (cartographer mapping) in heading. Added: (e.g., also denoted as a cartographer map, using d3.js adapted from: https://gist.github.com/tommaybe/5558084).]
2.5 Region validation analysis
We choose to interpret the p-value as an indirect measure of relative density as the permutation test considers the amount of connections it generates for testing equivalent to that found significant (N), thereby correcting for the total relative connection count. This analysis was repeated for the ACE/ADE model influences later discussed. The parcels were additionally binned based on their counts and visualized both in terms of their mapping onto hemispheres using a linear interpolation of the minimum to maximal parcel count per analysis (Pham et al., 2021), as well as the overall region counts onto a colorbar. Specifically, the color denoted by the figures generated using wb_view extensions display the color gradient from the lowest to highest intensity of the given regions of connections found that fell in the given ROIs. That is, after determining N connections to be significant at, for example, the group level, these N connections were counted as if the connection was broken in half (into one of two respective ROIs), and this total count was tabulated. A simple example is if we had two significant connections, one from area A to area B, and one from area A to area C; the highest peak intensity on the brain visualization would be A, as it has two counts when the connections are broken apart, followed by B and C with one count each. The same principle applies to visualization using ACE/ADE model estimates in Supporting Information Section S7 (Figure SA). These counts are linearly interpolated from white to the RGB color selected, so darker colors indicate more connections which had significant variances (MZ < DZ) in the cases of the F-test, and which had more highly influenced estimates (>0.4) in the ACE/ADE model.
2.6 Intraclass correlation validation analysis
To validate our findings, we computed from the significant rsFC measures—which were shown to be significant (and excluding opposite direction connections [3] where FMZ > FDZ) at the whole brain level from the F-test analyses—the average composite rsFC value of those found significant on a subject by subject basis. The intraclass correlation (ICC) (using the r-icc package, with options: model = oneway, type = consistency, unit = average; Wolak, 2015) of this average measure was calculated across MZ twin pairs and DZ twin pairs separately, for a single ICC measure per grouping (95% confidence intervals, α = .05, p-value testing using an F-test, distinct from proposed approach, to determine if the population parameter ICC is such that H0: ICC=0 or HA: ICC>0 for the respective ICCs).
2.7 Comparison with standard ACE/ADE model
The specific implementation for comparison in this study, therefore, was the ACE, ADE and nested models. Specifically, because the ICC validation results (Sections 2.6 and 3.3) demonstrated a considerable gap between that of the MZ and DZ ICC (ICCMZ >> ICCDZ), and in order to have a model-agnostic comparison when it comes to the classical ACE twin design, the ADE model was also implemented (as A, C, and D are known to confound and mask each other in practice, caution was carried in assuming preference of any model). In total, the ACE, AE, CE, ADE, DE, and E models were implemented using the direct variance version of the model in OpenMx version 2.19.5 (Neale et al., 2016; Verhulst et al., 2019) (which provides unbiased parameter estimates) and umx version 4.8.0 (Bates et al., 2019; Figure SF, right panel).
Prior to ACE/ADE model estimation, the necessary assumptions for the classical twin design aforementioned (mean and variance equality in twins) across all Fisher-Z transformed rsFC phenotypes were tested using modified versions of Dr. Hermine Maes' scripts (https://hermine-maes.squarespace.com/#/one/) via both OpenMx and umx, and using the SLSQP optimizer (CSOLNP results in similar ultimate assumption violations). Specifically, for any given rsFC phenotype, a saturated model was fit wherein no constraints are set between twins of a given zygosity. Constrained models based on the aforementioned assumptions (if expected means are equal across twin order, variances equal across twin order, mean and variance equal across zygosity) were then fit (successively building and incorporating constraints of the last), and if the fit for each constrained model was not significantly worse than the saturated model, then the assumptions were not violated (p > .05, α = .05, chi-squared test, for each model fit relative to the saturated model). [Correction added on 05 September 2023, after first online publication: added: Dr.]
For the marginal fraction in which the optimizers could not converge to a successful solution, the p-values from the closest converged results were used. If any assumption was violated, the data for the given rsFC phenotype were excluded. Additionally, multivariate normality testing was additionally performed using a robust generalized multivariate version of the Shapiro–Wilk test (Villasenor Alva & Estrada, 2009) in which bivariate normality was assessed per zygosity (assessed whether p > .05, α = .05, for each zygosity for no serious deviations from bivariate normality).
If any rsFC phenotypes encountered assumption violations, the data were excluded (see Supporting Information material Section S2. Figures S2–S4 for cases where the normality assumption is ignored at the cost of unbiased estimates).
2.8 Validation of DE and F-test inclusions
Given the statistical properties of the F-test, the median-based Levene's test (with Bonferroni correction) was employed with the proposed method to generate network heatmaps for referential and validation purposes (i.e., tabulation of VG estimates). Additionally, given the assumptions and potential implications inherent in including the DE model for the ACE/ADE model portion of the study, the variety of influence estimates were generated when not including the DE model for referential and validation purposes, as well (h2, d2, c2, e2). [Correction added on 13 March 2023, after first online publication: ACE/ADE was replaced with DE.]
3 RESULTS
3.1 Population (group) differences
3.1.1 Proposed Approach
Terminology and definitions
For the rest of this study, we define strictly the following networks: frontoparietal networks/FPNs (dorsolateral prefrontal, orbital and polar frontal cortex, sensorimotor paralobular and mid cingulate, insular and frontal opercular, superior parietal and IPS, inferior parietal, anterior cingulate), frontotemporal networks/FTNs (inferior frontal, posterior opercular, early auditory, auditory association), sensorimotor networks/SMNs (premotor, somatosensory and motor, sensorimotor paralobular and mid cingulate, early auditory, early visual, insular and frontal opercular), visual networks/VNs (primary visual, early visual, dorsal stream, ventral stream, MT+ complex), eDMN (posterior cingulate, inferior parietal, medial temporal, lateral temporal, anterior cingulate and medial prefrontal, temporal–parietal–occipital junction [TPOJ], hippocampus), fronto-occipital networks/FONs (involving VNs and frontal areas), and task positive networks/TPNs (primarily: insular and frontal opercular, premotor, superior parietal and IPS, inferior parietal, auditory association, early auditory, and generally throughout all lobes of the brain).
Similarly, we define strictly the anatomical grouping: occipital lobe (equivalent to visual networks), temporal (lateral, medial, TPOJ, early auditory, auditory association), parietal (superior parietal and IPS, inferior parietal), cingulate (anterior/posterior/midline), frontal (dorsolateral prefrontal, inferior frontal, orbital and polar frontal, medial prefrontal and anterior cingulate, premotor, insular and frontal opercular), operculum (posterior/insular and frontal opercular) and the subcortex (FreeSurfer regions; basal ganglia [caudate, putamen, globus pallidus]).
Any discussion denoting genetically influenced (or genetic effects) or environmentally influenced (or environmental effects) especially in the context of sex-specific comparisons, refer to comparisons of sex-specific individual differences in resting state functional connectivity influenced by genetics (or environment, if that is being discussed). For example, the statement “group A was more influenced by genetics than group B” would be strictly in terms of individual differences (and contingent on and with respect to the specific samples). Likewise, at the group level it would be population-level individual differences. And, for the trait(s) in question, influence is taken to mean that the trait is associated with that respective concept (e.g., for genetically or environmentally influenced, genetic factors are associated or environmental factors are associated, respectively). Dominance/Nonadditive Genetics, D, in the context of the ACE/ADE model findings is representing nonadditive genetics in total (inclusive of dominance and epistasis), and is therefore referred to as nonadditive genetics beyond this point. It should also be kept in mind that the findings are specific to the cohort investigated. Finally, any reference of the F-test beyond this point is in relation to that pertaining to variances and of the proposed approach.
Population results
Between MZ and DZ twin groups, 61,805 subtracted connection distributions were normal. Of those, 2580 out of 71,631 connections showed significant difference in variance (corrected p < .05), and all connections had greater variances in the DZ group. Out of 2580, 1321 were intrahemispheric (704 connections were left-hemispheric, 617 right-hemispheric), 1257 interhemispheric, and 2 bilateral brainstem (Figure 1).
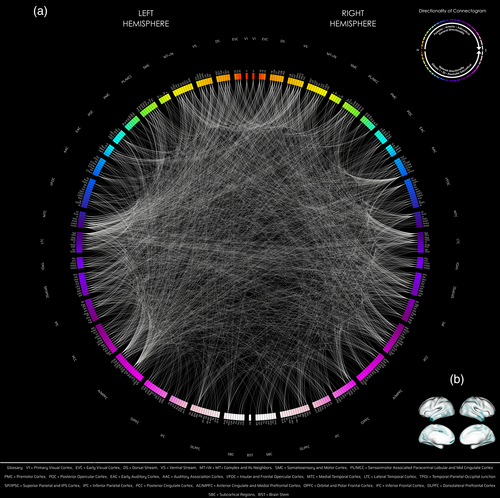
At the population level, there appeared to be a posterior to anterior gradient of more to less genetic influence, both at the parcel- and at the network-level, with visual, temporal, parietal > frontal (Figures 1 and 3, left panel).
There was a high number of genetically-influenced functional (GIF) connections involving posterior to posterior regions of the brain: occipital or VNs, temporal cortices, as well as midline brain cortices (Figures 1 and 3, left panel; Table 2 and Figure S5, left panel).
| Region/network no. (Glasser et al., 2016) | Parcel count | Region/network name | Parcels from significant connections/p-values (permutation) | ||
|---|---|---|---|---|---|
| All | Male | Female | |||
| 1 | 2 | Primary visual cortex (V1) | 45/<0.001* | 12/0.87986 | 4/0.11989 |
| 2 | 6 | Early visual cortex | 121/<0.001* | 49/0.47575 | 2/0.97692† |
| 3 | 12 | Dorsal stream visual cortex | 296/<0.001* | 141/<0.001* | 20/0.01004* |
| 4 | 14 | Ventral stream visual cortex | 343/<0.001* | 91/0.98641† | 21/0.02475* |
| 5 | 18 | MT+ complex and neighboring visual areas | 337/<0.001* | 116/0.99498† | 31/<0.001* |
| 6 | 10 | Somatosensory and motor cortex | 57/>0.999† | 99/0.02182* | 3/0.99592† |
| 7 | 16 | Paracentral lobular and mid cingulate cortex | 285/<0.001* | 188/<0.001* | 9/0.96581 |
| 8 | 14 | Premotor cortex | 82/>0.999† | 79/>0.999† | 5/>0.99715† |
| 9 | 12 | Posterior opercular cortex | 125/>0.999† | 85/0.89722 | 17/0.06324 |
| 10 | 10 | Early auditory cortex | 123/0.8876 | 108/0.00165* | 1/>0.999† |
| 11 | 16 | Auditory association cortex | 249/0.01627* | 114/0.91816 | 11/0.88759 |
| 12 | 26 | Insular and frontal opercular cortex | 205/>0.999† | 165/>0.999† | 9/>0.999† |
| 13 | 14 | Medial temporal cortex | 325/<0.001* | 127/0.09115 | 31/<0.001* |
| 14 | 18 | Lateral temporal cortex | 539/<0.001* | 283/<0.001* | 23/0.08574 |
| 15 | 10 | Temporo-parieto-occipital junction | 151/0.10144 | 49/>0.999† | 8/0.72373 |
| 16 | 20 | Superior parietal cortex | 347/<0.001* | 209/<0.001* | 34/<0.001* |
| 17 | 20 | Inferior parietal cortex | 267/0.63869 | 201/0.00107* | 17/0.69594 |
| 18 | 28 | Posterior cingulate cortex | 354/0.93705 | 175/>0.999† | 24/0.71046 |
| 19 | 30 | Anterior cingulate and medial prefrontal cortex | 426/0.18682 | 280/0.00501* | 41/0.01025* |
| 20 | 22 | Orbital and polar frontal cortex | 161/>0.999† | 104/>0.999† | 21/0.50318 |
| 21 | 16 | Inferior frontal cortex | 72/>0.999† | 72/>0.999† | 5/>0.999† |
| 22 | 26 | Dorsolateral prefrontal cortex | 160/>0.999† | 172/0.9974 † | 11/>0.999† |
| 23 | 2 | Amygdala | 0/>0.999† | 4/>0.999† | 0/>0.999† |
| 24 | 2 | Hippocampus | 43/0.00269* | 2/>0.999† | 3/0.28708 |
| 25 | 2 | Nucleus accumbens | 1/>0.999† | 0/>0.999† | 2/0.56216 |
| 26 | 2 | Caudate | 13/>0.999† | 32/<0.001* | 0/>0.999† |
| 27 | 2 | Globus pallidus | 0/>0.999† | 4/>0.999† | 0/>0.999† |
| 28 | 2 | Putamen | 1/>0.999† | 4/0.999† | 0/0.999† |
| 29 | 2 | Thalamus | 8/>0.999† | 52/<0.001* | 0/>0.999† |
| 30 | 1 | Brain stem | 2/0.999† | 0/0.999† | 1/0.60722 |
| 31 | 2 | Ventral diencephalon | 15/0.99705† | 17/0.44099 | 0/>0.999† |
| 32 | 2 | Cerebellum | 7/>0.999† | 14/0.73822 | 2/0.55978 |
- The (uncorrected, α = .025) p-values were determined from a right-tailed permutation test with 100,000 iterations. *p < .025, which indicates that there were significantly larger number of significant connections found in the region than random. †p > .975, which indicates that there were notably (visually, albeit nonsignificant given the nature of one-tailed/right-tailed test) smaller number of significant connections found in the region than random. Shading of colors respectively is denoted by the following: red denotes p < .025 across all groups (males, females, all); green denotes p > .975 across all groups (males, females, all); and blue denotes sex-specific individual differences where p < .025 for one sex, and p > .975 for the other. [Correction added on 10 May 2023, after first online publication: added: α = .025.]
There was a low number or paucity of GIF connections involving anterior to anterior/posterior regions of the brain: Frontofrontal, FPNs, FTNs, FONs involving dorsolateral prefrontal, orbital and polar frontal, inferior frontal, insular and frontal opercular, posterior opercular, premotor cortices as well as very low number of GIF connections involving subcortical/noncortical regions—hippocampus (also relative density) > basal ganglia, thalamus, nucleus accumbens, amygdala, ventral diencephalon, brainstem, and cerebellum. These findings were seen both at the parcel level as well as at the overall network level (Figures 1 and 3, left panel; Table 2 and Figure S5, left panel).
3.1.2 ACE/ADE model (with DE)
Assumption testing (population)
Between MZ and DZ twin groups (inclusive of males and females), 65,371 connections satisfied the classical twin design assumptions, and 43,476 connections satisfied the normality constraint. Jointly, 39,735 connections remained after exclusion by normality and classical twin design assumptions to investigate the influence from genetics and the environment. The following significant connections based on the four variance components using the ACE/ADE model approach were found of the aforementioned subset.
Additive genetics
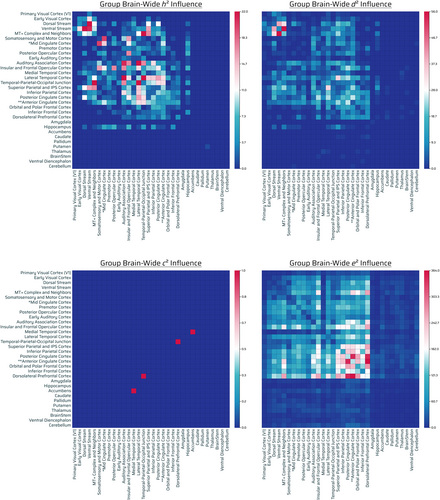
Nine hundred eighty-eight out of 71,631 connections showed significant influence by additive genetics. Out of 988, 489 were intrahemispheric (217 connections were left-hemispheric, 272 right-hemispheric) and 499 interhemispheric (Figure 4, top left panel). Relative contribution of narrow-sense heritability (additive genetics, h2) demonstrated highest additive-GIF connection number across posterior–posterior regions of the brain: temporal cortices, parietal cortices, cingulate, and sensory areas, with a general preference for posterior (parietal/temporal/occipital) > frontal (excluding anterior cingulate and insular and frontal opercular cortex). There was a paucity of frontofrontal, and frontoposterior (frontal to FPN, FTN, FON) (outside of anterior cingulate and insular and frontal opercular cortex) additive-GIF connections in general. The hippocampus, considering its relative capacity for connections, was highly influenced as well. Notably, insular and frontal cortex, an FPN, had high additive genetic number with a variety of sensory and eDMN regions (Figures 4, top left panel and S6, h2).
There was a very low to absent additive genetic count across the rest of the subcortical/noncortical regions (Figures 4, top left panel and S6, h2).
Nonadditive genetics
One thousand five hundred fifty-nine out of 71,631 connections showed significant influence by nonadditive genetic effects. Out of 1559, 810 were intrahemispheric (359 connections were left-hemispheric, 451 right-hemispheric), 747 interhemispheric, and 2 bilateral brainstem (Figure 4, top right panel).
There was highest nonadditive-GIF connection number across posterior–posterior brain regions (visual/parietal/temporal cortices), cingulate, and frontal cortices (insular and frontal opercular cortex).
There was moderate nonadditive-GIF count across the dorsolateral prefrontal cortex. Overall, there was medium count across frontofrontal and a low-medium count across frontal-posterior (frontal with FPNs > frontal with FTNs, FONs). The hippocampus was moderately influenced considering its relative size.
There was a very low nonadditive-GIF connection number across the rest of the subcortical/noncortical regions in general (less so than additive genetic influence, however: see caudate, thalamus, brainstem, ventral diencephalon, amygdala, cerebellum) (Figures 4, top right panel and S6, d2).
Shared environment
Two out of 71,631 connections showed significant influence by shared environmental effects. Out of 2, 1 was intrahemispheric (0 connections were left-hemispheric, 1 right-hemispheric) and 1 interhemispheric (Figure 4, bottom left panel). The relative contribution of shared environmental effects appeared marginal in quantity and had no appreciable pattern (Figure 4, bottom left panel).
Unshared environment
A total of 24,612 out of 71,631 connections showed significant influence by unshared environmental effects. Out of 24,612, 12,145 were intrahemispheric (5707 connections were left-hemispheric, 6438 right-hemispheric), 12,295 interhemispheric, and 172 bilateral brainstem connections (Figure 4, bottom right panel).
There was a general gradient of very high to low number of unshared environmental influence with high to low counts being: frontofrontal, FPNs, FONs, FTNs, and midline eDMN/cingulate > parietal/temporal > visual regions, albeit connections were influenced throughout. There was a substantially high density (normalized) of unshared environmentally influenced noncortical/subcortical regions in the brain with cerebellum, nucleus accumbens, thalamus, putamen and amygdala being most environmentally influenced (Figures 4, bottom right panel, S6, e2 and S9 G).
Overall findings
Overall there were notable additive-GIF connections (988), which were a fraction of that of nonadditive-GIF connections (1599), although overlap is possible. There were nearly negligible effects (2 counts) of the shared environment on connections, and there were substantial connections (24,612) (>9.5× relative to nonadditive or additive genetics together assuming no overlap) influenced by the unshared environment or measurement error. Compared with unshared environmental effects, genetic effects generally highly influenced in number posterior–posterior (parietal/temporal/occipital) regions more than anterior, with the exception of the cingulate regions and insular and frontal opercular cortex; conversely environmental effects generally had the opposite pattern (of frontal > posterior) with substantially greater number of connections throughout the brain. Generally, nonadditive > additive genetic effects, but had relatively similar overall count patterns with deviation (Figure S6, h2, d2; Figure 4, top left and top right panels; and Figure SA, row G). Specific sensory regions and processing were notable for both influences: audition, insular and frontal cortex, and vision. There was moderate genetic influence of the hippocampus compared with the rest of the subcortical structures, whereas the environment influenced the cerebellum, nucleus accumbens, thalamus, putamen and amygdala the most across the subcortex (Figure S9 G).
Comparison with proposed approach
Similar to the approach proposed, at the population level, the ACE/ADE model results corroborated the pattern of findings of a genetic influence gradient present primarily across a temporal/parietal/visual (posterior) > frontal fashion (Figure SA, row G).
