White matter hyperintensities at critical crossroads for executive function and verbal abilities in small vessel disease
Funding information: Netherlands Organization for Scientific Research, Grant/Award Numbers: 024.001.006, 451-17-003; Dutch Heart Foundation, Grant/Award Numbers: 2016 T044, 2014 T060; Innovational Research Incentive, Grant/Award Number: 016-126-351
Abstract
The presence of white matter lesions in patients with cerebral small vessel disease (SVD) is among the main causes of cognitive decline. We investigated the relation between white matter hyperintensity (WMH) locations and executive and language abilities in 442 SVD patients without dementia with varying burden of WMH. We used Stroop Word Reading, Stroop Color Naming, Stroop Color-Word Naming, and Category Fluency as language measures with varying degrees of executive demands. The Symbol Digit Modalities Test (SDMT) was used as a control task, as it measures processing speed without requiring language use or verbal output. A voxel-based lesion–symptom mapping (VLSM) approach was used, corrected for age, sex, education, and lesion volume. VLSM analyses revealed statistically significant clusters for tests requiring language use, but not for SDMT. Worse scores on all tests were associated with WMH in forceps minor, thalamic radiations and caudate nuclei. In conclusion, an association was found between WMH in a core frontostriatal network and executive-verbal abilities in SVD, independent of lesion volume and processing speed. This circuitry underlying executive-language functioning might be of potential clinical importance for elderly with SVD. More detailed language testing is required in future research to elucidate the nature of language production difficulties in SVD.
1 INTRODUCTION
Cerebral small vessel disease (SVD) is characterized by the degeneration of the cerebral small blood vasculature and can appear as white matter hyperintensities (WMH), lacunes, or microbleeds (Pantoni, 2010). The present study focuses on WMH, which are the most common lesion type seen on magnetic resonance imaging (MRI) scans of patients with SVD. The WMH are often symmetrical and commonly present in the periventricular or deep white matter though they can differ greatly in size (Wardlaw et al., 2013). In general, patients with SVD perform poorly on executive functioning and processing speed tasks (Prins et al., 2005). There is high agreement among voxel-based lesion–symptom mapping (VLSM) studies that WMH and lacunar volumes are associated with low processing speed and executive dysfunctions, particularly in relation to damage at strategic subcortical-frontal connections (Biesbroek, Weaver, & Biessels, 2017), such as the anterior thalamic radiation (ATR) (Biesbroek et al., 2016; Duering et al., 2011) and forceps minor (Biesbroek et al., 2016; Duering, Gesierich, Seiler, & Gonik, 2014).
While a relationship between WMH and poorer executive functioning is widely reported, the link between language functioning and WMH in SVD patients remains unclear (Ter Telgte et al., 2018). Language impairment is not typically documented as a symptom of SVD, but it remains to be established whether language abilities are affected. People with SVD do not typically report major language problems and are not routinely assessed for their language skills, so a subtle deficit may be present but undiagnosed or mislabeled. Few studies have investigated language abilities in patients with SVD and their association with WMH in strategic subcortical-frontal connections. For example, participants with SVD with different degrees of WMH burden, with and without cognitive impairment, have shown worse performance in verbal fluency than healthy controls (Charlton, Morris, Nitkunan, & Markus, 2006; Herbert, Brookes, Markus, & Morris, 2014) and Alzheimer's disease patients (Lafosse et al., 1997). Vasquez et al. have shown in their meta-analyses that people with vascular cognitive impairment without dementia, which is the most common consequence of SVD, had worse scores on language functioning when compared to healthy people or to people with mild cognitive impairment due to probable Alzheimer's disease. In their case, language functioning was operationalized as the compound score of the following neuropsychological tests: Category Fluency, the Boston Naming test, and the Token test (Vasquez & Zakzanis, 2015).
The location of WMH may be critical for verbal abilities since language is considered to have a more specific localization than executive functioning (Fedorenko, Behr, & Kanwisher, 2011). In addition, subcortical-frontal connections are also associated with language processing (Barbas, García-Cabezas, & Zikopoulos, 2013). Thus, damage of this circuit could be expected to influence language abilities in SVD patients as well. Recent studies conducted with healthy elderly provide some evidence that WMH in specific locations are more strongly associated with language abilities than global WMH measures (Hilal et al., 2020; Jiang et al., 2018).