Considering both additive and nonadditive genetics, there was in agreement with the method proposed, a high number of GIF connections involving primarily posterior cortices: occipital (dorsal/ventral stream, MT+ complex), parietal, temporal (excluding early auditory) as well as midline brain regions. Medial temporal cortex was more relatively influenced in the proposed approach, however. Additionally, the less influenced regions were similar in that respect as well, with an overall lower number of GIF connections involving frontofrontal, FPNs > FTNs, and FONs (with the exception of the insular and frontal cortex for the ACE/ADE model). Finally, the lowest in count connections were generally found to be subcortical/noncortical, in agreement with the proposed approach findings (however, there were seemingly no nucleus accumbens parcels influenced by genetics in the ACE/ADE approach, which may be due to the types of hypothesis testing chosen). The hippocampus, notably, was more influenced in the ACE/ADE model in general (both additively and nonadditively). [Correction added on 10 May 2023, after first online publication: complex.]
In terms of similarity in influence accounting for the amount of findings (e.g., 2580 in F-test), the proposed approach was most similar to the nonadditive genetic findings (and if not then additionally additive effects) as shown in Figure SA and Table S1 (using 100,000 permutations in the permutation test) across many regions of the brain for those most highly or lowly influenced, albeit with deviation as listed in the table (not highlighted blue—indicates either h2/d2 deviated from F-test in terms of p-values <.025 (significantly high relative density results) or p-values >.975 (visually [nonsignificant] low relative density results) or the F-test was outside these p-value ranges; Table S1). The p-values in general were in a close range (between ACE/ADE estimates and the F-test) for those that did not show significance, with the primary exception of medial temporal cortex and early visual cortex, in which the F-test demonstrated significant influence relative to the ACE/ADE model. The general concentrations across posterior (parietal/temporal (lateral)/visual) were comparable across the approaches (more so for nonadditive genetics than additive genetics, in ACE/ADE, however) (Figure SA, row G).
In comparison to the delineated results determined by separating additive and nonadditive genetics, visual network genetic effects were much more prevalent in general with respect to nonadditive genetic influence. In general, the proposed analysis results as a whole closely corroborated with strictly the nonadditive genetic influence components of the ACE/ADE model, albeit, both ACE/ADE model findings had patterns which overlapped with the distribution of the proposed findings, even if not identically (Figures 3, left panel and 4, top left and top right panels). [Correction added on 05 September 2023, after first online publication: added: panel.]
Any deviations from the two approaches may be due to proportionately more connections possible, the framework itself, or its assumptions. Although in proportion these results may vary across the different approaches, and the ACE/ADE model delineates more specifically, under certain assumptions and methodology that the genetic influence is teased apart as additive or nonadditive genetics, the general finding appears consistent with some deviation. One key point to note is that the comparison with the ACE/ADE model is essentially viewing its results as a binary “cartographer's map,” which is useful in practice and for visually understanding the general distribution of influenced traits in the brain; however, there was a difference in hypothesis testing compared with the approach proposed, and so the connections that are notable may necessarily be different ultimately.
3.2 Qualitative sex-level individual differences
3.2.1 Proposed Approach
Males
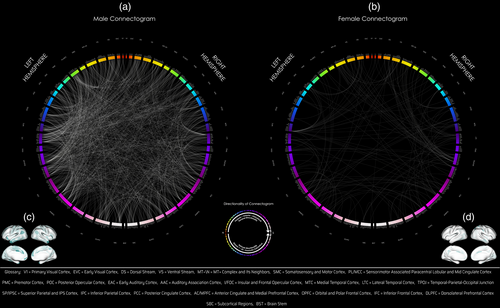
Comparing only male twins (F-test for MMZ versus MDZ), 64,373 subtracted connection distributions were normal. Of those, 1524 connections (794 intrahemispheric [519 left, 275 right], 730 interhemispheric) showed significant differences in variance (corrected p < .05) and all connections had greater variances in the MDZ group (Figure 3, middle panel, Table 2; Figure 2a,c). Two hundred seventy-seven of these 1524 connections were exclusive to the male group (these connections showed the opposite effect in females, i.e., larger variances in the FMZ twin group compared with FDZ). There were extensive number of GIF connections in males involving VNs (dorsal stream), eDMN with other TPNs (occipital, parietal, temporal, cingulate, frontal cortices) which were seen in the network–network and parcel–parcel interactions (Table 2, Figures 2, left panel and 3, middle panel). [Correction added on 05 September 2023, after first online publication: added: panel.]
Females
Comparing only female twins, 62,720 subtracted connection distributions were normal. Of those, 178 connections (102 intrahemispheric [46 left, 56 right], 75 interhemispheric, one from left hemisphere to brainstem [Bilateral BrainStem—Left Nucleus Accumbens]) showed significantly greater variance (corrected p < .05) in the DZ group, and three connections (Right Area V3B-Right Area 9-46d, Right Area V3B-Left Area 9-46d, Right Frontal Opercular Area 4-Right VentroMedial Visual Area 2) showed the opposite effect (Table 2, Figure 2b,d). Ten of these 178 connections were exclusive to the female group (these connections showed the opposite effect in males, i.e., larger variances in MMZ compared with MDZ). The most significant GIF connections and network interactions in females involved VNs (dorsal and ventral stream, MT+ complex), medial temporal (trending toward significance; Table 2), lateral temporal, superior parietal, anterior cingulate, posterior cingulate (high number of connections, although not significant in terms of relative density in Table 2) (Figures 2 and 3, right panel; Table 2).
Males versus females
Specific networks with high GIF connection number in males and low genetic influence in females include sensorimotor systems (somatosensory and motor cortex, paracentral lobule, early auditory cortex, early visual cortex), caudate, and thalamus. Specific networks with high relative density of genetic influence in females and low genetic influence in males include MT+ complex, neighboring visual areas and ventral stream visual cortex (Table 2, Figure 2).
Other cortical regions
Although at the network level (Table 2), frontal regions (excluding anterior cingulate) were not significant for males or females, specific parcels of these networks were significantly involved in males more so than females (Figure 2).
Subcortical/noncortical
Genetics appeared to influence a moderate amount of connections involving cortico-basal-ganglia-thalamic-cortical > ventral diencephalon, cerebellar, amygdala, globus pallidus, and putamen regions predominantly in males (with brainstem and accumbens marginally present for females and not males).
Overall patterns
There were over eight (>8.5) times as many significant connections in males (1524) compared with females (178) (Figures 2 and 3, middle and right panels). There was also a greater genetic influence in terms of intra and internetwork interactions of eDMN and TPNs in males than in females (Figures 2 and 3, middle, right panels). In terms of low-level or primary systems, there was greater genetic influence of involvement of primary sensorimotor networks (visual, auditory, paracentral lobular, somatosensory, and motor networks) in males than females (Figure 3, middle, right panels). Overall, therefore, the TPNs, eDMN, sensorimotor, subcortical/noncortical systems were significantly genetically influenced much more in males than females. See Appendix S1 for the full list of connections that were significant (Bonferroni: resultant p-values multiplied by number of tests) at the parcel level (Table S3a–c).
3.2.2 ACE/ADE model
Assumption testing (male)
Between Male MZ and DZ twin groups, 64,890 connections satisfied the classical twin design assumptions, and 61,059 connections satisfied the normality constraint. Jointly, 55,382 connections remained after exclusion by normality and classical twin design assumptions to investigate the influence from genetics and the environment. The following significant connections based on the four variance components using the ACE/ADE model approach were found of the aforementioned subset.
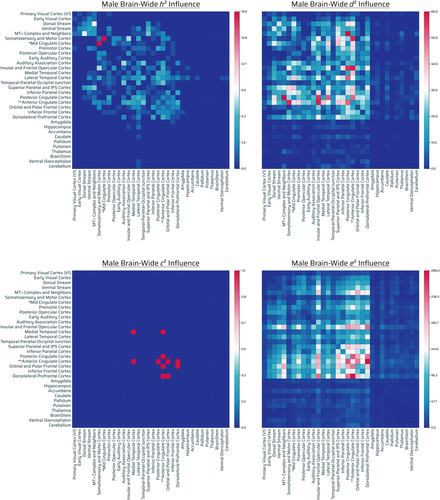
Additive genetics (male)
Three hundred thirty four out of 71,631 connections showed significant influence by additive genetics. Out of 334, 167 were intrahemispheric (78 connections were left-hemispheric, 89 right-hemispheric) and 167 interhemispheric (Figure 5, top left panel). Additive-GIF connections had highest number across cingulate (anterior, posterior, mid [borderline]), sensorimotor cortex (somatosensory and motor cortex), visual networks (MT+ complex and neighbors, ventral stream > dorsal stream), temporal cortices (lateral temporal, temporal–parietal-occipital junction, auditory association cortex), and frontal cortices (insular and frontal opercular, orbital and polar frontal cortices) (Figures 5, top left panel and S7, h2). There was modest/well-tempered interplay of eDMN and TPNs. [Correction added on 13 March 2023, after first online publication: added text: (anterior, posterior, mid [borderline]). Replaced dorsal stream, ventral stream with: ventral stream > dorsal stream.]
There was a very low number of additive-GIF connections across the subcortex: hippocampus, caudate, ventral diencephalon, amygdala, and cerebellum joint with lateral temporal cortex (Figures 5, top left panel and S7, h2). [Correction added on 13 March 2023, after first online publication: thalamus was replaced by ventral diencephalon.]
Nonadditive genetics (male)
A total of 3435 out of 71,631 connections showed significant influence by nonadditive genetic effects. Out of 3435, 1708 were intrahemispheric (975 connections were left-hemispheric, 733 right-hemispheric), 1724 interhemispheric, and 3 bilateral involving the brainstem (Figure 5, top right panel). Nonadditive-GIF connections were highest in number across eDMN with other TPNs (with superior/inferior parietal, dorsolateral prefrontal and insular and frontal opercular cortex as the main networks). Separately and overlapping, basic primary sensorimotor systems were involved (auditory, vision, somatosensory, and motor complex cortices) (Figure 5, top right panel).
There was a low-very low count (and in consideration of their relative connection capacity) of subcortical/noncortical region with the exception of: hippocampus, basal ganglia (caudate, putamen), ventral diencephalon, thalamus, and cerebellum (Figures 5, top right panel and S7, d2).
Shared environment (male)
Six out of 71,631 connections showed significant influence by shared environmental effects. Out of six, two were intrahemispheric (2 connections were left-hemispheric, 0 right-hemispheric) and four interhemispheric (Figure 5, bottom left panel). The relative contribution of shared environmental effects appeared marginal in quantity for males, with no appreciable pattern (Figure 5, bottom left panel).
Unshared environment (male)
A total of 18,105 out of 71,631 connections showed significant influence by unshared environmental effects. Out of 18,105, 8920 were intrahemispheric (4105 connections were left-hemispheric, 4815 right-hemispheric), 9024 interhemispheric, and 161 bilateral brainstem connections (Figure 5, bottom right panel). Environmentally-influenced connections were prominent throughout the brain. In general, there was a gradient of very high to low number of unshared environmental connections of frontofrontal, unrestricted FPNs, FONs, FTNs, and midline eDMN/cingulate > parietal/temporal > posterior/visual regions. Additionally, there was a substantially high density (normalized) of unshared environmentally influenced noncortical/subcortical regions in the brain (Figures 5, bottom right panel and S7, e2). The most prominent environmentally influenced regions were the nucleus accumbens, amygdala, cerebellum, and ventral diencephalon (Figure S9 M).
Overall findings (male)
Overall there were notable additive-GIF connections (334), but demonstrably more (10×) nonadditive-GIF connections (3435), although overlap is possible. There were nearly negligible effects (6 counts) of the shared environment on connections, and there were predominant connections (18,105) (>4.8× relative to nonadditive or additive genetics together assuming no overlap) influenced by the unshared environment or measurement error. Similar to the group (population) level, compared with unshared environmental effects, genetic effects generally influenced posterior regions (parietal/temporal > visual) more than anterior (frontal) (with the exception of midline brain regions, e.g., anterior cingulate and insular and frontal opercular cortex), whereas environmental effects generally had the opposite pattern (of frontal > posterior) when considering amount of connections influenced (but overall still influenced throughout the brain, and especially subcortical regions when considering normalization). Interactions of sensorimotor areas (SMNs/TPNs) were evident for both additive and nonadditive genetics as well (Figures 5, top left and top right panels and SA, row M; Table S1). While the hippocampus, basal ganglia (caudate, putamen), ventral diencephalon, thalamus, and cerebellum were the most genetically influenced of the subcortex, there was overlap in that the nucleus accumbens, amygdala, cerebellum, and ventral diencephalon were the most influenced by the environment (or measurement error). Notably, the delineation between how additive genetics and nonadditive genetics influence the brain did not necessarily show the same overall brain-wide trend (gradient) (Figures 5, top left and top right panels and SA, row M). [Correction added on 05 September 2023, after first online publication: added: panels.]
Comparison with proposed approach (male)
Similar to the method proposed, for males, the ACE/ADE model results in general corroborated the pattern of findings of a genetic influence gradient across parietal/temporal > frontal regions. Specifically, in agreement was high number of GIF connections across midline brain regions (cingulate), parietal, temporal cortices (lateral temporal, medial temporal, auditory association cortex; Figures 5, top left and top right panels, S7, VG, h2, d2, and SA, row M). Broadly this result agrees with the overall influence across eDMN with TPNs in males as in the proposed approach, albeit this finding was far more substantial in nonadditive genetics as opposed to additive genetics (10× connections). [Correction added on 13 March 2023, after first online publication: added: finding.]
The proposed approach had agreement (relative to the maximum of the results found in either approach) with nonadditive genetics, and in this circumstance, nonadditive genetics overshadows additive genetics in amount, perhaps denoting an overall direction of genetic influence (albeit both should be considered, and additive genetics had moderate deviation with the proposed approach across several regions; Figure SA, row M). Sensorimotor areas also had a high number of connections across both approaches (regardless of additive or nonadditive genetics; Figure SA, row M and Table S1). Insular and frontal opercular cortex and dorsolateral prefrontal cortex findings had agreement (more so for nonadditive genetics, particularly again as nonadditive genetics overshadows additive genetics). There was high interactivity of the eDMN with TPNs in general agreeing with the proposed approach (pattern of nonadditive > additive effects).
Hippocampus, caudate, thalamus, ventral diencephalon, and cerebellum (as well as marginally putamen, amygdala, and globus pallidus) were influenced in both approaches (with more substantial influence in nonadditive as opposed to additive genetics). Higher proportionate findings were in the ACE/ADE model framework, in general.
There were substantially more notable connections (again attributing to the cartographer's map concept) in the case of the ACE/ADE model (3435 + 334: ACE/ADE > 1524: proposed). Considering both additive and nonadditive genetics, there was general agreement with the method proposed, although in proportion and in specifics the results may vary.
Assumption testing (female)
Between female MZ and DZ twin groups, 67,186 connections satisfied the classical twin design assumptions, and 48,334 connections satisfied the normality constraint. Jointly, 45,341 connections remained after exclusion by normality and classical twin design assumptions to investigate the influence from genetics and the environment. The following significant connections based on the four variance components using the ACE/ADE model approach were found of the aforementioned subset.
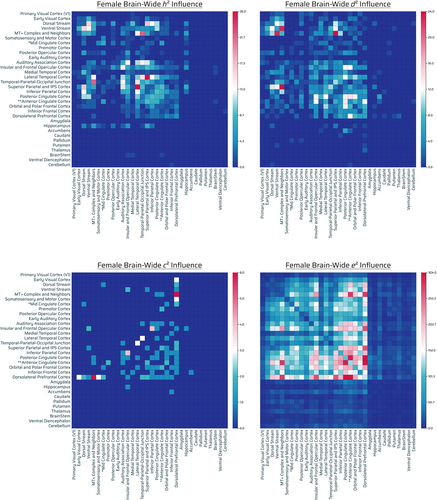
Additive genetics (female)
Six hundred ninety-nine out of 71,631 connections showed significant influence by additive genetics. Out of 699, 379 were intrahemispheric (165 connections were left-hemispheric, 214 right-hemispheric), 317 interhemispheric, and 3 bilateral from the brainstem (Figure 6, top left panel).
The highest additive-GIF connection counts were parietal, temporal cortex (lateral, TPOJ, auditory association cortex), midline eDMN/cingulate (posterior cingulate cortex), VNs (MT+ complex, ventral stream, dorsal stream), and frontal (insular and frontal opercular cortex).
There was a low-moderate number of additive genetic influence across FPNs. There was a very low number across the rest of the subcortical/noncortical regions in general, with the exception of the hippocampus considering its relative connection capacity, and marginally the brainstem and cerebellum (Figures 6, top left panel and S8, h2).
Nonadditive genetics (female)
Six hundred eighty four out of 71,631 connections showed significant influence by nonadditive genetic effects. Out of 684, 376 were intrahemispheric (161 connections were left-hemispheric, 215 right-hemispheric) and 308 interhemispheric (Figure 6, top right panel).
The highest number of nonadditive-GIF connections was across parietal cortex (superior parietal and IPS cortex), midline eDMN/cingulate (posterior/anterior cingulate), visual networks (MT+ complex, ventral stream > dorsal stream), temporal cortex (lateral), and other frontal regions/FPNs (insular and frontal opercular cortex, dorsolateral prefrontal cortex, orbital and polar frontal cortex). There was a moderate number across the nucleus accumbens and hippocampus, considering their connection capacity (Figure 6, top right panel).
There was a low-very low count across subcortical/noncortical regions, however, certain regions did have minimal frequency in the subcortex (e.g., marginally: cerebellum, thalamus, basal ganglia [putamen, globus pallidus, caudate]) (Figures 6, top right panel and S8, d2).
Shared environment (female)
Sixty-six out of 71,631 connections showed significant influence by shared environmental effects. Out of 66, 38 were intrahemispheric (15 connections were left-hemispheric, 23 right-hemispheric) and 28 interhemispheric (Figure 6, bottom left panel). The relative contribution of shared environmental effects was predominantly involving the dorsolateral prefrontal cortex, and with its most notable effects with primarily visual areas (FONs; dorsolateral prefrontal with: early visual cortex, dorsal stream, ventral stream, MT+ Complex) and somatosensory/motor cortex (Figure 6, bottom left panel).
Unshared environment (female)
A total of 26,554 out of 71,631 connections showed significant influence by unshared environmental effects. Out of 26,554, 13,001 were intrahemispheric (6332 connections were left-hemispheric, 6669 right-hemispheric), 13,413 interhemispheric, and 140 bilateral brainstem connections (Figure 6, bottom right panel). Environmentally-influenced connections were prominent throughout the brain. In general, there was a gradient of very high to low number of unshared environmental connections of frontofrontal, unrestricted FPNs, FONs, FTNs, and midline eDMN/cingulate > parietal/temporal > posterior/visual regions. Additionally, there was a substantially high density (normalized) of unshared environmentally influenced noncortical/subcortical regions in the brain (Figures 6, bottom right panel and S8, e2). The most prominent environmentally influenced regions were the nucleus accumbens, amygdala, thalamus, cerebellum, and globus pallidus (Figure S9 F).
Overall findings (female)
Overall, there was comparable additive-GIF connections (699) to that of nonadditive-GIF connections (684), although overlap is possible. There was a moderate (albeit magnitude 10× lesser than either set of genetically-influenced connections) amount of shared environment influenced connections (66), and there were substantial connections (26,554) (>19× relative to nonadditive genetics or additive genetics together assuming no overlap) influenced by the unshared environment or measurement error. There was a genetic influence gradient across parietal, temporal, and visual (posterior) > anterior regions of the brain, with moderately similar but slightly different distributed patterns when it came to delineating between the two genetic effects (Figure SA, row F—relative number, gradient; Table S1—accounting for total connections found, permutation test). Additionally, genetics broadly influenced the eDMN and visual networks in females with certain frontal involvement (dorsolateral prefrontal, orbital/polar, insular/frontal). Relative to genetic influence, shared environmental effects appeared to influence dorsolateral prefrontal cortex (with visual cortices) (FON) and somatosensory/motor cortex, and unshared environment effects (as well as environmental influence in the widest sense) had an influence with the opposite directionality (frontal to posterior) to that of genetics when considering amount of connections influenced. There was substantial presence of environmental influence across the brain, and the subcortical regions after considering the capacity for connections were notably affected: nucleus accumbens, amygdala, thalamus, cerebellum, and globus pallidus.
Comparison with proposed approach (female)
Relative to the method proposed, for females, the ACE/ADE model results corroborated the pattern of findings in that genetics (additive and nonadditive) influenced primarily visual networks (MT+ complex, dorsal/ventral stream), superior parietal and IPS cortex, cingulate (anterior/posterior), and lateral temporal cortex, with additive and nonadditive effects displaying different GIF connection number magnitude depending on the region (Figure S8, VG, h2, d2). Visually, nonadditive genetics as compared with the proposed approach results (Figure 6, top right panel vs. Figure 3, right panel) appeared to potentially have the same highly influenced locations of connections, ignoring the greater eDMN and TPN involvement in females for the ACE/ADE model (at least partially due to higher proportionate findings). [Correction added on 05 September 2023, after first online publication: changed “last” to “right” panel.]
There were substantially more genetically-influenced connections in general (699 + 684: ACE/ADE > 178: proposed), as well as those involved in temporal, frontal, and midline brain regions in the ACE/ADE model compared with the approach proposed (Figure SA, row F).
Lateral temporal cortex also remained in high counts (relatively) regardless of approach and influence, but medial temporal cortex was relatively moderately less influenced in the ACE/ADE model than the proposed approach. Parietal lobe findings across either approach were closely matched (albeit with slight differences based on additive vs. nonadditive genetics). Less influenced primary sensory areas (somatosensory and motor cortex, mid cingulate cortex, premotor cortex, early auditory cortex, auditory association cortex) matched in relative magnitude as well across approaches (Figure S8, h2, d2). However, again, dorsolateral prefrontal cortex was more pronounced in its influence by nonadditive genetics (Figure S8, h2, d2). Taken altogether, any conflicting findings may be due to a difference in hypothesis testing, estimation, or the assumptions of the respective methods. Relative to the proposed approach, there was much greater eDMN with TPN involvement in females in the ACE/ADE approach, especially given the substantial difference in amount of genetically-influenced connections.
Again, divergently, there being many more connections present in the ACE/ADE model could potentially be due to the aforementioned limitations, as well as intrinsic model assumptions.
Qualitative sex-level individual differences, overall findings
Additive genetics (male vs. female)
Cortex: Females had over double the amount of additive genetic influenced connections (699) as compared with males (334). Although connections influenced were distributed throughout the brain in general for each group, additive genetic influence across males was higher (relative to count) across mid cingulate and paracentral lobular cortex, the frontal lobe, and lower than females with respect to eDMN in general whereas for females there was greater focus on namely superior parietal and IPS cortex with notable hippocampal involvement out of all subcortical parcels. The magnitude of these counts in females was stronger than that for males. Additionally, auditory association cortices were similarly influenced (in proportion to other region counts) in females and males (Figures S7 and S8; Table S1). The overall gradient of findings demonstrated overlap in eDMN (e.g., lateral temporal cortex) regions of the brain for males and females, but much stronger concentration for somatosensory areas for males (Figure SA, Additive genetics (h2), row M/F).
Subcortex: Males had additive genetic influence across hippocampus, caudate, ventral diencephalon, amygdala, and cerebellum, whereas females only had influence across the hippocampus, brainstem and cerebellum. [Correction added on 13 March 2023, after first online publication: replaced thalamus with ventral diencephalon. Added hippocampus.]
Nonadditive genetics (male vs. female)
Cortex: There was demonstrably more nonadditive-GIF connections across males (3435) as compared with females (684) (5×). For males more than females, nonadditive genetics was very notable across the eDMN (with eDMN still being influenced in both cases), and with greater visual networks being influenced in the case of females (MT+ complex, ventral stream, V1, early visual; Table S1), albeit, females overall had 5× less connections than males so this finding is relative to within the group. Additionally, somatosensory cortices (somatosensory and motor cortex, paracentral lobular) were much more influenced in males than females (Figure SA Nonadditive genetics (d2), row M/F; Table S1). Nonetheless, given the amount of connections for males present, there was still more notable in number visual network involvement for males. Males in general had greater eDMN with TPN involvement than that of females. There was greater influence of nonadditive genetics in males than in females for parietal and temporal lobes, but both agreed in the paucity of influence in frontal areas of the brain, with the exception of insular/frontal opercular and dorsolateral prefrontal (albeit, females also had high genetic number in orbital and polar frontal; Figures S7 and S8).
Subcortex: Males had influence across primarily hippocampus, basal ganglia (caudate, putamen), ventral diencephalon, thalamus, and cerebellum, whereas females had a moderate number of genetically influenced connections from the nucleus accumbens and hippocampus, and a marginal amount from cerebellum, thalamus, basal ganglia (caudate, putamen, globus pallidus).
Shared environment (male vs. female)
There was marginal shared environmental influences in males (6) as relative to substantially in females (66) (~11×). Given the relative absence of influenced connections, there were no discernable patterns across the brain for males, whereas females had predominant influences across the dorsolateral prefrontal cortex, with the most notable influence involving the interaction of the dorsolateral prefrontal cortex with primarily visual areas (early visual cortex, dorsal stream, ventral stream, MT+ complex) (FON).
Unshared environment (male vs. female)
Relative to the other influences, environmental influences vastly outnumbered the others with >25% of the male resting state brain being uniquely environmentally influenced (18,058 connection counts) but still substantially less (~1.5×) than that of females (>37%, 26,526 connection counts). For both males and females, the same pattern emerged in unique environmental influence of frontal cortices/FPNs (orbital and polar frontal cortices, inferior frontal cortices, dorsolateral prefrontal cortices, insular and frontal opercular cortex) and a broad gradient of influence of high–low across the frontal–posterior regions of the brain (Figures S7, S8, and S9 (M, F)). For highly influenced subcortical regions (when considering the normalized density of connections possible), the findings were as follows.
Subcortex: Males—The most prominent environmentally influenced regions were the nucleus accumbens, amygdala, cerebellum and ventral diencephalon (Figure S9 M).
Females—The most prominent environmentally influenced regions were the nucleus accumbens, amygdala, thalamus, cerebellum and globus pallidus (Figure S9 F). However, in terms of resting state functional connectivity individual differences in females were more environmentally influenced than individual differences in males with respect to the subcortex in general (i.e., higher count of environmentally influenced connections). [Correction added on 10 May 2023, after first online publication: added: individual differences in, twice.]
Qualitative sex-level individual differences, comparison with proposed approach
In comparison to the proposed approach, although there were many more genetically-influenced connections in the ACE/ADE model approach, notably in terms of nonadditive genetics (additive genetics is comparable across males/females and may overlap with nonadditive genetics as well), males had roughly 5× more connections (3435) than females (699), in the same direction but disparate and not proportionate to the >8.5 times in the proposed approach (1524 genetic connections for males, 178 genetic connections for females). In contrast with the proposed approach, auditory systems were relatively equally distributed in genetically-influenced count in females compared with males in the ACE/ADE model approach, but that is not considering the substantial amount of total connections males had with respect to females (for which male nonadditive genetic influence is overall higher in these systems), and visual networks were comparably genetically influenced in males and females (although proportionately different). In agreement with the proposed approach although not to the same scale, there was greater genetic influence in terms of intra and internetwork interactions of eDMN and TPNs for individual differences in males compared with individual differences in females. [Correction added on 05 September 2023, after first online publication: added: individual differences in, twice.]
There was high genetic influence across individual differences in males and low genetic influence across individual differences in females in terms of sensory areas (somatosensory and motor cortex, paracentral lobule, early auditory cortex), caudate (nonadditive genetic influence primarily), and thalamus (nonadditive genetic influence primarily) as in the proposed approach. The same pattern was found in the ACE/ADE approach in that females had high relative density for MT+ complex and ventral stream whereas males did not, at least in terms of nonadditive genetics (which is 10× more than additive genetics for males). For additive genetics, males had partially (visually high [significant, p < .025] or low [nonsignificant, p > .975]) comparable relative density in these regions to that found in the proposed approach (Figures S7 and S8; Table S1). [Correction added on 05 September 2023, after first online publication: added: across individual differences, twice.]
The cortical regions found in the proposed approach for both males and females (dorsolateral prefrontal, inferior frontal, insular/frontal opercular, orbital and polar frontal premotor) were all found to be highly influenced by genetics (additive and nonadditive) as in the ACE/ADE model approach as well, with nonadditive genetics influence highly present on all, but most strongly on dorsolateral prefrontal and insular/frontal opercular. Comparatively, males had a much greater count than females, albeit females had a higher relative count for orbital/polar frontal (Figures S7 and S8).
In agreement with the subcortical findings of the proposed approach, individual differences in males had greater genetic influence across cortico-basal-ganglia-thalamic-cortical regions, ventral diencephalon, cerebellum, amygdala, globus pallidus, and putamen as relative to individual differences in females (typically with respect to nonadditive genetics). Additionally, females appeared to have more nucleus accumbens nonadditive-GIF connections as relative to males, similar to the proposed approach (Figures S7 and S8). [Correction added on 05 September 2023, after first online publication: added: individual differences in, twice.]
Overall, as in the proposed approach, although the proportion and specifics may differ, in terms of rsFC individual differences, individual differences in males were in general more notably genetically influenced in terms of TPNs, eDMN, sensorimotor, and subcortical systems compared with individual differences in females, generally in the context of nonadditive genetics. [Correction added on 05 September 2023, after first online publication: added: individual differences in, twice.]
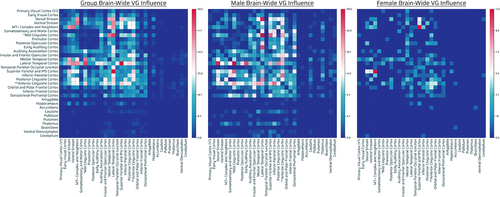
3.3 Network-network (unshared) genetic influence cartographer map summary (of proposed approach)
The breakdown of network–network interactions and the extent of genetically-influenced connections per network–network interaction can be seen in Figure 3. Given the arbitrary nature of normalization methods, the raw data tabulation was picked to illustrate the interaction. Overall, there appears to be a posterior to anterior gradient of more to less genetic influence of network–network interaction with visual, temporal, parietal > frontal (Figure 3, left panel). [Correction added on 05 September 2023, after first online publication: added: panel.]
Males showed a large number of genetically-influenced network–network interactions involving visual, parietal, and temporal regions with a paucity of network–network involvement of frontal regions (Figure 3, middle panel). Individual differences in males overall showed much greater genetically-influenced network–network interactions (with a few exceptions) than females (Figure 3, right panel). There was genetic influence of eDMN involvement in the overall population as well as in males (Table 2, Figure 3). In addition, individual differences in males showed more genetically-influenced network interactions of eDMN with TPNs (occipital, parietal, temporal and frontal cortices), as well as primary sensorimotor systems (vision, audition, and somatosensory and motor systems), subcortical systems, and cerebellum than individual differences in females (Figure 3, middle, right panels). These results were overall convergent with what was noted at the parcel level. [Correction added on 05 September 2023, after first online publication: added: panel, twice. Added: individual differences in, twice. Added: panels.]
3.4 Validation with ICC
The correlations for the average constructed metric for all MZ pairs, regardless of sex, were shown to be higher than that for all DZ pairs (ICCMZ ≤ 0.87, ICCDZ ≤ 0.27), lending validation to our approach (Table 3). Albeit, it should be noted that the MDZ ICC value is negative, implying that the true ICC is low (Bentley et al., 2017; Taylor, 2009).
| Group | ICC | p-value | CI (lower) | CI (upper) |
|---|---|---|---|---|
| MZ | 0.80 | 2.34E−10 | 0.68 | 0.88 |
| DZ | 0.27 | .10 | −0.19 | 0.56 |
| MMZ | 0.87 | 1.64E−07 | 0.73 | 0.94 |
| MDZ | −0.20 | .68 | −1.56 | 0.44 |
| FMZ | 0.86 | 3.37E−08 | 0.73 | 0.93 |
| FDZ | 0.26 | .18 | −0.42 | 0.62 |
3.5 ACE/ADE model results
3.5.1 Population (group) rsFC phenotype model distribution
After excluding connections which violated the classical twin design and normality assumptions (total remaining: 39,735), based on the model offering the smallest AIC at the population (group) level, per connection the best model fit was ACE (60 counts), AE (13,250), CE (4386 counts), ADE (986 counts), DE (18,944 counts), and E (2,109 counts) out of 71,631 connections. As illustrated in Section 3.1.2, the respective counts per variance component influence can be seen graphically in Figure 4.
3.5.2 Male rsFC phenotype model distribution
After excluding connections which violated the classical twin design and normality assumptions (total remaining: 55,382), based on the model offering the smallest AIC at the male level, per connection the best model fit was ACE (76 counts), AE (7786), CE (3245 counts), ADE (5153 counts), DE (29,070 counts), and E (10,052 counts) out of 71,631 connections. As illustrated in Section 3.2.2, the respective counts per variance component influence can be seen graphically in Figure 5.
3.5.3 Female rsFC phenotype model distribution
After excluding connections which violated the classical twin design and normality assumptions (total remaining: 45,341), based on the model offering the smallest AIC at the female level, per connection the best model fit was ACE (488 counts), AE (12,731), CE (11,537 counts), ADE (830 counts), DE (12,601 counts), and E (7,154 counts) out of 71,631 connections. As illustrated in Section 3.2.2, the respective counts per variance component influence can be seen graphically in Figure 6.
3.6 Quantitative sex differences
3.6.1 Proposed approach (unshared genetic effects)
Out of the 1524 connections that were significant for males, and the 178 (MZ variance < DZ variance) that were significant across females, after requiring the joint normality (from the individual male and female analyses) across both of these sets of connections, there were a total of 1467 connections that could be compared between MZ and DZ (and which also overlapped to a degree between males and females in terms of significance). That is, of this initial set, 1299 connections were significant for males, 3 for males and females, and 165 for females.
The result of the FDR (Bonferroni) correction demonstrated that of the significantly influenced genetic effects across males and females, there were 1177 (54) that were rejected. 1140 (53) connections were males > females, and 37 (1) were females > males. However, of the 1140 (53) connections where males > females, 3 (2) were of those in which significantly FMZ < FDZ, and not MMZ < MDZ.
Overall, this corroborated the trend that males had substantially more genetically-influenced connections than that of females (with directionality to males > females and MMZ < MDZ).
3.6.2 ACE/ADE model (additive genetics and nonadditive genetics)
Additive genetics
Out of the 334 connections that were highly influenced for males, and the 699 that were highly influenced across females, after requiring joint normality and valid classical twin design assumptions (from the individual male and female analyses) across both of these sets of connections, there were a total of 807 connections that could be compared between MZ and DZ (and which also overlapped to a degree between males and females in terms of significance). That is, of this initial set, 230 connections were highly influenced for males, 9 for males and females, and 568 for females.
The result of the FDR (Bonferroni) correction demonstrated that of the highly influenced additive genetic effects across males and females, there were 648 (582) that were rejected. 171 (156) connections were males > females, and 477 (426) were females > males.
Overall, this corroborated the trend that females had more additive-genetically-influenced connections than that of males.
Nonadditive genetics
Out of the 3435 connections that were highly influenced for males, and the 684 that were highly influenced across females, after requiring joint normality and valid classical twin design assumptions (from the individual male and female analyses) across both of these sets of connections, there were a total of 2625 connections that could be compared between MZ and DZ (and which also overlapped to a degree between males and females in terms of significance). That is, of this initial set, 2083 connections were highly influenced for males, 44 for males and females, and 498 for females.
The result of the FDR (Bonferroni) correction demonstrated that of the highly influenced nonadditive genetic effects across males and females, there were 2056 (909) that were rejected. 1796 (776) connections were males > females, and 260 (133) were females > males.
Overall, this corroborated the trend that males had substantially more nonadditive genetically-influenced connections than that of females.
3.7 Proposed Approach with F-test validation
In order to validate the results of the F-test paired with the proposed approach, considering the statistical differences and limitations with the F-test even with normality, Levene's test (Brown-Forsythe's test) was used for validation and reference (Brown & Forsythe, 1974). Specifically, both the results generated by Levene's test with the proposed approach unconstrained by normality (as it is less sensitive to normality), and constrained by the same normality test constraints as that with the F-test was considered. Overall, any absence of a finding (and other more nuanced differences) in Levene's test relative to usage of the F-test may be due to the F-test false positive rate, Levene's test false negative rate (reduction of true positives), absence of constraints of normality, or the error rates in general of the study, all also contingent on the data distributions themselves. For further detail on more nuanced differences specific to between Levene's test with and without normality constraints, see Appendix Section S12. For the full set of connections significant (without normality constraints) for Levene's test (again with the p-value being multiplied by the number of tests for Bonferroni correction), see Tables SLa-c in the appendix. [Correction added on 13 March 2023, after first online publication: added: Brown and Forsythe citation. Replaced minimization with reduction. Replaced “or the error rates in general of the study” with “absence of constraints of normality … in the appendix.”]
3.7.1 Levene's test: population (group) level
For results that were not constrained by normality, between MZ and DZ twin groups, 1768 connections were significant out of 71,631 connections, with 906 intrahemispheric (455 left-hemispheric, 451 right-hemispheric), 861 interhemispheric, and one bilateral brainstem connection significant.
For results constrained by normality, there were a total of 1511 connections significant out of 71,631 connections after normality reduction. Out of the 1511 connections, 775 were intrahemispheric (385 left-hemispheric, 390 right-hemispheric), 735 interhemispheric, and one connection was bilateral brainstem.
In terms of the number of connections found in the F-test, there was a substantial drop (2580 to 1511 considering the same normality constraints). This finding may be attributed to the more anticonservative versus more conservative nature of the F-test and Levene's test, respectively.
However, in terms of the pattern of findings of Levene's test in either case relative to that mentioned with the F-test in Section 3.1.1, there (ignoring relative density) were no notable discrepancies in the overall network count trends mentioned previously other than nuances and namely the absence of the pallidum, nucleus accumbens and amygdala in Levene's test (Figures 3 left panel, S6 VG, 7, 9 left panel, SV1 left panel). [Correction added on 04 September 2023, after first online publication: added: (ignoring relative density). Added: nucleus.]
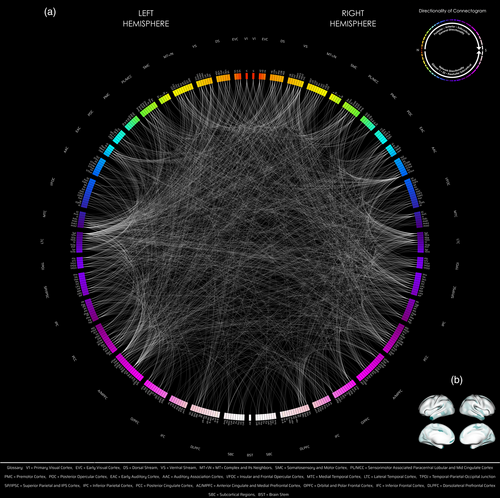
The general regions of high notability were temporal (lateral temporal, medial temporal, TPOJ), cingulate, superior parietal and IPS, early auditory cortex, and visual networks (early visual, dorsal/ventral stream, MT+ complex), with the same overall trend as in the F-test of posterior-posterior (temporal/parietal/visual) > frontal (Figures 9 left panel, SV2 VG). [Correction added on 13 March 2023, after first online publication: added: stream. Added: as in the F-test. Replaced: posterior with posterior-posterior. Added: (Figures 9 left panel, SV2 VG).]
Detailed view
Comparing total trending patterns across Figure S6 (VG—F-test) vs. SV2 (VG—Levene's test without normality), of the more prevalent counts, there were more notable relative concentrations of dorsal stream, MT+ complex, auditory association cortex, and inferior parietal cortex using the F-test than Levene's test (with the full set of connections). Otherwise, the trends were similar (other than magnitude of count which the F-test had considerably more connections).