VLSM allows to investigate the association between specific areas and a particular behavior by comparing performance of individuals with and without a lesion at every voxel. Previous VLSM studies in SVD (reviewed above) have focused only on executive functions and processing speed (Biesbroek et al., 2017). However, these studies either analyzed a single executive task or grouped together executive and language tasks in a single compound measure. The use of compound scores or isolated cognitive tasks in VLSM studies limits the possibility for understanding whether WMH disrupts connections important for performance in either the language or executive domains or for cognitive function more generally.
In the present study, we separately analyzed tasks with different degrees of language and executive demands by using VLSM (Bates et al., 2003) in a sample of 442 people with SVD without dementia. We used Category Fluency and the Color-Word Naming task of the Stroop test as linguistic tasks with a high degree of executive demand (Eng, Vonk, Salzberger, & Yoo, 2018; Wilson, 2017). Category Fluency and Color-Word Naming tap into executive function and language, although Color-Word Naming may be slightly less reliant on language because it involves choosing from a limited set of words (i.e., typically three) while Category Fluency entails self-generating words from a bigger set of possibilities (i.e., one's own vocabulary). We employed the Word Reading and Color Naming tasks of the Stroop test as measures of linguistic performance with a low executive component (Jensen, 1965). Finally, we also employed the Symbol Digit Modalities Test (SDMT), which is the least demanding in terms of linguistic performance because only the instructions are verbal but the execution of the test does not depend on language use (Fellows & Schmitter-Edgecombe, 2019; Spreen & Strauss, 2006). Our first aim was to clarify the relation between WMH location and language abilities in patients with SVD. A second aim was to examine whether executive and language measures are associated with the same location of WMH. We hypothesized that all tasks would be associated with lesioned voxels in anterior white matter tracts (Biesbroek et al., 2017), but possibly with a differential lateralization such that tasks depending more on language would be associated with left, rather than right areas.
2 METHODS
2.1 Sample
Patients in our study were included from the Radboud University Nijmegen Diffusion tensor and Magnetic resonance imaging Cohort (RUN DMC) study, which prospectively investigated the risk factors and clinical consequences of SVD among 503 nondemented older adults with SVD. The selection procedure of the participants and study protocol have been described previously in detail (van Norden et al., 2011). Based on established research criteria, SVD was characterized by the presence of lacunes and/or WMH on neuroimaging scans in combination with symptoms (Pantoni, 2010). Consecutive patients referred to the department of neurology between October 2002 and November 2006 were selected for participation. Symptoms of SVD included acute symptoms such as transient ischemic attacks or lacunar syndromes (n = 219), or subacute manifestations such as cognitive (n = 245) and motor (gait) disturbances (n = 97) and/or depressive symptoms (n = 100) or a combination thereof (Pantoni, 2010). Inclusion criteria were age between 50 and 85 years and presence of lesions caused by SVD detectable on MRI scans. Main exclusion criteria were dementia, psychiatric disease, severe language impairment interfering with cognitive testing or follow-up, WMH or SVD-like pathologies due to other causes than SVD, and MRI contraindications.
As we specifically wanted to study the effect of WMH on language and executive tasks, 57 patients with infarct in the cerebral cortex were excluded as these lesions can cause cognitive and linguistic impairment, which would confound the negative effect of WMH on cognition. One patient was excluded because of unsuccessful normalization of the scan and three patients had incomplete neuropsychological data. The final sample size comprised 442 individuals.
2.2 Measures
We employed the following neuropsychological tests: the Category Fluency test (van der Elst, van Boxtel, van Breukelen, & Jolles, 2006a, 2006b), the three tasks of the Stroop Color Word Test (SCWT, Jensen, 1965), that is, Word Reading, Color Naming, Color-Word Naming, and the SDMT (Smith, 1982). These tasks span a language-executive continuum, from tapping more into language abilities and less into executive functioning (e.g., Color naming, Word Reading) to the reverse (i.e., Color-Word Naming and SDMT). Even though all tasks have a verbal component, for example because verbal instructions are given, here we consider tasks to depend more on language functioning if they require language use for a response to be generated. The RUN DMC study protocol was designed independently from the goal of the present study.