The absence of findings (e.g., in the subcortex, and other more nuanced differences) in Levene's test may be due to the F-test false positive rate, Levene's test false negative rate (reduction of true positives), absence of constraints of normality for Levene's test, or the error rates in general of the study, all also contingent on the data distributions themselves. [Correction added on 13 March 2023, after first online publication: replaced paragraph with: Detailed view sub-section.]
3.7.2 Levene's test: sex-specific level
Males
For results that were not constrained by normality, between male MZ and male DZ groups, 1760 connections were significant in total out of 71,631 connections. Out of 1760, 868 connections were intrahemispheric (563 left-hemispheric, 305 right-hemispheric), 888 were interhemispheric, and 4 were bilateral brainstem. There were 291 connections exclusive to males (as opposed to females) unconstrained by normality. [Correction added on 13 March 2023, after first online publication: added: There were 291 connections exclusive to males (as opposed to females) unconstrained by normality.]
For the results constrained by normality, there was a total of 1228 connections significant out of 71,631 connections after normality reduction. Out of 1228 connections, 626 were intrahemispheric (410 left-hemispheric, 216 right-hemispheric), 599 interhemispheric, and 3 were bilateral brainstem. There were 196 connections exclusive to males (as opposed to females) under constraints of normality. [Correction added on 13 March 2023, after first online publication: added: There were 196 connections exclusive to males (as opposed to females) under constraints of normality.]
In terms of the number of connections found in the F-test, there was a moderate drop (1524 to 1288 considering same normality constraints). This finding may be attributed to the more anticonservative versus more conservative nature of the F-test and Levene's test, respectively.
In terms of the pattern of findings of Levene's test in either case relative to that mentioned with the F-test in Section 3.2.1, in the previously described network count trends (ignoring relative density), the main difference was an alteration in the notability of the dorsal versus ventral stream (though not remarkably), and more pronounced dorsolateral prefrontal cortex involvement (Figures 3, after 3 middle panel, S7 VG, 8a,c, 9, after 9 middle panel, SV1 middle panel). The areas with notable count were lateral temporal, superior parietal and IPS, cingulate, dorsolateral prefrontal (being a high-counted region for clarity exception relative to the F-test findings), somatosensory and motor cortex, and visual networks (dorsal and ventral stream). Otherwise, for the normality constrained Levene's test, and similarly for the full-set of connections using Levene's test, the trends were present as previously with additional deviation (Figure SV1).
Detailed view
Comparing total trending patterns across Figure S7 (VG—F-test) vs. SV3 (VG—Levene's test without normality, i.e., not constrained to a subset of connections as with the F-test), of the more prevalent counts, there were more notable relative concentrations of dorsal stream, superior parietal and IPS cortex, inferior parietal cortex, and moderately higher concentration (again relative to other counts) for mid cingulate cortex, anterior cingulate cortex, orbital and polar frontal cortex, caudate, thalamus and dorsolateral prefrontal cortex for the F-test than Levene's test; there were more notable instances of early auditory cortex and ventral stream for Levene's test as opposed to the F-test. Otherwise, there were general similarities for the remaining regions. [Correction added on 05 September 2023, after first online publication: deleted: male exclusivity and. Added: 8a,c. Added: The areas with notable … visual networks (dorsal and ventral stream). Replaced last sentence with: Detailed view sub-section.]
Females
For results that were not constrained by normality, between female MZ and DZ groups, 92 connections were significant in total out of 71,631 connections. Out of 92, 58 were intrahemispheric (27 left-hemispheric, 31 right-hemispheric), 34 interhemispheric, and there were no bilateral brainstem connections. [Correction added on 13 March 2023, after first online publication: replaced: male with female.]
For the results constrained by normality, there was a total of 79 connections out of 71,631 after normality reduction. Out of 79 connections, 52 connections were intrahemispheric (24 left-hemispheric, 28 right-hemispheric), 27 interhemispheric, and there were no bilateral brainstem connections. There were 7 connections exclusive to females regardless of normality constraints. [Correction added on 13 March 2023, after first online publication: added: There were 7 connections exclusive to females regardless of normality constraints.]
Given the small amount of connections in general for females, there were marked differences in that shown in Levene's test due to a less discernible pattern in general from females, much of which may be due to the dropping of significant results by about half (Figures 3, before right panel, S8 VG, 8b,d, 9, after 9 right panel, after SV1 right panel). In general, however, the top most notable network counts belonged to: ventral stream, temporal cortices (medial, lateral, both on lower end), superior parietal and IPS cortex, inferior parietal cortex, posterior cingulate cortex, anterior cingulate cortex, MT+ complex, and orbital and polar frontal cortex (Figures SV4 VG, SV6 F), which agreed with the findings for females from the F-test (ignoring relative density).
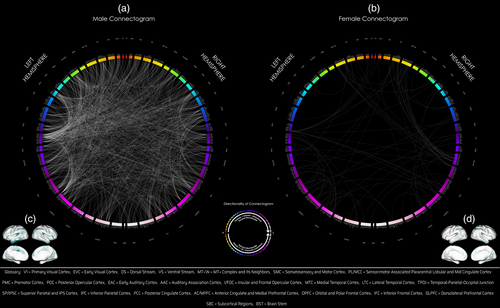
Detailed view
Comparing total trending patterns across Figure S8 (VG—F-test) vs. SV4 (VG—Levene's test without normality), perhaps the most notable deviations from the F-test result were that of the dorsal stream (more prominent using the F-test), ventral stream, inferior parietal, MT+ complex, and orbital polar frontal cortex (less pronounced using the F-test); additionally Levene's test had a loss of one brainstem connection (Figures SV4 VG, SV6 F, 9 right panel). [Correction added on 05 September 2023, after first online publication: added: panel. Added: b, d. Added: panel. Added: panel. Added: MT+ complex. Deleted: female exclusivity and. Replaced last sentence with: Detailed view sub-section.]
Males versus females
Using the more stringent constrained and unconstrained by normality Levene's test, the difference in male and female significantly influenced connections notably increased in amplitude from the F-test result (range: >15.5 [different subsets of possible connections for males vs. females] to >19.1 [no normality, all connections considered]), where the least biased proportion of male to female genetic influence connection count is arguably >19.1×.
The same overall pattern of eDMN and TPN interactions for males being greater (in count) than that for females, along with (albeit with proportionate differences) sensorimotor systems, basal ganglia (excluding globus pallidus), ventral diencephalon, cerebellum, amygdala, thalamus, putamen, and frontal regions being more notable in males as opposed to females is comparable between the F-test and Levene's test. These findings support the validity of the F-test result, with the main exceptions of globus pallidus being absent and a reduction in the presence of connections across the cortico-basal-ganglia-thalamic-cortical loop in Levene's test for males, and brainstem being absent in Levene's test for females (Figures 9 middle, right panels, SV1 middle, right panels).
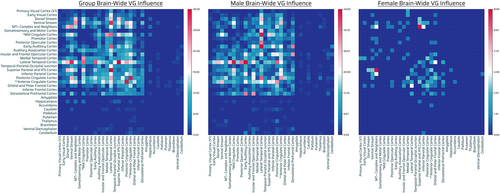
For normalization (similar to relative density) type results, the normalization figures shown in the appendix of Levene's test and F-test differ in respects (Figures S5, SV7, SV12), however.
Again, for any differences, any difference in findings in the results may be due to the F-test false positive rate, Levene's test false negative rate (reduction of true positives), absence of constraints of normality for Levene's test, or the error rates in general of the study, all also contingent on the data distributions themselves. [Correction added on 05 September 2023, after first online publication: added: (in count). Replaced scaling with proportionate. Added: and a reduction in the presence of connections across the cortico-basal-ganglia-thalamic-cortical loop. Added: For normalization (similar to relative density) type results … contingent on the data distributions themselves.]
3.8 ACE/ADE model without DE
- Group: Of 1365 connections which had d2 > 0.4 in the best fit DE model, only 1 connection also had d2 > 0.4 in the ADE model, indicating a possibility of low power, DE marginality limitations, or amalgamation and competing effects of d2 with h2 (and possibly e2).
- Male: Of 1729 connections which had d2 > 0.4 in the best fit DE model, only 2 connections also had d2 > 0.4 in the ADE model, indicating a possibility of low power, DE marginality limitations, or amalgamation and competing effects of d2 with h2 (and possibly e2).
- Female: Of 493 connections which had d2 > 0.4 in the best fit DE model, no connections also had d2 > 0.4 in the ADE model, indicating a possibility of low power, DE marginality limitations, or amalgamation and competing effects of d2 with h2 (and possibly e2).
3.8.1 Population (group) level
Using the same normality constraints as for the ACE/ADE model with DE incorporated, the results for h2 and d2 are as follows. The c2 and e2 estimates were relatively unaffected (c2 not altered in general, e2 higher in count, but overall pattern the same). The model choice estimates based on AIC are as follows: after excluding connections which violated the classical twin design and normality assumptions (total remaining: 39,735), based on the model offering the smallest AIC at the group level, per connection the best model fit was ACE (60 counts), AE (27,248), CE (4386 counts), ADE (5649 counts), and E (2392 counts) out of 71,631. [Correction added on 13 March 2023, after first online publication: replaced “and can be seen in Appendix S12” with: The model choice estimates … out of 71,631.]
Additive genetics
A total of 1405 out of 71,631 connections showed significant influence by additive genetics. Out of 1405, 708 were intrahemispheric (319 connections were left-hemispheric, 389 right-hemispheric) and 697 interhemispheric (Figure 10, top left panel).
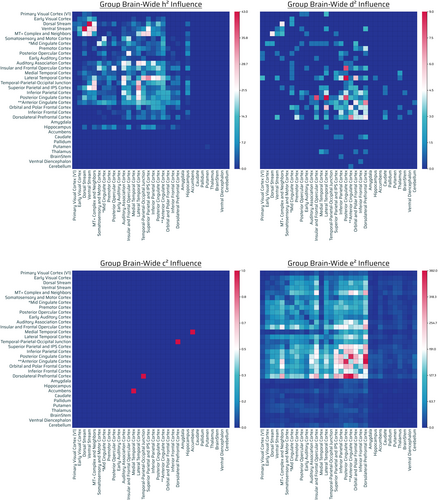
There was a high relative level (count) of influence across visual networks (dorsal stream, ventral stream, MT+ complex), insular and frontal opercular cortex, parietal cortices, cingulate (posterior, anterior), and temporal cortices (lateral temporal, TPOJ, auditory association cortex). In general, in agreement with that with the DE model, there was a pattern of posterior (visual/temporal/occipital) > frontal (excluding anterior cingulate, insular and frontal opercular cortex), with there being a paucity of frontofrontal, frontoposterior connections, in general. [Correction added on 13 March 2023, after first online publication: replaced paragraph with: There was a high relative level … frontoposterior connections in general.]
The hippocampus was highly influenced as well considering its connection capacity. There was a very low to absent presence of influence across subcortical/noncortical regions (highest: hippocampus, very low: putamen, otherwise absent) (Figure 10, SV2 h2), results which in pattern generally agreed with that of the original ACE/ADE model (without DE). [Correction added on 13 March 2023, after first online publication: added: (highest: hippocampus, very low: putamen, otherwise absent).]
Nonadditive genetics
A total of 195 out of 71,631 connections showed significant influence by nonadditive genetics. Out of 195, 95 were intrahemispheric (30 connections were left-hemispheric, 65 right-hemispheric), 99 interhemispheric and 1 bilateral brainstem (Figure 10, top right panel).
Influence count was high across frontal cortices (dorsolateral prefrontal cortex, orbital and polar frontal cortex), cingulate (posterior, anterior), inferior parietal cortex, and lateral temporal cortex. There was medium to high influence count of insular and frontal opercular cortex, and medium influence across visual networks as well as TPOJ. There was low to very low influence count of caudate, thalamus, hippocampus, brainstem, and cerebellum, and absent presence across the rest of the subcortex. There was a considerable drop in number of nonadditive-GIF connections when excluding DE, and there were demonstrable differences (e.g., less noteworthy visual networks) in this circumstance, possibly instead migrating across other influences when DE is excluded (Figures SV2 d2, 10, top right panel).
Shared environment
A total of 2 out of 71,631 connections showed significant influence by shared environmental effects. Out of 2, 1 was intrahemispheric (0 connections were left-hemispheric, 1 right-hemispheric) and 1 was interhemispheric (Figure 10, bottom left panel). The pattern remains similar as that with the DE model (Figure 4, bottom left panel).
Unshared environment
A total of 25,983 out of 71,631 connections showed significant influence by unshared environmental effects. Out of 25,983, 12,841 were intrahemispheric (6059 connections were left-hemispheric, 6782 right-hemispheric), 12,964 interhemispheric and 178 bilateral brainstem (Figure 10, bottom right panel). The pattern remains similar as that with the DE model (Figure 4, bottom right panel).
Detailed view
Comparing total trending patterns across Figure S8 (with DE) vs. SV4 (without DE), in terms of the total effect of h2, there was a general similar trend across either model selection inclusion/exclusion. In terms of the total effect of d2, there were notable differences in the distribution of parcel concentrations; namely, inferior parietal, superior parietal and IPS, insular and frontal opercular, visual networks (ventral/dorsal stream), TPOJ, and subcortical regions were reduced when the DE model was excluded. Nucleus accumbens and inferior frontal cortex were more notable when DE was excluded, albeit with respect to the smaller total max count (and similarly for other frontal regions). In terms of c2 there appeared to be no notable difference (possibly identical), and in terms of e2, there was no considerable difference.
Comparison to proposed approach
The proposed approach findings in terms of visual networks, and in terms of the general pattern (e.g., visual/temporal/parietal > frontal) are in general agreement with the additive genetic influence findings. Nonadditive genetics had a notably different pattern in this circumstance, and most of the effect was found to be in additive genetics. For subcortical findings, additive genetics influenced hippocampus and putamen, while nonadditive genetics influenced cerebellum, brainstem, ventral diencephalon, thalamus, hippocampus, and caudate (again for both no influence across nucleus accumbens was present). [Correction added on 13 March 2023, after first online publication: replaced paragraph with: Influence count was high across frontal cortices … 10, top right panel). And the following sub-sections were included: Shared environment, Unshared environment, Detailed view and Comparison to proposed approach.]
3.8.2 Sex-specific level
Using the same normality constraints as for the ACE/ADE model with the DE incorporated, the results for h2 and d2 are as follows. The c2 and e2 estimates were relatively unaffected (c2 not altered in general, e2 higher in count, but overall pattern was the same).
Male rsFC phenotype model of ACE/ADE (without DE) distribution
After excluding connections which violated the classical twin design and normality assumptions (total remaining: 55,382), based on the model offering the smallest AIC at the group level, per connection the best model fit was ACE (79 counts), AE (26,403), CE (3245 counts), ADE (13,905 counts), and E (11,750 counts) out of 71,631. [Correction added on 13 March 2023, after first online publication: deleted: and can be seen in Appendix Section S12. Deleted last sentence. Added: (c2 not altered in general, e2 higher in count, but overall pattern the same). Also, the following sub-section was included: Male rsFC phenotype model of ACE/ADE (without DE) distribution.]
Additive genetics (male)
A total of 763 out of 71,631 connections showed significant influence by additive genetics. Out of 763, 374 were intrahemispheric (202 connections were left-hemispheric, 172 right-hemispheric) and 389 interhemispheric (Figure 11, top left panel).
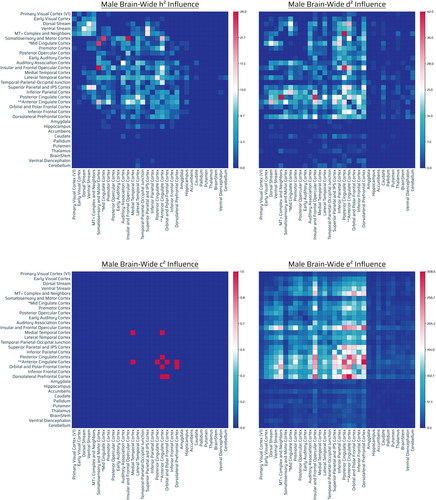
There was high influence count across cingulate (anterior, posterior, mid [borderline]), temporal cortices (lateral temporal, auditory association), superior parietal and IPS cortex, sensorimotor cortex (somatosensory and motor cortex), frontal cortex (insular and frontal opercular cortex), and visual networks (ventral stream > dorsal stream, MT+ complex). There was a medium influence across frontal cortices (excluding anterior cingulate, insular and frontal opercular), TPOJ, inferior parietal cortex, and medial temporal cortex. There was a low presence of connections for hippocampus, amygdala, caudate, ventral diencephalon, and cerebellum joint with lateral temporal cortex; and there was absent influence presence across the rest of the subcortex (Figures SV3 h2, 11, top left panel). [Correction added on 05 September 2023, after first online publication: replaced paragraph with: There was high influence count across cingulate … (Figure SV3 h2, Figure 11, top left panel).]
In comparing Figure 11 top left panel with that of Figure 5 top left panel, there was an enhancing effect (due to additional additive-GIF connections), but a general subset of connections remained the same. The most notable differences were in orbital/polar frontal cortex and TPOJ; otherwise, the pattern, along with well-tempered interplay of eDMN and TPNs, generally agreed with the result of including DE. [Correction added on 13 March 2023, after first online publication: added: top left panel. Added: top left panel. Added: The most notable differences were … result of including DE.]
Nonadditive genetics (male)
A total of 1708 out of 71,631 connections showed significant influence by nonadditive genetics. Out of 1708, 864 were intrahemispheric (469 connections were left-hemispheric, 395 right-hemispheric), 841 interhemispheric and 3 bilateral brainstem (Figure 11, top right panel).
There was a high influence presence across parietal cortex, cingulate (posterior, anterior > mid [borderline]), and frontal (dorsolateral prefrontal cortex, insular, and frontal opercular cortex). Visual networks (dorsal stream, ventral stream, MT+ complex, early visual cortex), sensory areas (premotor, somatosensory and motor, mid cingulate), temporal (medial, lateral), and frontal (orbital and polar frontal, inferior frontal) had a medium connection count. There was a low to very low count across the subcortex, with pallidum, brainstem, nucleus accumbens (absent), and amygdala being the lowest of the counts in agreement with the result with the DE model (Figure SV3 d2, Figure 11, top right panel). In agreement with the result with DE, nonadditive-GIF connections were highest across eDMN with other TPNs as listed (superior/inferior parietal, dorsolateral prefrontal, and insular and opercular cortex as the most notable networks). Also in agreement are the primary sensorimotor systems involved (albeit, there is a reduction in influence count, most notably in terms of vision). [Correction added on 13 March 2023, after first online publication: replaced paragraph with: There was a high influence presence across parietal cortex … most notably in terms of vision).]
In comparing Figure 11 top right panel with that of Figure 5 top right panel, there was an alteration effect (due to removal of substantial nonadditive-GIF connections), but much of the general pattern remained in-tact (with deviation, also as discussed above).
Shared environment (male)
A total of 6 out of 71,631 connections showed significant influence by shared environmental effects. Out of 6, 2 were intrahemispheric (2 connections were left-hemispheric, 0 right-hemispheric) and 4 were interhemispheric (Figure 11, bottom left panel). The pattern remains similar to that with the DE model (Figure 5, bottom left panel).
Unshared environment (male)
A total of 20,853 out of 71,631 connections showed significant influence by unshared environmental effects. Out of 20,853, 10,303, were intrahemispheric (4769 connections were left-hemispheric, 5534 right-hemispheric), 10,365 interhemispheric and 185 bilateral brainstem (Figure 11, bottom right panel). The pattern remains similar to that with the DE model (Figure 5, bottom right panel).
Detailed view (male)
Comparing total trending patterns across Figure S7 (with DE) vs. SV3 (without DE), in terms of the total effect of h2, there was a general similar trend outside of specifically for the more notable concentrations of counts, TPOJ and orbital and polar frontal cortex being higher with DE than without, and insular and frontal opercular cortex, hippocampus, and superior and IPS cortex being higher without DE than with DE; otherwise, in general the patterns were similar for the more pronounced effects. In terms of the total effect of d2, there were notable differences in the distribution of parcel concentrations; namely in SV3 (without DE), dorsolateral prefrontal, orbital and polar frontal, and inferior frontal were notable, and there was a lack of marked concentration of visual networks (dorsal/ventral stream, MT+ complex), somatosensory and motor cortex, mid cingulate, lateral temporal cortex, and TPOJ. There was also a lessening of influence for hippocampus (DE > no DE) and greater effect (relative to count) for caudate (no DE > DE), among other subtle but generally reductive effects (across no DE) in the subcortex. The most notable regions generally remained similar in ranking, regardless of model exclusion, other than TPOJ. In terms of c2 there was identical distribution, and in terms of e2, there was no considerable difference.
Female rsFC phenotype model of ACE/ADE (without DE) distribution
After excluding connections which violated the classical twin design and normality assumptions (total remaining: 45,341), based on the model offering the smallest AIC at the group level, per connection the best model fit was ACE (492 counts), AE (23,020), CE (11,537 counts), ADE (2594 counts), and E (7698 counts) out of 71,631. [Correction added on 05 September 2023, after first online publication: added: top right panel. Added: top right panel. Added at last sentence: “also as discussed above.” Also, the following sub-sections were included: Shared environment (male), Unshared environment (male), Detailed view (male) and Female rsFC phenotype model of ACE/ADE (without DE) distribution.]
Additive genetics (female)
A total of 898 out of 71,631 connections showed significant influence by additive genetics. Out of 898, 488 were intrahemispheric (208 connections were left-hemispheric, 280 right-hemispheric), 407 were interhemispheric and 3 were bilateral brainstem connections (Figure 12, top left panel).
Visual networks (dorsal stream, ventral stream, MT+ complex), temporal (auditory association cortex, lateral temporal, TPOJ), parietal, cingulate (posterior cingulate cortex), and frontal (insular and frontal opercular cortex) had high influence count. There was medium-low count across FPNs (e.g., anterior cingulate, frontal regions), hippocampus, medial temporal, early visual, and somatosensory and motor cortex. There was very low to negligible counts (marginal: brainstem and cerebellum) across the rest of the subcortex (Figures SV4 h2, 12, top left panel). [Correction added on 13 March 2023, after first online publication: replaced paragraph with: Visual networks (dorsal stream, ventral stream, MT+ complex) … (Figures SV4 h2, 12, top left panel).]
In comparing Figure 12 top left panel with that of Figure 6 top left panel, there was general agreement in the pattern displayed (given the same subset of connections retained), and the general pattern listed remained the same. [Correction added on 13 March 2023, after first online publication: added: top left panel. Added: top left panel. Added: and the general pattern listed remained the same.]

Nonadditive genetics (female)
A total of 191 out of 71,631 connections showed significant influence by nonadditive genetics. Out of 191, 104 were intrahemispheric (40 connections were left-hemispheric, 64 right-hemispheric), and 87 were interhemispheric (Figure 12, top right panel).
There was a high influence count across the cingulate (posterior, anterior), frontal (dorsolateral prefrontal and orbital and polar frontal), lateral temporal cortex, and MT+ complex. There was a high-medium influence count across inferior frontal cortex, insular and frontal opercular cortex, and inferior parietal cortex.
There was a medium-very low count across visual networks (excluding MT+ complex), temporal cortices (medial temporal, auditory association > TPOJ), superior parietal and IPS cortex, posterior opercular cortex, early auditory cortex, somatosensory and motor cortex, and the subcortex (nucleus accumbens > hippocampus, basal ganglia [caudate, globus pallidus], and thalamus, the rest absent) (Figures SV4 d2, 12, top right panel).
These findings are in partial agreement (ignoring count, given the substantial decrease between that with DE and without DE) but in general have a large degree of variation from the model with DE incorporated.
Shared environment (female)
A total of 66 out of 71,631 connections showed significant influence by shared environmental effects. Out of 66, 38 were intrahemispheric (15 connections were left-hemispheric, 23 right-hemispheric), and 28 were interhemispheric (Figure 12, bottom left panel). The pattern remains similar to that with the DE model (Figure 6, bottom left panel).
Unshared environment (female)
A total of 27,803 out of 71,631 connections showed significant influence by unshared environmental effects. Out of 27,803, 13,661 were intrahemispheric (6646 connections were left-hemispheric, 7015 right-hemispheric), 13,997 interhemispheric and 145 bilateral brainstem (Figure 12, bottom right panel). The pattern remains similar to that with the DE model (Figure 6, bottom right panel).
Detailed view
Comparing total trending patterns across Figure S8 (with DE) vs. SV4 (without DE), in terms of the total effect of h2, there was a general similar trend across either model selection inclusion/exclusion. In terms of the total effect of d2, there were notable differences in the distribution of parcel concentrations; namely, inferior parietal, superior parietal and IPS, insular and frontal opercular, visual networks (ventral/dorsal stream), TPOJ, and subcortical regions were reduced when the DE model was excluded. Nucleus accumbens and inferior frontal cortex were more notable when DE was excluded, albeit with respect to the smaller total max count (and similarly for other frontal regions). In terms of c2 there appeared to be no notable difference (possibly identical), and in terms of e2, there was no considerable difference. [Correction added on 04 September 2023, after first online publication: replaced last clause with: “(nucleus accumbens > hippocampus, basal ganglia [caudate, globus pallidus], and thalamus, the rest absent) (Figures SV4 d2, 12, top right panel)”. And included: “These findings are in partial agreement … DE incorporated”. Also, the below mentioned sub-sections are included: Shared environment (female), Unshared environment (female), and Detailed view.]
Qualitative sex-level individual differences, without DE
Additive genetics (male vs. female)
Females had more, although not substantially more, connections across additive genetics (female: 898, male: 763). Females had more notable eDMN and visual network involvement across additive genetics relative to males (albeit males had involvement as well), and males had greater additive-GIF connections present across sensorimotor and certain frontal areas than that of females. These trends (including higher superior parietal and IPS cortex involvement and hippocampal involvement for females) are in agreement with the findings with the DE model. Both males and females also had comparable auditory association cortex involvement (Figure SV3 h2 and Figure SV4 h2). In terms of the subcortex, females had hippocampus, brainstem, and cerebellum connections, and males had hippocampus (mainly) and putamen (marginally) connections, which differ from that found while including DE. For further specifics on the differences (relative to max count), see Figures 11 top left panel, SV3 h2, 12 top left panel, and SV4 h2. [Correction added on 13 March 2023, after first online publication: replaced paragraph with: Females had more … top left panel, and SV4 h2.]
Nonadditive genetics (male vs. female)
There were demonstrably more nonadditive-GIF connections across males (1708) as compared with females (191) (>8.9×), and subsequently, greater eDMN, TPN and visual network interactions in males as opposed to females (in partial agreement with the model including DE) (Figure SV3 d2 and Figure SV4 d2), and in general agreement with the proposed approach findings (in terms of count and general comparative pattern). Males had notable influence across the majority of the subcortex and in count (outside of nucleus accumbens) relative to females. Females had influence primarily across nucleus accumbens, hippocampus > thalamus, basal ganglia (globus pallidus, caudate). These subcortical findings are in general agreement (with some exceptions—no putamen for females) with the results with DE. However, unlike that with DE, influence across females generally was less concentrated visually. There was also higher influence (in count) across temporal and parietal cortices in general for males than females, with the main exception (in terms of relative to proportion) of females having greater influence across lateral temporal cortex than males. For further specifics on the differences (relative to max count), see Figures 11 top right panel, SV3 d2, 12 top right panel, and SV4 d2. [Correction added on 13 March 2023, after first online publication: replaced paragraph with: There were demonstrably … top right panel, and SV4 d2.]
Takeaways of ACE/ADE model with and without DE
In comparison to the models with DE, there are a few takeaways. First, one potential speculative argument against the idea that the DE model should not be selected due to the principle of marginality is that, while it is true the D term represents interaction effects in theory, in the means it is actually being calculated, the model itself is possibly agnostic to that information (that domain knowledge), and the principle of marginality may not apply. Additionally, the D term is often underestimated and A overestimated when parameters are dropped from the model (Ozaki et al., 2011). And, the case with the DE model will result in a more unlikely outcome, but which may be more all-encompassing and agnostic (less presumptive) of possibilities, as opposed to this corresponding result without the DE model, which is conservative in general (but present with less potential statistical limitations). In either case, what remains true for both circumstances (with and without DE) is that there is a substantial difference between males and females in terms of d2 influence (>8.9× in this instance, >5× otherwise, albeit limited by normality constraints and different subsets of connections between males and females). What differs between ACE/ADE without DE versus with DE is an increase in overall attribution to h2 and e2 for all groups moderately to substantially, and an overall decrease substantially of d2, but still highly notable across males. The results for h2 are generally comparable regardless of group with that without the DE model (with some deviation); however, for d2, the pattern that remains most intact and in proportion is that for males, and not the other groups. Additionally, considering the alteration of nonadditive genetics and additive genetics, instead of a 10× difference for males (nonadditive: additive), the proportion drops to 2.2×. Similarly, for females, the proportion of additive:nonadditive genetics increases to 4.8× from approximately an even distribution (1:1). For normalization (similar to relative density) type results, the normalization figures shown in the appendix of DE versus no DE show differences in that respect (Figures S13-15, SV13-15), which was not a primary focus of this study, however. [Correction added on 05 September 2023, after first online publication: replaced paragraph with: In comparison to the models … was not a primary focus of this study, however.]
4 DISCUSSION
4.1 Novelty of method
The proposed approach models the variance of the difference in phenotypes in pair-wise connectivity similarity of MZ and DZ twins separately, and then compares these two variations with one another. Again, phenotypic variance is known to be composed of VP = VG + VE,total (excluding/not considering genotype by environment covariance and genotype by environment interaction) (Falconer & Mackay, 1996) where VG is the variance due to genetic sources and VE,total is the variance due to environmental sources. Given that MZ twins have identical genomes, and DZ twins have half identical genomes on average, we presuppose that the influence of environmental effects is similar among monozygotic and dizygotic twins. This approach provides insight into the likelihood of genetic influence on the phenotypic variance as outlined in Section 2.3. Overall, although it does not further delineate sources of variation as does Falconer's Formula or the ACE/ADE model, it avoids the assumptions and issues that may accompany these approaches. For example, Falconer's Formula allows for heritability estimates greater than 1 or less than 0, causing interpretability issues (Corruccini et al., 1990). Further discussion is presented in Section 4.3. [Correction added on 05 September 2023, after first online publication: added: /not considering.]
4.2 Overall findings
Our study aimed to investigate the genetic and sex influence of individual rsFC connections based on the Glasser and FreeSurfer subcortical parcellation mapping. This is the first study to our knowledge that specifically investigates rsFC unshared genetic variance and heritability in healthy young adults with regard to both males and females. Furthermore, this is the first study to our knowledge which incorporates the D component of the ACE/ADE framework across resting state functional connectivity. We discuss these main findings at the population level and sex-specific level via the proposed approach as well as via the ACE/ADE model comparison for elucidation of the unique aspects of the approach proposed. [Correction added on 05 September 2023, after first online publication: added: unshared genetic variance and
4.2.1 Population level
At the population level, there was a high number of GIF connections involving posterior to posterior regions of the brain: VNs (primary visual cortex, early visual cortex, dorsal stream and ventral stream visual cortices, MT+ complex), parietal, and temporal regions; these regions are implicated for example in low-level visual, dorsal (“where”), ventral (“what”) stream visuospatial processing (VNs) or “what” and “where” pathway, categorization, auditory processing, and long term memory processes, as well as midline brain regions (the cingulate, implicated in attentional processes, emotional responses to pain).
There was also a paucity or low number of GIF connections involving anterior to anterior/posterior regions of the brain: frontofrontal, frontoparietal networks (FPNs), frontotemporal networks (FTNs) and frontooccipital networks (FONs)—dorsolateral prefrontal, insular and frontal opercular, orbital and polar frontal, inferior frontal, posterior opercular, and premotor cortices. These anterior regions are implicated for example in high-level cognitive and affective processes such as working memory, executive function, reasoning, attentional and impulse control, emotional judgment, and decision making (FPNs) (Bor et al., 2003; Duncan, 2001; Duncan et al., 2012; Prabhakaran et al., 1997; Prabhakaran et al., 2000; Prabhakaran et al., 2001), language processes (FTNs, FONs) (Blau et al., 2010; Friederici, 2009; Hernandez, 2009; Hernandez et al., 2001; Hodges et al., 1992; Hommel et al., 2001; Matsumoto et al., 2004; Morillon et al., 2010; Spitsyna et al., 2006; Van Atteveldt et al., 2004), and action-planning and movement processes (SMNs) (Andersen & Cui, 2009; Grafton & Hamilton, 2007; Janes et al., 2012; Johnson-Frey et al., 2005; Miller & Cohen, 2001; Penfield & Boldrey, 1937).
This result was corroborated by our network analyses which showed a gradient of higher to lesser genetic influence of network–network interaction from posterior to anterior regions, respectively: visual, temporal, parietal > frontal networks. The result was further corroborated and demonstrated similar, albeit proportionately different findings, with regard to the ACE/ADE model approach, with further delineation that nonadditive genetics and additive genetics influence was present with different intensity contingent on each of these areas, with the inherent assumptions in the respective models. However, there was a distinction in that, the FPN/TPN, insular and frontal opercular cortex, had greater influence of additive/nonadditive genetics in general, relative to the proposed approach findings (where it was only moderately notable except for males where it was highly notable in count)—this occurrence repeated several times; this finding may be due to just a higher proportion of these networks findings, due to differences in hypothesis testing, or due to the native assumptions and methodology specific to the ACE/ADE framework. However, interestingly, insular and frontal opercular cortex is known to be implicated in considerable multisensory integration and is involved in the salience network which aids in performing dynamic task switching between executive control network and the default mode network (external to internal attention) as well as decision-making, bodily/self-awareness, and empathy (Gogolla, 2017). Furthermore, the insular and frontal opercular cortex it is implicated in emotion control and reinforcement learning, in general (Gogolla, 2017). Relatedly, the superior parietal cortex is also an additional multisensory integration site, which was found notable in our analyses (regardless of the approach), which is part of a network involved in cognitive functions, namely working-memory, spatial attention, and decision-making (Katsuki & Constantinidis, 2012).
We found a very low number of GIF connections involving subcortical/noncortical networks such as basal ganglia, thalamus, nucleus accumbens (involved in processing speed, motor control and learning, reward and reinforcement, habit formation) and cerebellar regions (involved in motor learning, coordinating movements, balance, gait, and posture), amygdala (involved in emotion), ventral diencephalon (involved in autonomic control), and brainstem (cardiorespiratory control, automatic survival reflexes—“fight” or “flight” response). In terms of the ACE/ADE results, these results were corroborated in terms of nonadditive genetics, outside of the putamen (where additive genetics was also present), but with the deviation that there were seemingly no parcels found influenced in terms of the nucleus accumbens. Furthermore, if the DE model is excluded, only nonadditive genetics explains these findings, with the absence of the amygdala, nucleus accumbens, and basal ganglia components outside of caudate.
There was a high number of GIF connections involving the eDMN (Amft et al., 2015). The eDMN is involved in cognitive and affective processes such as those involved in episodic memory retrieval, mental imagery, introspection, rumination, and evaluation of self and others (Amft et al., 2015; Göttlich et al., 2017). These findings were comparable with the ACE/ADE model framework in proportion, with the delineation that nonadditive genetics had greater influence than additive genetics on these regions in general (albeit, both had influence), and that the genetic influence gradient was similar. Specifically, under the ACE/ADE model framework, it is both single allelic additive effects genetically, but also its dominance deviation from the genotype (i.e., genetics which selection tends toward and which is not explained by additive/linear allelic effects alone and instead represents nonlinear allelic interactions at a given locus) and potentially genetic effects due to allelic interactions across loci (epistasis) that more substantially influence the eDMN as well as the VNs previously mentioned. This finding is notable because of the different network processes which may be influenced by genetics in young adults.
These differences in genetic influence on posterior (more) versus anterior (less) brain regions may have implications in terms of the environmental influence (e.g., education, school and work environment, family and home environment, social interaction with friends and peers, medications, nutrition, sports, and physical exercise) on posterior (less) versus anterior (more) portions of the brain during development and later in life. This finding was corroborated by the unshared environmental influences in the ACE/ADE model, in which anterior (frontal parietal networks) regions were substantially more prevalent, albeit, with predominant environmental influence across almost all of the brain (>30% of connections possible), demonstrating that it may be that environmental influence has an undeniable in magnitude role in influencing individual differences in the resting state brain not only frontally (in terms of personality, etc.) but also brain-wide in general.
Other regions that were significantly genetically (relative density limited to the F-test) influenced at the network level for the population included the hippocampus which has been implicated in long term memory processes (Squire et al., 2004). These findings (in count and relative density) were also evident in the ACE/ADE model approach, particularly with the hippocampus interactions being comparably influenced by both additive and nonadditive genetics (if DE included, otherwise, only additive genetics was present and relative density was not investigated); additionally, the hippocampus interactions with other regions were influenced substantially by the environment as well, suggesting both environmental influence and genetic influence effects on these interactions. [Correction added on 13 March 2023, after first online publication: added: limited to the F-test. Added: genetics was present and relative density was not investigated.]
It is important to note again that the ACE/ADE model provides essentially a binary “cartographer's map” (as does the proposed approach with different restrictions) with the chosen threshold of significantly influenced connections, which is useful in practice and for visually understanding the general distribution of influenced connections in the brain; however, there was a difference in hypothesis testing performed and assumptions, compared with the approach proposed, and so the connections that are notable may necessarily be different ultimately in quantity.
4.2.2 Sex-specific level
The results found at the population level extend to males and females in differing ways.
Overall patterns
There were over 8.5-19.1 (validated with Levene's test to be in the range of >15-19) times more significant functional connections that were genetically influenced in males (1528) than females (178). There were also greater genetically-influenced network–network interactions noted in males than females. These patterns suggest males may be more functionally influenced by genetics than females in terms of sex-specific individual differences (of the resting state brain). With respect to the ACE/ADE model findings, although proportionately the connections differed, as was noted in terms of nonadditive genetics (as additive genetics was compared to nonadditive genetics, relatively smaller in difference across males and females), males had greater than five times more highly influenced connections (3435 with DE, 1708 without) than females (684 with DE, 191 without) (relative to 2× more females than males for additive genetics specifically). This proportionate finding (>5-8.9×) was comparable to the approach proposed (>8.5-19.1×), and also may suggest greater influence of genetics in males as opposed to females in terms of sex-specific individual differences. [Correction added on 10 May 2023, after first online publication: influence.]
Cortical regions
As noted at the population level, individual differences in primarily males were under extensive genetic influence in terms of network interactions involving visual, parietal, temporal cortices, as evidenced by the findings of either approach, as both the ACE/ADE model and the proposed approach agreed, with the delineation that both males and females had additive genetic influence in these visual, parietal, temporal network interactions; but males had even greater nonadditive genetic influence (in count) and in proportion relative to that of females in these networks. In addition, individual differences in males were more genetically-influenced in terms of network interactions involving auditory-language related cortices compared with individual differences in females, as evidenced by both the proposed approach findings, and the ACE/ADE model findings across nonadditive genetics but not additive genetics in these areas (in terms of count, females had more additive genetic influence in auditory areas, and relative density significance (limited to the F-test) but nonadditive genetic effects were much higher (in count) than additive genetic effects in males, and higher overall (in count) than additive and nonadditive genetic effects in females). This ACE/ADE finding remained true when excluding the DE model for females (in terms of count), but had comparable nonadditive and additive effects in males. This finding suggests that in terms of individual differences in resting state functional connectivity, males may be more influenced by genetics and that females may be more environmentally influenced in terms of auditory–language systems than males. This outcome may explain some of the differences in auditory–language system functions reported between males and females (Clements et al., 2006). [Correction added on 05 September 2023, after first online publication: added: individual differences in, three times. Added: in count, twice. Added: in terms of count. Added: (limited to the F-test).]