SCWT Word Reading and Color Naming, although considered measures of processing speed, are heavily based on linguistic performance (Jensen, 1965). In SCWT Word Reading, participants read aloud color words written in black ink (e.g., say “red” to “red” written in black ink), whereas in SCWT Color Naming, participants name the color of patches (e.g., say “green” to the green patch). The SCWT Color-Word Naming subtest is considered primarily a measure of executive functioning (Scarpina & Tagini, 2017), although it is again a language-based task. In this subtest, participants name the ink color of color words, whereby the ink color is incongruent with the word (e.g., say “yellow” to “green” written in yellow ink). Each subtest was presented on a card containing 5 rows with 10 items each. For each of the SCWT subtests, the time in seconds to complete a card with all stimuli is the final score for that subtest.
In the Category Fluency test (semantic-based word retrieval), participants were given 60 s to retrieve as many profession names as they could, avoiding repetitions. The final number of retrieved words was recorded. Category Fluency is considered a neuropsychological measure of both language and executive capacity (Henry & Crawford, 2004; Shao, Janse, Visser, & Meyer, 2014; Tao, Zhu, & Cai, 2020; Vonk et al., 2019). Recent studies have found that this test has a strong language component (Tao et al., 2020; Whiteside et al., 2016).
In the modified SDMT, little language is involved, except for task instructions. Participants are instructed to write down digits (1–9) that correspond to symbols, following a symbol-digit key given in the example matrix. The test was composed of 8 rows of 15 symbols, with the 9 symbols presented randomly. The number of completed items in 60 s is recorded as the final score. No language use is required to perform the task.
2.3 MRI acquisition parameters
MRI scanning was performed in a 1.5-T Magnetom scanner (Siemens, Erlangen, Germany). The scanning protocol relevant for the present study included a whole brain 3D T1 magnetization-prepared rapid gradient-echo sequence (time repetition [TR]/TE/TI 2,250/3.68/850 ms; flip angle 15°; voxel size 1.0 × 1.0 × 1.0 mm) and fluid-attenuated inversion recovery images (FLAIR) pulse sequences (TRTE/TI 9,000/84/2,200 ms; voxel size 1.0 × 1.2 × 6.0 mm, including slice gap of 1 mm).
2.4 Radiologic markers of SVD
WMH was defined as hyperintense lesions on FLAIR MRI without corresponding cerebrospinal fluid-like hypointense lesions on the T1 weighted image. Gliosis surrounding lacunes and infarcts in the cerebral cortex was not considered to be WMH (Hervé, Mangin, Molko, Bousser, & Chabriat, 2005). The WMH were segmented on FLAIR images in native space using an in-house developed semi-automatic WMH segmentation method (Ghafoorian et al., 2016). First, the algorithm was trained to discriminate WMH from normal white matter based on a subset of participants from this dataset with this voxel size, which was manually segmented. Second, the classifier was then run for all individuals to segment WMH, after which the segmentations were visually checked for segmentation errors by one trained rater blinded for clinical data and if necessary manually adjusted to improve the segmentation results. If a WMH was not visually present on an axial slice, a WMH was not delineated; conversely, if a WMH was present on the FLAIR but not included by the algorithm, this was manually corrected. The WMH masks included hyperintensities present in the basal ganglia nuclei. This is an area where both white and gray matter tissues are present and where white matter lesions might also occur in the gray matter (Wardlaw et al., 2013). We note that the anisotropic size of the FLAIR image is a limitation and WMH might thus be underestimated. Individual WMH maps were transformed into Montreal Neurological Institute 152 (MNI) standard space. First, the T1 images of individuals were nonlinearly registered to the MNI standard space using FLIRT (FMRIB's Linear Image Registration Tool 2002) and FNIRT (FMRIB's Non Linear Image Registration Tool, Andersson, Jenkinson, & Smith, 2010). Second, the FLAIR images were linearly registered to T1 images, after which these transformation matrices were combined to register the WMH masks to the MNI space for each individual and subsequently binarized using the tool fslmaths from the software FSL (version 5.3, Jenkinson, Bannister, Brady, & Smith, 2002). The images of the WMH of all patients were visually inspected to check the quality of the MNI alignment.