As noted at the population level, individual differences in primarily males (and relatively marginally females) were under extensive genetic influence in terms of interactions involving the eDMN which is considered a central hub of the brain for various low-level cognitive and affective processes such as internal monitoring, rumination, and evaluation of self and others, as noted previously (Ames et al., 2008; Buckner et al., 2008; De Brigard et al., 2015; Denny et al., 2012; Hagmann et al., 2008; Jenkins et al., 2008; Leech & Sharp, 2014; Lois & Wessa, 2016; Mitchell et al., 2005; Nejad et al., 2013; Posner et al., 2013; Raposo et al., 2011). In addition, individual differences in males also were more genetically influenced (and teased apart by the ACE/ADE model as primarily nonadditive genetic influence, albeit additive genetics was present) compared with individual differences in females in terms of intranetwork and internetwork interactions between eDMN and other brain regions (occipital, temporal, parietal, and frontal regions) involved in various task-oriented processes and attending to and interacting with the environment which comprise part of the Task Positive Networks (TPNs) (Adolphs, 2006; De Brigard et al., 2015; Denny et al., 2012; Jenkins et al., 2008; Mahy et al., 2014; Raposo et al., 2011; Saxe, 2006; Schurz & Perner, 2015). [Correction added on 05 September 2023, after first online publication: removed: in terms of individual differences. Added: individual differences in, three times.]
Sensorimotor regions
In addition, individual differences in males were more genetically-influenced in terms of network interactions involving primary sensorimotor cortices (audition, vision, somatosensory and motor cortices) compared with individual differences in females, as evidenced by both the proposed approach findings, and the ACE/ADE with DE model findings across both additive but more substantially nonadditive genetics for males; for females, nonadditive and additive contributions were generally similar outside of audition in these areas. If the DE model is not considered, then there is a similar pattern for males, but for females, additive effects are more explanatory than nonadditive effects, in general. [Correction added on 10 May 2023, after first online publication: individual differences in, twice.]
Subcortical/noncortical regions
Thalamus and basal ganglia (caudate) regions demonstrated a significant number of connections (and notable influence of primarily nonadditive genetic connections in the ACE/ADE approach) with each other as well as cortical regions in males which may be due to the cortico-basal-ganglia-thalamic-cortical feedback loops needed to maintain or sustain activity in these lateral cortical regions involved in various high-level and low-level cognitive processes (Alexander, 1986). Ventral diencephalon showed significant connections (and which were influenced by nonadditive genetics in ACE/ADE) in males which control autonomic functions. Cortico-cerebellar involvement was noted more in males (and which was influenced by nonadditive genetics in ACE/ADE) than females which are involved in processes such as providing error correction and feedback to cortical processes (Habas et al., 2009).
There were also over 8.5-19.1 times more genetically influenced functional connections for males (1524 proposed method, 3435 nonadditive genetic connections if including DE, 1708 if excluding, in ACE/ADE) than for females (178 proposed method, 684 nonadditive genetic connections if including DE, 191 if excluding, in ACE/ADE, a 5-8.9× difference in ACE/ADE) suggesting that individual differences in male resting state brains may be more genetically influenced than that of females replace with. This result may suggest individual differences due to genetics in males (more) versus females (less) in terms of interplay of attending to task-oriented interactions with the environment (TPNs) versus internal and external interactions with self and others (eDMN), SMN, and subcortical/noncortical regions. Overall, these findings suggest that for the young adult cohort investigated, individual differences in males may be more functionally influenced by genetics and that individual differences in females may be more environmentally-influenced in terms of TPN, eDMN, sensorimotor, subcortical networks, and cerebellum. This outcome may explain some of the differences in functions reported between males and females (Andreano et al., 2013; Clements et al., 2006; Jäncke, 2018). [Correction added on 05 May 2023, after first online publication: Added: individual differences in, three times.]
4.3 Uniqueness of the proposed approach based on ACE/ADE model findings
In this study we have proposed an alternative framework which is supported and relies on general quantitative genetics theory regarding the genetic dissimilarity across monozygotic and dizygotic twins. In doing so, we investigated what is another means of assessing what is cited by Falconer as the MZ and DZ “within pairs of twins” variance components (Falconer & Mackay, 1996), and in essence compared across these components using a variance test based on the within-pair differences of rsFC phenotypes of the two zygosities. In performing the analysis as such, we restrict our assumptions minimally, which provides strictly the (unshared) genetic influence binary indication, or in the lower bound case, a binary indication of the unnormalized broad-sense heritability (and the upper bound, the unnormalized narrow-sense heritability), across a phenotypic trait, based on the difference in MZ and DZ unshared phenotypic variances. Therein lies the limitation and yet the parsimony of the approach, as we do not make further assumptions pertaining to broad genetic influence.
Furthermore, as we use the F-test for equality of two variances (in this study) which is highly susceptible to normal distribution deviations, because of the manner of subtraction of the approach creating in a sense an artificial symmetric and possibly normal distribution (based on subtracted Fisher-Z transformed data and normality testing), the assumption of normality can be supported in this approach. Additionally, normality is dependent on the subtracted distributions, and not the independent raw data; this fact is in contrast to normal ACE/ADE, which expects the raw data to be bivariate normal distributed to avoid unbiased estimate coverage. Furthermore, comparison of the proposed approach with Levene's test also corroborated the general pattern of results of the proposed approach with the F-test, with deviation due to the inherent differences in statistical properties. Moreover, as is similar to Falconer's Formula, our approach allows the variance components to differ, and specifically relies on this fact to tease apart the genetic influence across monozygotic and dizygotic twins. This fact is in sharp contrast to the ACE/ADE model, which specifically assumes that the variance components (and means) across the given twin zygosities are fixed or the same and decomposes the variance subsequently based on structural equation modeling. Again, the ACE/ADE model assumes that the phenotype is expected to be bivariate normally distributed. Any violations of these assumptions may lead to biased estimates and coverage (Arbet et al., 2020).
Therefore, the two approaches are fundamentally tackling the same problem but in a different manner, the approach proposed by having the variances differ, and the ACE/ADE model having the variances fixed. The proposed approach hones in on the notion of unshared genetic influence, as opposed to explicitly normalized heritability. Additionally, the ACE/ADE model, while providing the additional advantage in delineating further components of interest (A, C, D, E), is confounded in the assumptions made by typical biological literature, in that, if (a) ICCMZ < 2 ICCDZ, then D (nonadditive genetics) is present, and vice versa (b) if ICCMZ > 2 ICCDZ, then shared environmental effects is present. This rule of thumb is problematic, because as noted in (Ozaki et al., 2011), these merely represent that (a) the D (nonadditive genetics) component, d2 < 2c2, or (b) that, d2 > 2c2, not that any particular effect is not present at all, and not necessarily elucidating that the d2 term is smaller than the c2 term in the first case (note: ICC is used in lieu of r as in Ozaki et al., 2011) as it conveys the same variation concept, albeit with potentially different statistical properties). Using that logic, and because the ACDE base model is not possible in this context, and as these variables naturally confound for each other even as far as nested models of ACE and ADE, was the primary rationale of choosing a more model-agnostic approach strictly based on AIC (albeit with the limitations therein, such as potential confounding of DE with ADE, but studies have demonstrated that this may be justified in their applications (Barclay et al., 2015; Den Hoed et al., 2013; Herskind et al., 1996; Nybacka et al., 2012; Skousgaard et al., 2016; Tholin et al., 2005; van der Linden et al., 2018; L. Wang, Mohammadnejad, et al., 2021; Xu et al., 2020)).
Overall, the method proposed, therefore, provides concrete findings of genetic influence under less assumptions than that of Falconer's Formula and the ACE/ADE model (albeit the base assumptions are identical [e.g., equivalent shared environmental and unshared environmental effects across twin zygosities]), and operates under more general contexts without violation of assumptions as in the ACE/ADE model, which can potentially provide subsequent biased estimates and coverage. At the cost of parsimony, the ACE/ADE model can give more delineation to what this approach determines, but the parameter estimates, and the overarching findings may be different due to violations of the assumptions or the different framework in which the model is operating entirely (e.g., assuming variance components are equal). It is evident, though, in our analyses, that both approaches overall converge to a similar outcome (with some deviation), and perhaps it can be said that both benefit each other in complementary ways and may be used in different contexts or applications as needed, the proposed approach seeking to investigate strictly the genetic influence, and the ACE/ADE model to further delineate those genetic components (as well as environmental components).
Notably, both approaches appeared to converge on genetic influence trends in the same way as one another, albeit with some deviation and potential different minute findings when considering all parcels or other model specification (without DE) and with different proportionate findings due to differences in hypothesis testing.
4.4 Reliability of findings
Resting state functional connectivity is known to be a reliable measure, particularly when derived from longer scan lengths, or when considering concatenated scans together as in (Birn et al., 2013). As we used four scans in the Human Connectome Project specific to this population, the reliability of functional connectivity in this instance should be more notable than in the alternative case of less scans (two or one). An intrinsic assumption in using the data over two sessions (days) is that it generalizes (e.g., the individual data does not change considerably over the acquisition period, which can be up to a month in time). There are a few additional scans specific from the HCP re-test validation at least in the cohort picked in this analysis (21 scans, all MZ), but which would not be sufficient in sample to fully validate the findings in that manner. Finally, the overall trend of our results appears to be corroborated by the proposed approach with Levene's test as well as the ACE/ADE model analyses which should give further support to our model approach. It should also be noted that, based on the proposed approach, conservatively, genetic influence across the resting state brain for males was >1.7% versus >0.1% for females (and >2.1% for the population level) out of all connections possible; and, for both males and females there was greater [than genetic influence] and substantial environmental influence (based on ACE/ADE model findings, females [>37%] > males [>25%]) in comparison, in terms of resting state functional connectivity sex-specific individual differences. Additionally, under the assumption of similar environmental effects across zygosities, there were three connections (post-normality testing) for females that demonstrated variance MZ > DZ (potentially akin to negative heritability). This finding may be due to noise or the relatively small sample size, or it may be due to recently proposed mechanisms (Verhulst et al., 2019; Steinsaltz et al., 2020).
The Glasser parcellation scheme with its predefined networks (regions) were used as functional networks for the sake of this analysis (i.e., the network–network heritability and significant influence matrices) because they have been established on specific criteria (Glasser et al., 2016). That is, Glasser parcellation was specifically developed on the Human Connectome Project (HCP) data via multimodal features inclusive of resting state fMRI, which demonstrates its utility for this study. Additionally, the term “network” based on this parcellation scheme in general includes more than one parcel/area based on the neuroanatomical supplementary results Table 1 from Glasser et al. (2016). For the subcortical regions, instead of considering the subcortex as a specific network, it was decided to break it down by each parcel for the network–network matrix calculation. This decision provided a suitable way to tease apart the effects of individual subcortical regions, and, in so doing, provided the resolution to tease out the main contributors in the subcortex to other networks.
4.5 Genetics and environmental factors
Fu et al. demonstrated in a pediatric population using rsfMRI (resting state eyes closed) data greater heritability among the posterior (visual), and lesser in the anterior brain regions (auditory, attention, executive control) resting state networks (Fu et al., 2015), which is consistent with our results that posterior compared with anterior regions were more genetically influenced in general. A study examining heritability in the HCP cohort involving twins, as well as nontwin siblings with rsfMRI data found low heritability of 20–40% throughout the brain including anterior and posterior brain regions (Adhikari et al., 2018). This finding is consistent with our proposed approach region analysis findings which finds only 11/32 networks demonstrating (higher connection count than random) significant relative density (limited to the F-test) of genetic influence at the overall population level, although somewhat difficult to compare given that genetics and environmental factors as well as twins and nontwin siblings were taken into account in how heritability was calculated in their study, versus our approach which examines mainly the influence of genetics in twins exclusively. [Correction added on 05 September 2023, after first online publication: added: (limited to the F-test).]
Task fMRI studies have implicated brain regions involved in certain tasks as heritable (Jansen et al., 2015). One study investigating heritability of signal in the N-back fMRI task found heritability specific to the left supplementary motor area, inferior, middle, and superior frontal gyri, precentral and postcentral gyri, middle cingulate cortex, superior medial gyrus, angular gyrus, and the superior parietal lobule (Blokland et al., 2011). Another study examined the shared genetic etiology between cognitive performance and brain activations in language and math tasks using the HCP dataset. They found several parts of the language network along the superior temporal sulcus, as well as the angular gyrus belonging to the math processing network, are significantly genetically-correlated with these indicators of cognitive performance (Le Guen et al., 2018). The differences in heritability/genetic influence results between rsfMRI datasets (Adhikari et al., 2018; Elliott et al., 2019) and other results which use task fMRI datasets may be due to the differences in what is being assessed by resting state vs. task fMRI (Koten et al., 2009). Resting state fMRI data is commonly utilized to assess the functional connectivity of the brain regions during rest, while task fMRI data is commonly utilized to assess the activation of brain regions while performing a task. Moreover, factors such as the amount of rsfMRI data collected or the scan length also appear to influence the amount of variance in rsFC attributable to genetic influence (Elliott et al., 2019). Additionally, the specific parcellation used to define regions of interest, as well as primarily examining connections at the region level and not the connection level may lead to differences in our results. Overall, however, rsfMRI and task fMRI are measuring two different aspects of brain function or state which may show differences in heritability or genetic influence. (Gritsenko et al., 2018). [Correction added on 05 September 2023, after first online publication: Gritsenko et al., 2018.]
Our results suggest that rsFC involving anterior brain regions implicated in high-level cognitive and affective processes such as working memory, executive function, reasoning, attentional and impulse control, decision making and emotional judgments (Frontofrontal, FPNs), language (FTNs, FONs), action planning and movement (premotor, region) may have both environmental and genetic input which is suggested by more recent work (Fu et al., 2015) while rsFC involving posterior regions implicated in visual, perceptual, low-level cognitive and affective processes, internal monitoring, rumination and evaluation of self and others may be influenced more by genetics. Individual differences in males, may be more genetically influenced than individual differences in females in terms of the interplay of TPNs with eDMN as well as primary sensorimotor systems (vision, audition, somatosensory and motor cortex), and subcortical/noncortical systems. Additionally, since the results for the proposed approach population and qualitative connection analyses are Bonferroni corrected, they serve as robust findings that may not otherwise be found in typical ACE/ADE modeling approaches (albeit using the cartographer map concept, they are comparable and have results which validate our findings). These connections and the regions which they constitute may in turn serve as specific endophenotypes in young, healthy individuals. Furthermore, these connections may also have implications in aging and development as well as in disease and other disorders. [Correction added on 05 September 2023, after first online publication: added: individual differences in, twice.]
4.6 Limitations and future work
4.6.1 Proposed Approach
This work was limited by the following listed factors. First, to determine genetic influence for the method proposed, it was assumed that the difference between MZ and DZ “within pairs of twins” variance components was primarily attributable to genetics. In other words, it was assumed that, on average, the shared and unshared environmental effects on the brain were similar between the MZ and DZ groups (however, it should be noted that, the HCP does not explicitly assess whether the twins are reared together). This similarity is a base assumption of the ACE/ADE model as well, and the statistical comparisons in terms of environmental factors under demographics (education, employment, household income), were found to not demonstrate significant differences in these respects (as proxies for environmental factors), contributing to the validity of this assumption. More so, just as in the ACE/ADE model, this assumption is the basis for the analysis, although it has generally been a given assumption in typical ACE/ADE model studies of which while contentious (in terms of the special MZ twin environment which is said to potentially have the ability of mimicking nonadditive genetic effects), when tested on other phenotypes of interest (not specifically rsFC) has generally been found to be valid or have deviation of which has marginal to moderate effect on heritability (Neale & Cardon, 2013; Felson, 2014; Hwang et al., 2021). For further opposing views and debate on the special MZ environment topic and implications, see (Joseph, 1998, 2014; Moore & Shenk, 2017; Wood, 2020). Additionally, the data was limited to LFO rsfMRI which focuses on functional connectivity involving various brain regions. Further analyses of other data types (e.g., task fMRI, diffusion tensor imaging (DTI), other frequency bands outside of low-frequency oscillations, for example, using MEG, and morphometrics) which have shown involvement of genetics and environment on other aspects of brain function and structure (Jansen et al., 2015; Jin et al., 2011) need to be investigated in conjunction with our method. Additionally, a future study should investigate how these results relate to smoking and alcohol habits of twins, as such habits has notable impact in the developing brain, although the HCP data is primarily localized to immediately past alcohol and smoking metrics.
For descriptive statistics (mean, median, mode, standard deviation, and frequency counts) relating to the distribution of a more cumulative smoking history metric and another associated metric (which is less localized to the past and more cumulative of life history), and additional comments, see Table SS; for further relevant discussion specific to sex differences see Section 4.6.3 (sex differences). [Correction added on 05 September 2023, after first online publication: added: Moore & Shenk and Wood citations. Added: environment such habits. Added: For descriptive statistics … (sex differences).]
The use of the F-test is highly sensitive to nonnormally distributed data, and therefore even slight deviations from normality (e.g., especially if involving heavy-tailed, or leptokurtic, data) may have influenced the resulting variance findings in terms of the error rate (i.e., higher Type I/false positive error rate if heavy-tailed, and otherwise, higher Type II/false negative error rate if light-tailed). See also: https://stats.stackexchange.com/a/210832, https://www.johndcook.com/blog/2018/05/16/f-bartlett-evene/, https://stats.stackexchange.com/a/24024. [Correction added on 05 September 2023, after first online publication: added relevant links for discussion.] Additionally, the data distributions at the group, male, and female level may differ in characteristics. However, by subtracting twin pair data both ways, this subtraction creates an artificial symmetric and possibly normal distribution, thereby helping mitigate the normality issue; additionally, by using sensitive normality testing such as Shapiro–Wilk, the amount of false positive results can be mitigated. While the Shapiro–Wilk test—which takes into account the tails of the distribution in assessing normality—was used to exclude subtracted connection distributions (of which the original connections were Fisher-Z transformed) which were nonnormal, future work and application should focus on and investigate less sensitive (to violations of normality) variance tests than the F-test, such as the median-based Levene's test [Brown-Forsythe test] (which was used for validation in this study), as it is noted to be robust to nonnormality as well as potentially possess stronger (lower) Type I error rates (but possibly weaker/higher Type II error rates) or alternative Type I and Type II error rates in general (dependent on the data and deviations from normality). See Section 4.6.5 on Levene's test vs. F-test for more details. And, as with both post-selection inference and assumption testing in general (e.g., surviving connections used for the quantitative sex-level differences analysis after AIC-based, model selection, ICC analysis post-connection selection, and effect of all assumption testing and connection exclusion performed prior to analysis) in the study (including the ACE/ADE model), the respective potential (biased) statistical impact (e.g., on error rates) should be considered when interpreting the results. [Correction added on 05 September 2023, after first online publication: added: Additionally, the data distributions at the group, male, and female level may differ in characteristics. Added: subtraction.]
It should also be mentioned that the F-test (as well as other variance approaches, and normality testing) expects that the input data are independently distributed; however, given the arbitrary order of subtraction in the case of twins and the double entry method as aforementioned, the dual subtraction was considered acceptable despite this independence assumption. Another limitation is that given the multitude of methods possible for normalizing the connections for the network-network interaction summarization, the raw data was discussed in general. In terms of the region validation analysis (for either the F-test or ACE/ADE model), only a one-sided permutation test was performed, hence, significance could not be determined for low relative density regions [p > .975 nonsignificant], and instead a high p-value indication. In terms of the region validation analysis (for either the F-test or ACE/ADE model), only a one-sided permutation test was performed, hence, significance could not be determined for low relative density regions [p > .975 nonsignificant], and instead a high p-value indication. Additionally, no multiple comparison correction scheme based on the number of regions was performed for the region permutation-based analyses (F-test and ACE/ADE model).
Although we are aware of correcting for test statistics in twin double entering methods such as in ACE model studies, in this study we have not implemented this correction since we did not make any assumptions about the direction of twin ordering leading implicitly to proper phenotypic variance by definition in the subtraction as well as an appropriate covariance term (see Section S10); a future study, if desired, could consider incorporating the degrees of freedom into the double entry method as a means of correction. In either case (double entry correction or lack thereof), the respective statistical power implications should be understood and considered. Similarly (by the same token), for the quantitative analyses, correction can be applied in terms of the double entry sample size number. Furthermore, the effect size of the findings and relevant confidence intervals are left for work in a future study. Finally, it should be kept in mind that as with specific ACE model implementations, the proposed approach is a double-entry method, and the statistical power will necessarily be different than other traditional significance testing concerning mean and variance testing unless corrected; therefore, the resultant differences in power and other statistical characteristics between double entry methods and traditional methods should be kept in mind holistically in this and any respective study. [Correction added on 10 May 2023, after first online publication: added: last sentence].
An additional limitation of this study is the low sample size (e.g., which increases sensitivity to outliers in twin studies) due to education, employment status, household income, age and sex matching. Future studies should investigate the effect of sample size on the results (including the ACE/ADE model). We also did not incorporate other siblings or related family members in the analyses to minimize assumptions about genetics and environment. While there was a significant [e.g., see Section 4.6.3] difference in age between males and females, all subjects were young healthy adults and brain maturation is thought and assumed in this study to have plateaued around this time resulting in no significant changes in brain plasticity or connectivity during this age range. Furthermore, the direct statistical comparisons that were examined were between the DZ and MZ groups with no significant age difference in these groups, which would result in removal of any age effects (more elaboration of genotype by age interactions, and the contingency of heritability on sample is discussed in Section 4.6.3). Interestingly, matching for cases like education difference (or group/male absolute difference in absolute RMS, and absolute difference in relative RMS in Section S8) may indicate (if significant, albeit, uncorrected in motion Table S2) not necessarily a confound, but potentially an unconventional notion toward genetic influence in this specific case of monozygotic and dizygotic twins. As with the ACE/ADE model, genotype by environment covariance (assumed not present in ACE/ADE), genotype by environment interaction (assumed not present in ACE/ADE), are not explicitly modeled; additionally, random mating is presumed in these estimates. Given the nature of rsFC being intrinsically a set of correlations across different nodes (the correlations/connections of which in and of themselves may then not be independent), this fact should be kept in mind when interpreting the results (such as in the ACE/ADE model), or applying correction schemes like Bonferroni (which assumes independence of outcome variables/connections, and which may be more conservative due to this fact if nonindependent). There are other approaches that may take into account for example the independence related limitation, such as a correction for Bonferroni as in (Shi et al., 2012). Any violations of assumptions may result in biased estimates. The proposed approach should be well-suited to quantitative phenotypes, yet investigation is needed for generalizability to non-quantitative phenotypes given the subtraction involved (e.g., given implications of subtracting ordinal variables, to which one possibility is to transform the data from ordinal to interval prior to subtraction, for example). Finally, there may be functional heterogeneity in regions defined as eDMN and TPNs. [Correction added on 10 May 2023, after first online publication: added: Given the nature … (Shi et al., 2012).]
4.6.2 ACE/ADE model
Limitations to the ACE/ADE model are listed in Section 4.3, but additionally, in consideration to not using the domain knowledge of ICCMZ > 2 ICCDZ and its implications on the C or D component of the ACE/ADE model and therefore subsequent nested models, this may have altered the results given a lack of comparison between CE and DE models if this alternative approach would have been done (for the distribution of alternate model counts based on AIC see the Section S5). Albeit we preferred the agnostic approach to be completely unbiased based on Section 4.3.
It should be noted, nested model preference may reflect a lack of power and ability to detect estimates, e.g., A, or aliasing of dropped components into remaining components, in certain circumstances (leading to upward or downward biased estimates). Similar biased estimates may result from violation of assumptions. For a more expansive list of over and under-estimated parameters contingent on model selection (e.g., the impact of not selecting ACDE due to data constraints) and further limitations (e.g., potential impact of higher order epistasis on the shared dominant genetic variance coefficient may lessen the coefficient in DZ twins, and the modeled relationship between A and D could lead to random chance spurious findings for D) of the ACE/ADE model and quantitative genetics in general see (Ozaki et al., 2011) and (Keller et al., 2005; Keller 2005).
Furthermore, by allowing the DE model as a possibility, there is risk in overinflated D estimates (related to the principle of marginality, more on this in Section 3.8.2 and 4.6.5) in lieu of A and or an indication that there is low power if this model is selected, and so these limitations should not be understated; relatedly, caution has been advised for the DE model, as it is considered unlikely for nonadditive but no additive effects to be present (e.g., in polygenic phenotypes), albeit possible. In response to this limitation, inclusion of the ACE/ADE approach without DE, and the respective validation section has been addressed (see again, Section 3.8.2, and 4.6.5). Additionally, the DE model has been included in a variety of recent studies (Barclay et al., 2015; Den Hoed et al., 2013; Herskind et al., 1996; Nybacka et al., 2012; Skousgaard et al., 2016; Tholin et al., 2005; van der Linden et al., 2018; L. Wang, Mohammadnejad, et al., 2021; Xu et al., 2020), and for the sake of agnostic investigation, it was incorporated as ICCMZ >> ICCDZ in the validation findings preceding, because it is known that C and D mask for each other (possibly nullifying each other), and that even A and D are confounded to a degree (Posthuma et al., 2003; Chen et al., 2015), and this effect can become more apparent when considering submodels as the relationships are different. Finally, we used the most recent unbiased direct variance estimation approach of the ACE model (Verhulst et al., 2019); while this approach offers unbiased estimates and corrects the Type I and Type II error rates as compared with the more biased approach, this benefit is at the cost of interpretability of the standardized estimates (estimates can exceed the range of 0 to 1, including their confidence intervals). For a comparison to the standard biased approach of the ACE model (no D component) see Section S6 and Figures S10–S12. We preferred the unbiased approach as it would offer more accurate Type I and Type II error rates (Verhulst et al., 2019), and as we based the significance of an estimate based on the lower bound of its confidence interval being greater than 0.4 (Fu et al., 2015), interpretability was satisfied (along with selecting for the base model ACE/ADE model with the positive parameter estimate). Findings illuminated by the D component may subsume dominance/epistasis (as previously noted), assortative mating genetic effects, epigenetics, and age influence if present, and these, at least in the ACE/ADE model must be noted. As is by design, the E component that was computed is not only indicative of unshared environmental factors, but also measurement error, and so these findings should be viewed with that aspect in mind. Additionally, in terms of optimization, NaNs were used instead of status codes to assess optimization failures (and E estimates of nested models generally had a starting value of 0.5 to aid convergence), which may lead to a subset of false positives. One other consideration which can be done in a follow-up study is to allow the means between males and females to differ at the group level, as this may influence group-level variance estimates, at the cost of an additional degree of freedom; in this study we assume that as it is the group level, the phenotypic mean should be across both male and female twin pairs, treating them as a “group” instead of sex-specific differences, which are demonstrated by the sub-analyses (albeit, otherwise, this lack of assumption could lead to biased effects due to mean phenotypic differences per sex, if present). [Correction added on 10 May 2023, after first online publication: effect.]
In the case of the ACE/ADE model, thresholding occurred at 0.4 for the lower bound confidence intervals of the estimates; however, given the basis of the cartographer map analogy of these ACE/ADE results, and given that this threshold demonstrates in general a binary cut-off of the “highly” influenced connections (that is, a map of elevated (highly) influenced connections) under this idea for comparison with our method, we accept this as a more general binary “cartographer's map” (approximate overview and map) of highly influenced connections with respect to the ACE/ADE model (to which the proposed approach application may be described as a differently defined cartographer's map). The results should be noted to be contingent on this chosen cut-off, (and also, for example, selection of the model–if it were E, there will always be a lower bound CI of 1, which has implications on bias). In this circumstance, we do not choose to use any specific kind of correction technique in this study based on the above viewpoint, and in general, the results, given the imperfect comparison to our method, would not necessarily be beneficially altered if this were to be done in this specific context.
4.6.3 Sex differences
Our findings include certain caveats when considering the proportion of connections that were significant for males versus females, as well as the quantitative comparisons (only performed with the F-test and ACE/ADE with DE) in terms of FDR correction. In the first case, we have demonstrated qualitative findings that are also to a degree contingent on avoiding violation of assumptions (e.g., certain connections were excluded due to nonnormality, classical twin design violations—future investigation of these untested connections is needed); however, despite this fact, the trends when considering not excluding these nonnormal connections remain similar for genetics for ACE/ADE model results (e.g., Figures 4-6 vs. Figures S2–S4, albeit if nonnormality included, estimates risk being over/under-estimated) to when connections are excluded. Relatedly, the qualitative analysis is done on the specific proportion and subsets of connections that survived assumption testing, which differs between males and females; future work should investigate approaches to address those which did not survive assumption and normality testing with the F-test and ACE/ADE model. However, Levene's test corroborated the broad patterns, in general (without being constrained by normality, albeit qualitatively), of the proposed approach paired with the F-test. In the second case, the quantitative analysis (specific to ACE/ADE with DE and the F-test) assumes normality of the variance components, which is the case specifically asymptotically but otherwise should be viewed with this caveat in mind (and other approaches such as potentially involving permutations can be done in the future with more nuance). Furthermore, by definition, if the SEs are 0 for one sex (due to an absent component of a subnested model, e.g., D in AE) and not the other, this will affect the Z-score with an optimistic bias. [Correction added on 13 March 2023, after first online publication: added: (only performed with the F-test and ACE/ADE with DE). Added: (specific to ACE/ADE with DE and the F-test).]
It has been recently noted that there may be a sex-specific participation bias which can affect the results of what is deemed sex-specific given the specific-sex participants who do end up participating (e.g., despite the plateauing of brain function in young adults, there was still a degree of age (significant age, two-sample t-test/Wilcoxon Rank Sum test, p < .05) difference (∼3-4 years) in males and females in our analysis, and this could be a product of the types of females who have chosen to participate versus the types of males who have chosen to participate; Pirastu et al., 2021); future study should investigate any potential age dependence across group and sex (e.g., genotype by age interaction) on variance estimates and genetic influence from this study and in general. Furthermore, there are known sex-specific factors when it comes to brain maturation and remodeling (hormones, sex-specific chromosomes, pregnancy-induced hormonal and brain changes) which may affect, explain, or modulate the results found herein (Kaczkurkin et al., 2019; McCarthy, 2008; Rawlik et al., 2016; Martinez-Garcia et al., 2021; Barba-Müller et al., 2018). Relatedly, although this study assumes nonsignificant brain plasticity changes in young adulthood, studies have suggested that males and females have differing brain maturation trajectory rates dependent on age and brain region (e.g., adolescence [prefrontal cortex rate: females > males], early to mid 20s); these differences, and the idea that metabolic brain age (relative to chronological brain age) may be older for males than for females in young adulthood onwards, should be taken into account when considering the results (and e.g., cohort ages: females > males) in this study (Goyal et al., 2019; Fish et al., 2020; Perry et al., 2020). And further studies should consider the nuances that may be present in spite of this plasticity assumption, due to other more subtle plasticity effects, such as possible region-specific plasticity, gene expression variation due to aging, reactive oxygen species due to aging, changes due to experience or seasonal effects, or plasticity due to outliers/violations to the plasticity assumption itself also noting in relation to this plasticity assumption any potential earlier age plasticity effects and which may differ by sex, as for males [and at the group level], the minimum participant age was 22, and for females the minimum participant age was 26 (∼3-4 year average age difference between sexes); (Garcia-Segura, 2009; Hofman & Swaab, 1992; Lu et al., 2004; Massaad & Klann, 2011).
For this set of twin cohorts (group, male, female), there appeared to be different distributions of one smoking history burden metric (as referenced in Table SS) based on each cohort (e.g., across zygosities, males had an unremarkable difference [0.06 mean difference, the group level had a minor difference (0.14 mean difference), and females had a notable (0.3 mean difference) albeit moderate difference in smoking history burden across zygosities (i.e., slightly shifted range of low/non-smoking status (0) and high/regular smoking status (3) frequency percent ranges across female MZ and DZ twins)]). This metric, in conjunction with the years smoked distribution (for those who scored 3 in the previous metric, and which on average was >10 years per group) provides a cohort-specific distribution of smoking habits (also in Table SS). For the regular smokers for females, there was an ~1.3 difference between zygosities, relative to 0.4 for the other groups. Whether these differences (across zygosity in particular) are notable in terms of impact (e.g., as a covariate/confound) on the brain, or whether they are a byproduct of the specific individual differences in genetics in the sample, or both, remain as questions for future investigation.
Specifically relating to females, it has been noted that structural brain volume changes for males, not females, may be affected by smoking based on one study (the implications of this potential finding and its correspondence with rsFC requires future investigation); and, this specific burden metric is limited to zero (score of 0) to five plus (score of 3) cigarette packs (assuming approximately 20 cigarettes in a pack), indicating it may be better used in conjunction with other smoking metrics (such as SSAGA_TB_Yrs_Smoked) given the potential limited information provided due to its saturated and small scale (Prom-Wormley et al., 2015; Seshadri et al., 2004). One study which does investigate rsFC in conjunction with smoking and alcohol metrics, and which uses this specific metric using the HCP database also included other smoking metrics in their investigation which may be useful for future investigation (Cheng et al., 2019).
It is noted that smoking and alcohol usage have been suggested to have an impact on chronological brain age (Ning et al., 2020). Similar to as described before, but specific to rsFC, there are differing effects of acute nicotine and chronic nicotine exposure, and with the anterior cingulate, prefrontal cortex, eDMN, and insular cortex particularly involved (although more regions may be implicated), which may relate in differing ways to the results in this study (e.g., insular and frontal opercular cortex was one noted region influenced by genetic effects found; Cheng et al., 2019; Fedota & Stein, 2015; Ghahremani et al., 2023; Sutherland et al., 2012). Additionally, the effects of time-period of quitting from cigarette, alcohol, or other substances used, or time of last substance use (ignoring other effects such as tiredness or time of day of scan, elaborated on in a comment in Cheng et al., 2019), and rsfMRI acquisition periods in relation to these events can have differing effects on rsFC (albeit, concatenating the rsfMRI time series across multiple time points may help with generalizability in this respect; Sweitzer et al., 2016). There are also sex-specific effects when it comes to rsFC and smoking (Zhang et al., 2017).
Nonetheless, when it comes to generalizability of the results, until further replication studies are performed to validate the findings, these specific smoking traits help elucidate that it is important to note the results (of the proposed approach and the ACE/ADE model) being contingent to this specific sample characteristics such as smoking, alcohol, other phenotypes/traits possible, of which themselves may have sex-specific relationships/effects. These additional traits and their potential impact/relationship on the results would be alongside the differential power of the samples (male sample size was smaller than female sample size) and corresponding potential impact/relationships as well. For further comments on smoking, see Table SS, and for a collection of other available metrics of investigation from HCP, please see the data dictionary here: https://wiki.humanconnectome.org/display/PublicData/HCP-YA+Data+Dictionary-+Updated+for+the+1200+Subject+Release. [Correction added on 05 September 2023, after first online publication: added: (∼3-4 years). Added: Relatedly, although this study assumes nonsignificant brain … https://wiki.humanconnectome.org/display/PublicData/HCP-YA+Data+Dictionary-+Updated+for+the+1200+Subject+Release.]
Additionally, it is feasible that medications that males versus females may be taking potentially documented or undocumented and the fact that certain females in the study were present with irregular menstrual cycles may have an explanatory effect on the results. To that end, there may be underlying variables at play that are subsets of each group, e.g., Simpson's Paradox. Although we have taken caution to match between many factors in terms of age, education, education difference, and socioeconomic status more broadly between monozygotic and dizygotic twin groups, it is feasible that there are certain proxy measures, some specific to sex, which are not available or which could also be looked at which may further explain some of the differences that have been found in this study. Furthermore, we did not explicitly match environmental variables across sex (males vs. females [Wilcoxon Rank Sum test], employment status and income significant in practice (p < .15), education not significant; one missing FDZ twin's data dropped), but this was acceptable based on the assumption that the effect of unshared and shared environmental effects of twins should be identical across zygosity, implying that any environmental effects (not due to genetics) should wash out when determining the final effect of genetics in the proposed approach. This “washing out” effect, again, under the presumption of shared and unshared environmental effects, should allow for comparison between males and females of the respective resultant unshared genetic variance. This fact similarly should hold for the basic ACE/ADE model. One caveat to mention related to this fact is that this study did not investigate gene by environment interaction (as well as covariance) under basic model formulation. To that effect, future studies should investigate this hypothesis with respect to group and sex and relate it to this study's findings.
This study did not take into account the parenthood status of twins, the age of parents (e.g., the mother) of the twin pairs at birth, which can affect the results, twin birth order, mitochondrial DNA contributions from parents in relation to twins, sport habits, gender identity, as well as the prenatal hormonal environment (of male vs. female twins). To this effect, whether twins were bichorionic or monochorionic was not accounted for. It is also known that parents may treat their children differently immediately from birth based on gender (Johnson et al., 2014), which may have implications on our results. In a similar vein, sibling interaction effects, parental environment effects, potential additional siblings (third siblings), epigenetic effects (MZ twin pair epigenetic supersimilarity and potential epigenetic differences with increase in age), and the special twin environment were not considered (Fraga et al., 2005; Neale & Cardon, 2013; van Baak et al., 2018). It should also be noted that the study is focused on the LFO-band frequency between males and females, which should be kept in mind in interpretation of the results. Future studies can also consider using objective sex markers via genotyping data if available/accessible. [Correction added on 05 September 2023, after first online publication: added: sport habits, gender identity. Added: and potential epigenetic differences with increase in age. Added Farga et al., citation. Added: last sentence.]
For the ACE/ADE model, given that the variance components are constrained to be the same across zygosities, this same conclusion can be made. Furthermore, as in the case for general limitations, the effect of motion (see Section S8 for additional metric on absolute motion (pre-ICA-FIX), which is known not to usually affect bold rsFC analyses, but which may have more of an effect contingent on the type of acceleration scheme, such as SMS (used in this study) and GRAPPA), while addressed by ICA-FIX which is known to be a strong filter against motion artifacts, can still affect rsFC in ways that are difficult to pinpoint, and in a nonobvious (or nonlinear) fashion (Couvy-Duchesne et al., 2014). However, as noted in Couvy-Duchesne et al. (2014), this impact is less likely the case for estimates which are known to be higher in heritability. Furthermore, in terms of reliability, as in Elliott et al. (2019), the features must be reliable in order for them to be heritable, lending credence to our results in this respect. Additionally, there may be cases in which state-of-the-art motion techniques such as ICA-FIX may not perform as ideally but which is an open question (Afyouni & Nichols, 2018); future study should investigate the choice of consistent, but alternative, motion correction schemes.
As we matched the sample prior to our analyses, we did not explicitly model sex, age, age2, motion, and the variety of socioeconomic measures directly in any particular model; but, as the matching was already performed, this lack of modeling was presumed to be acceptable (albeit, future work should investigate any potential nonlinearities due to age2). A future study can investigate specifically the effect of including these as explicit covariates in the respective desired analyses. There was a significant difference in the DVARS between males and females, and no significant difference between males and females in terms of average absolute RMS and average relative RMS (two sample t-test/Wilcoxon Rank Sum Test, p < .05 indicates significance), pointing to potentially a biological difference in this metric after ICA-FIX is performed (but prior to further processing steps and bandpass filtering); as there was no significant difference in DVARS across MZ and DZ within a sex, this may point toward more biological differences specifically, and is of interest for future investigation as they relate to the results herein. Although, notably, the scale of DVARS units, which would relate to this difference, has often been a point of contention, and is still open to interpretation. It is additionally known that heritability estimates from the twin design are known to be sample-specific (further generalization of which would also be contingent on unbiased and minimal self-selection sampling, e.g., implications of a homogeneous sample environment may lessen environmental impact to that of genetics in the estimate, or implications of twins potentially more likely to be born due to assisted reproductive technology or genetic factors may limit generalizability) (Maloy & Hughes, 2013; Neale & Cardon, 2013; Chambers et al., 2014; Mbarek et al., 2016). Heritability estimates may change as a function of historical time as the variation due to genetics, environment or correlation between genes and environment can change, although this change is expected to be slowly for polygenic inheritance (Wray & Visscher, 2008; Maloy & Hughes, 2013). [Correction added on 10 May 2023, after first online publication: replaced men with males and women with females.]
Similarly, the findings are specific to the young adult cohort/sample (males and females) investigated, and the relative proportion of genetic versus environmental influences for each sex per phenotype of interest, again contingent on the sample. It is also assumed that twin populations generalize to that of singleton populations (e.g., twins often are born prematurely compared to singletons, which may matter more for younger aged twins dependent on the trait; Haworth et al., 2008). Relatedly, it should be noted that the HCP excluded prematurely born twins (prior to 34 weeks). Further investigation should pertain to generalizability of these results across the lifespan and using alternative methods, as heritability (or unshared genetic variance) can change throughout time in a population (and e.g., due to different genetic and environmental factors over time) (Wray & Visscher, 2008). This study also does not consider (e.g., in terms of results interpretation, albeit which may or may not have implications, based on the hypotheses of the approaches being asked) genetic influence pertaining to phenotypic traits with little or no variability [low heritability], which may still be influenced by genetics (e.g., the phenotype of having a brain) (Vitzthum, 2003). Additionally, we did not consider the impact of race in this study, which may limit generalizability of our findings (Maloy & Hughes, 2013). Nor did this study account for the degree to which participants may have fallen asleep versus remained awake during scan acquisition. Other covariates in future studies could factor in intracranial volume-based considerations (which itself can be a function of age and sex), age by sex for the group level analyses, and proxies for statistical power (Colclough et al. 2017). [Correction added on 05 September 2023, after first online publication: added last two sentences.]
It is also important to mention that in terms of the quantitative (Z-score) analyses (albeit important to note in general), the analyses suggest greater genetic variation (proportionate to other influences) between males or females in the respective findings (e.g., the unshared genetic variance, based on the proposed approach, or nonadditive and additive genetics based on the ACE/ADE model, is greater between males or females assuming equal environments within-sex). While it is true that the proportions may differ between sexes, this difference can be a product of less or more environmental variability within sex leading to stronger or weaker proportion of variance toward genetics (contingent on the sample). This caveat may relate to the significant difference between environmental factors between sexes, to which larger samples should be investigated for corroboration, especially given the aforementioned limitations of sample-specific heritability (and given the sample size); further limitations (such as heritability potentially varying with population, trait distribution, and measurement validity, albeit in the context of items) are shown as in (Vitzthum, 2003; Wicherts & Johnson, 2009; Neale & Cardon, 2013). In looking at the comparison of sex-specific individual differences in rsFC, for the connections that were found to be influenced, the magnitude may be more concentrated in certain areas (e.g., in certain cases, lesser frontal as opposed to posterior) which should be kept in mind for an accurate view of the findings, rather than solely the quantity/magnitude of difference itself. Finally, given the relatively small twin sample size (and of differing sizes/power [males < females], which can affect ACE/ADE model estimate detectability and nested model selection preference, e.g., DE instead of ACE/ADE etc. if lower power is present in males compared to females) of sex-specific analyses, these findings should be considered preliminary but of useful value for future studies. And, carrying the sentiment from earlier, as with all studies, these findings should be investigated, validated and replicated in further studies and with larger samples to ensure reproducibility and generalizability. [Correction added on 05 September 2023, after first online publication: added: difference. Added: In looking at … solely the quantity/magnitude of difference itself. Added: carrying the sentiment from earlier.]
4.6.4 Covariates in modeling
For future studies, one approach toward modeling confounding effects in our approach would be to residualize the components using a multiple linear regression in terms of head motion and whatever other covariates are of interest into the variance test (F-test, or alternative variance test, e.g., median-based Levene's test [Brown-Forsythe test]) analysis. Although, again, as stated in Couvy-Duchesne et al. (2014) addressing head motion such as in the case of treating it as a covariate directly in the model should for the most part be a proper means of addressing the confound of motion, it still would not entirely eliminate any nonlinear effects that may be attributed to it. Given the nature of variance tests (such as the F-test, or alternative variance test), this suggestion may be the simplest advocated approach, although there may be more advanced techniques that could be explored or developed in this context. Again, while there has been found to be heritability of head motion, at the very least, the same study also indicated that when higher heritability estimates are concerned, which is of interest in our study, this estimate would be more shielded from the effect of the covariate. [Correction added on 05 September 2023, after first online publication: estimate.]
Additionally, in the specific context of ICA-FIX (although this fact is not generalizable when such techniques are not employed), absolute and relative head motion (pre-ICA-FIX) should be less of a concern, as these are the absolute/relative head motion metrics prior to processing via ICA-FIX which is designed to account for it. But that is limited to the context of this study (where ICA-FIX specifically was used, or studies which employ similar techniques).
4.6.5 Cartographer's map suggested guidelines
Levene's test vs. F-test
There are strong proponents for the exclusion of usage of the F-test, especially when normality is in doubt, given the false positive rate and false negative rate issue when it comes to very high or low kurtic data which cannot always be assessed (Hosken et al., 2018). As these limitations of the F-test are established, with the caveat that in general when sample sizes are large, or when normality is not in doubt, the F-test can be a more effective test in general than other variance equality tests (Nordstokke & Zumbo, 2007), further studies should be cautious in their immediate usage of the F-test, especially if not considering any normality tests in conjunction. At the same time, it could be argued that (without reference to the ground truth), that by pairing the F-test with a strong normality test such as Shapiro-Wilk (it too being dependent on the sample size, and data distribution), the anticonservative nature of the F-test in conjunction with the normality tests to restrict the issue could lead to the balance between anticonservative and conservative effects. However, that is at the additional cost of 1) pre-testing effects on the error rate, and 2) the exclusion in the case of the application at hand, of certain connections. One view in this study is that normality testing can be viewed as a kind of filter for the cartographer's map concept (although this view has limitations statistically).
Regardless, this study validated and corroborated the general trend count of the F-test findings of the proposed approach with Levene's test (although in terms of raw count of connections there was moderate to substantial difference, e.g., at the group level >1000 connections, and the differences between males and females became more pronounced in proportion, upon using Levene's test). The deviation of findings may possibly be due to any of the trade-offs above mentioned, and what also may be due to the conservative nature of Levene's test (potential high reduction of true positives). Therefore, keeping all of these factors in mind for future studies, it is recommended that, when it comes to application of the proposed method, the pairing between the F-test and stringent normality testing be further investigated to ensure appropriateness, and when in doubt about normality, avoid using the F-test by itself and instead compare with Levene's test (or use Levene's test solely). Additionally, as per (Nordstokke & Zumbo, 2007), the median-based Levene's test may be preferable (as in this study) in general (although specifics will vary), and other versions may give coverage that is poor and has similar performance to when the F-test is not accounted for in terms of its sensitivity to normality or sample size. [Correction added on 13 March 2023, after first online publication: replaced and relates with: and has similar performance.]
DE vs. no DE (and parameter indeterminacy)
There is contention in the field regarding whether the DE model should be used. The DE model was incorporated in an agnostic fashion prior to validation of the ACE/ADE model results without the DE model. However, upon validating the results with model selection which excludes the DE model, there are considerable changes to the cartographer maps that are generated (in principle it is explainable because by excluding DE as a possibility, that biases the model preference to A, C or E, and vice versa). The discrepancy highlights, outside of the limitations of the DE model itself, the confounding of variables in the ACE model framework (e.g., A and D), and given lack of ground truth, especially in the context of generating cartographer maps, more emphasis should be directed perhaps away from using strictly AIC (and the limitations of unsubstantial model fit differences between models). Upon viewing the results provided (with and without DE), inclusion of DE could be considered more conservative in terms of assumptions, whereas exclusion of DE could be more conservative in terms of likelihood and potential arguable avoidance of certain statistical limitations that inclusion of DE may be susceptible to. In either case, it is recommended that, even though there is the benefit of being agnostic to the outcome for the DE model, given its consequential potential power and statistical issues, as well as theoretical low-likelhood, if it is used, relevant estimates from ADE (with those respective tradeoffs and benefits) should also be included (if using traditional AIC). That is to say, evaluate both (and consider avoiding using DE if in doubt, or when not considering it in a probabilistic sense as discussed next). [Correction added on 13 March 2023, after first online publication: replaced statistical limitations with: avoidance of certain statistical limitations that inclusion of DE may be susceptible to.]
Relatedly, parameter indeterminacy is a concept that (Keller, 2005) discusses at length, and proposes an interesting solution, which may be logical in the future application of the ACE/ADE model portion of cartographer mapping. Specifically, the idea of generating a space of possible outcomes, and generating maps based on multiple possible models may be of interest in the future, especially given the importance of the estimated maps in how they may guide future researchers, and given the lack of the ground truth being present. Specifically, (Keller, 2005) stresses that the value of r (which has a relationship of rVNA = 1/4VD + VI,S,DZ and of (1-r)VNA = 3/4VD+ΔVI as formulated in this study), used for VNA which encompasses VD and higher order epistatic effects (VI) and which can range from 0 – 0.25 for DZ twins, along with the various models (ACE, ADE, AE, DE, CE), are assumptions that are inherent in generating estimates. Given this fact, it was suggested that the models and r could be used altogether to create a joint space of what is unlikely (albeit possible) to likely, and that these assumptions should be made explicit and kept in mind since they affect the result ultimately. [Correction added on 10 May 2023, after first online publication: replaced an indirect relationship of Δ in ΔVI with: a relationship of rVNA = 1/4VD + VI,S,DZ and of (1-r)VNA = 3/4VD+ΔVI.]
Additional future directions
When considering the results represented as part of a cartographer's map of influence in terms of individual differences, a few factors should be noted. 1) The cartographer's map, as indicated by AdminNeale, should be considered a rough map which is useful for future exploration, but may have errors in specifics (as with age-old cartographer maps), and which would be most appropriate for those applications which are low-risk, (e.g., not clinical trials). 2) The cartographer map is dependent on a few factors: A) the data (and its distribution), how it is acquired, sampled and processed (e.g., even the data may generate spurious connections in terms of rsFC and has its own true positive and false positive rate, ignoring statistical tests), B) the technique, or instruments to analyze the map (in this case, the statistical tests used, their limitations and cut-offs, or the software such as OpenMx), and C) how the model or problem itself is formulated and inherent assumptions (the proper or guided question and problem formulation that are being considered, for example, when considering model types—e.g., including or excluding DE—may change the outcome). It might be argued that each statistical test, with their own limitations, may allow the user to glean different information from the cartographer's map created, as each test has distinct properties contingent on the data—that is, each will have a different set of true positive, false positive, true negative and false negative rates, which can be beneficial dependent on the application and purpose. [Correction added on 13 March 2023, after first online publication: replaced old-age with: age-old. Replaced “the data and how it is acquired and sampled” with: “the data (and its distribution), how it is acquired, sampled and processed (e.g., even the data may generate spurious connections in terms of rsFC and has its own true positive and false positive rate, ignoring statistical tests)”. Added: and inherent assumptions. Replaced “true positives, false positives, true negatives and false negatives” with: “true positive, false positive, true negative and false negative rates”.]
Although for the sake of comparison this study did not incorporate any correction scheme to the ACE/ADE model results (e.g., the cartographer maps relevant), in future studies, it may be of value to do so. Based on the cartographer map concept, it may be beneficial to apply additional correction to obtain a different set of properties which would elucidate different information (e.g., the level of correction itself can result in a different signal-to-noise-ratio, or SNR of the map, which may highlight different features). And, similar to other cases, it is also possible that each rendition of a cartographer map may encode intrinsically different covariate information if not previously addressed. It may be of value in the future to provide maps with different covariates accounted for distinctly, and to see whether that accounts for changes in certain regions of the map (e.g., visually), as that may provide unique sets of information based on the application. Another element which could be focused on as well, and which ultimately will result in different maps of use for validation and alternative information, would be the parcellation scheme (and relatedly, resolution, definition, and parcels) used (e.g., the parcellation choice affects the amount of connections possible when comparing across groups). [Correction added on 05 September 2023, after first online publication: added paragraph.]
Specific to the ACE/ADE model, the maps reported in this study were restricted by normality and classical twin design assumption constraints and hence are incomplete—given the amount of connections possible, future studies should focus on amending this restriction issue, while still maintaining quality of the result. Modeling the problem (without DE, for example) dynamically changes the best fit model possibilities, which may be more insightful with regard to certain information as opposed to others, when the ground-truth expected model is unknown, and with trade-offs in statistical and theoretical ramifications. However, as mentioned previously, a manner of designing a joint space of all model possibilities and assumptions may be of future interest which is informative and agnostic. Finally, more objective means to quantify and validate the maps (e.g., the counts that are represented in this study, from high to low, or relative density and density in general, which was not as emphasized as counts and relative counts), may be of interest in the future. In any case, for whatever set of choices are determined, it is recommended it be stated explicitly, justified, and the corresponding limitations and implications stated.
5 CONCLUSIONS
Overall, our approach allowed for a parsimonious determination of genetic influence at the population and sex-specific levels. The method highlighted genetically-dependent resting state connections particularly across visual, parietal, temporal (i.e., visual, ventral stream, and dorsal stream regions, MT+ complex) and cingulate cortices. These posterior and midline brain regions which are more genetically influenced are implicated in vision, perception, low-level cognitive and affective processes. There was also a paucity of connections involving anterior regions of the brain which are less genetically-influenced, that is, frontoparietal cortices, which are implicated in high-level cognitive and affective processes such as working memory, executive function, reasoning, emotional judgment, attentional and impulse control, decision making and action planning, in addition to frontotemporal and frontooccipital cortices which are implicated in language and auditory processes. These regional differences in genetic influence may have implications in terms of the brain's ability to be influenced by environmental input (e.g., education, school and work environment, family and home environment, social interaction with peers, medications, nutrition, sports, and physical exercise). [Correction added on 10 May 2023, after first online publication: added: + complex.]
There were over 8.5-19.1 times more connections that were genetically influenced in males than females which may suggest that, for the young adult cohort investigated, individual differences in the resting state brain may be more genetically influenced in males and more environmentally influenced in females. There were also greater intra and internetwork interactions (in count) between TPNs and eDMN, primary sensorimotor, subcortical systems and cerebellum in males than females, which may suggest individual differences of males compared with females being more genetically influenced across the resting state brain in terms of interplay of interaction with environment and task-oriented processes vs. interaction with self and others. Furthermore, based on traditional approaches, environmental influences on individual differences may be substantially greater than that of genetics, for either sex, frontally and brain-wide. These results reveal the similarities and differences of genetics and environmental influences on different parcels, connections, and networks of the resting state functional brain in young healthy males and females with implications in development and aging. [Correction added on 05 September 2023, after first online publication: added: -19.1. Added: in count.]
AUTHOR CONTRIBUTIONS
Arman P. Kulkarni: Conceptualization (Proposed Approach, ACE/ADE Model, Sex Differences), Data curation, Funding acquisition, Methodology (Proposed Approach, ACE/ADE Model), Software (Proposed Approach, ACE/ADE Model), Validation, Visualization, Writing-original draft, Writing-reviewing & editing (Lead + Extra + ACE/ADE + Levene's test). Gyujoon Hwang: Conceptualization (Proposed Approach), Data curation, Methodology (Proposed Approach), Software (Proposed Approach), Validation, Writing-original draft, Writing-reviewing & editing. Cole J. Cook: Conceptualization (Proposed Approach [Idea: link to Falconer's Formula]), Methodology (Proposed Approach, ACE/ADE Model), Software (Proposed Approach), Writing-review & editing. Rosaleena Mohanty: Methodology (Proposed Approach), Writing-reviewing & editing. Akhil Guliani: Software (ACE/ADE Model). Veena A. Nair: Methodology (Proposed Approach), Validation, Writing-review & editing. Barbara B. Bendlin: Writing-review & editing. Elizabeth Meyerand: Writing-review & editing. Vivek Prabhakaran: Conceptualization, Funding acquisition, Methodology, Writing-review & editing (Lead). [Correction added on 13 March 2023, after first online publication: added: (Lead + Extra + ACE/ADE + Levene's test). Added: (Lead).]
ACKNOWLEDGMENTS
The authors would like to thank all the participants involved in the Human Connectome Project and Washington University in St. Louis for data collection and grant support from NIH UF1AG051216, R01NS105646, R01EB027087, R01NS117568 and R01AG063849. The research presented was supported under NIH award TL1TR002375. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Data were provided by the Human Connectome Project, WU-Minn Consortium (Principal Investigators: David Van Essen and Kamil Ugurbil; 1U54MH091657) funded by the 16 NIH Institutes and Centers that support the NIH Blueprint for Neuroscience Research; and by the McDonnell Center for Systems Neuroscience at Washington University. This work is an extension of the initial work and method proposed in the oral talk at Organization for Human Brain Mapping (OHBM) 2018: “Studying Genetic Impact on Resting State Connectivity using Twin Brains,” and several abstracts incorporating the ACE model “Evaluating Resting State Connectivity Heritability at the Whole Brain Level” (Alzheimer's Association International Conference (AAIC) 2019), and “Genetic and Environmental Influence on Resting State Networks In Young Healthy Adults” (OHBM 2020). We would like to sincerely thank one of the Human Brain Mapping (HBM) editorial office assistants, Vinitha Kannaperan, and both the HBM and Wiley editorial offices (i.e., especially the editor(s) and HBM Production Team for being instrumental [such as Vineela Bandaru, Vishnampettai Swaminathan (V.S.) Balasubramanian, Sally Syzmanski, and Ramesh Baalaji] in general for the assistance throughout the manuscript process. We would like to additionally thank the anonymous reviewers for their feedback and questions throughout the process, leading to a more thorough manuscript. Thanks also to Rick Reynolds from Medical College of Wisconsin for his input on AFNI, as well as the OpenMx/umx moderators (AdminRobK, AdminNeale, AdminJosh/jpritkin, tbates, mhunter), for ACE/ADE model guidance. And thanks to Dr. Abhejit Rajagopal of University of California, San Francisco, Basil Saeed of Stanford University, and Dr. Neset Hikmet of University of South Carolina for valuable conversations for the manuscript, as well as both Dr. Nagesh Adluru and RadIT at University of Wisconsin-Madison and Jivan Kulkarni for technical assistance. Thanks also to Dr. Moo K. Chung and Dr. Walter Block of University of Wisconsin-Madison, Dr. Jean-Baptiste Poline of McGill University, Dr. Eric Tortosa, and University of Wisconsin-Madison TL1 writing workshop members (e.g., Dr. Laura Hogan and Karly Cody). Finally, thank you to all of the environmental factors (such as the professors (e.g., Dr. David Goldsman Dr. Charles Isbell, Dr. Duen Horng Chau, and Dr. Constantine Dovrolis) at Georgia Institute of Technology for their great lectures/course material, Dr. Andrew Alexander's and Dr. Rasmus Birn's joint class at University of Wisconsin-Madison. International Statistical Genetics Workshop lectures, Stanford Human Behavioral Biology lecture series by Dr. Robert Sapolsky, Stack Overflow/Stack Exchange content, hcp-users mail-archive and staff (e.g., Dr. Jennifer Elam of Washington University in St. Louis), previous journal paper reviewers, the people who may have indirectly led to this manuscript in its current format, family and friends for discussion and support, and the circumstances that allowed it to be finalized) that have made this manuscript possible. [Correction added on 05 September 2023, after first online publication: Added: the editor(s). Added: for being instrumental [such as Vineela Bandaru, Vishnampettai Swaminathan (V.S.) Balasubramanian, Sally Syzmanski, and Ramesh Baalaji]. Added: Thanks also to … Karly Cody. Added: Dr. Charles Isbell, Dr. Duen Horng Chau and Dr. Constantine Dovrolis. Added: /course material, Dr. Andrew Alexander's and Dr. Rasmus Birn's joint class at University of Wisconsin-Madison. Added: Stanford Human Behavioral Biology lecture series by Dr. Robert Sapolsky … paper reviewers. Added: Dr. several times.]
CONFLICT OF INTEREST
No authors had any financial or potential conflicts of interest to report.
Open Research
DATA AVAILABILITY STATEMENT
All imaging data is freely available for download at https://www.humanconnectome.org/study/hcp-young-adult/data-releases. For extensive demographic and family structure details on the subject level, interested readers will need to apply for restricted data access using this form: https://humanconnectome.org/storage/app/media/data_use_terms/DataUseTerms_HCP_RestrictedAccess_30Nov2017.pdf. All code used for the analysis by the authors will be available upon reasonable request.