2.5 VLSM analysis
In order to identify WMH locations critical for language and executive functions, participants' WMH maps and neuropsychological data were analyzed using univariate VLSM2 (version 2.55) in MATLAB. In VLSM, a t test is performed at every voxel, comparing test scores of individuals with and without a lesion in each voxel. We compared test scores of each test (i.e., Category Fluency, all tasks of the SCWT test and SDMT) separately. A minimum threshold for lesion voxels overlap was set at 4% of the total sample (n = 18). Setting a minimum lesion overlap threshold is common practice in VLSM studies in order to ensure statistical power, reduce the spatial bias and improve the anatomical validity (Sperber & Karnath, 2017). The voxel-wise alpha threshold was set at .05. The Type 1 family-wise error rate was controlled for at the nominal alpha level of .05 through permutation testing. The cut-off for a significant cluster size was determined based on 6,000 permutations. In addition, all VLSM analyses included lesion volume, age, education, and sex as covariates because these variables are known to influence the used cognitive measures. Statistical power was calculated using the Dixon-Massey approximation, as implemented in the VLSM2 toolbox, based on an effect size of d = .8 at an alpha-level of .05. Even though this power calculation may be overestimated, it still enables an evaluation of power in one hemisphere or site (anterior vs. posterior) relative to the others, assuming similar effect sizes across tasks.
Additional VLSM analyses were also conducted. The VLSM analyses of the Category Fluency test and the SCWT Color-Word Naming subtest were repeated after correcting the scores for processing speed. We adjusted the scores of the SCWT Color-Word Naming subtest and Category Fluency for the baseline performance on the SCWT Word Reading and Color Naming subtests (see for example, Periáñez, Lubrini, García-Gutiérrez, & Ríos-Lago, 2020) using the interference ratio score (van der Elst et al., 2006a, 2006b) in order to obtain a more valid estimate of executive function and language abilities.
Finally, the VLSM analyses were repeated post hoc after excluding participants with microbleeds and lacunes in the caudate nucleus and adding the presence of microbleeds and lacunes elsewhere than in the caudate nucleus as covariates (categorical, present or absent) for the remaining sample. This was done to ensure that the results of the analyses were not driven by the presence of microbleeds and lacunes that were not included in the lesion masks.
3 RESULTS
Our sample included 442 participants with a mean age of 65.4 years (SD 8.8); 55% of the participants were male. The mean number of years of education was 10.8 (SD 3.5) and their mean Mini-Mental State Examination score was 28.2 (SD 1.6). A summary of the demographic and neuroimaging characteristics of the sample is given in Table 1. Means and standard deviations of the participants' scores on the Category Fluency (number of professions in 60 s), the SCWT Word Reading, Color Naming and Color-Word Naming (time to complete a card in s), and the SDMT (number of correct items completed in 60 s) are shown in Table 2. Figure 1a shows the distribution map of WMH overlap, indicating the number of people with overlapping lesion in each voxel. Figure 1b shows the distribution map of the statistical power. Voxels in red indicate where we had 96% power to detect an effect. The power map shows that anterior and posterior areas in both hemispheres had sufficient power coverage.
| Demographics | Study cohort (N = 442) |
|---|---|
| MMSE mean (SD); range | 28.2 (1.6); 22–30 |
| Age, mean (SD); range | 65.4 (8.8); 50–85 |
| Male (%) | 246 (55) |
| Education years (SD); range | 10.8 (3.5); 5–17 |
| Neuroimaging | |
| White matter lesion volume (ml) mediana | 3.4 |
| Lacunar infarct (%) | 110 (25) |
| Microbleeds (%) | 74 (17) |
- Note: MMSE, maximum score is 30, cutoff of cognitive impairment is <24.
- Abbreviations: ICV, intracranial volume; MMSE, Mini Mental State Examination; WMH, white matter hyperintensity.
- a
Corrected for ICV formula:
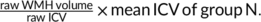
| Cognitive test | Study cohort (N = 442) |
|---|---|
| Category Fluency: mean number of professions (SD); range | 16.4 (5.3); 3–35 |
| SCWT (in s) | |
| Word Reading (SD); range | 25.8 (6.4); 15–60 |
| Color Naming (SD); range | 33.5 (8.2); 18–87 |
| Color-Word naming (SD); range | 64.1 (2.3); 31–183 |
| SDMT: mean number correct (SD); range | 27.3 (2.3); 6–55 |
- Abbreviations: SCWT, Stroop Color Word Test; SDMT, Symbol Digit Modalities Test.
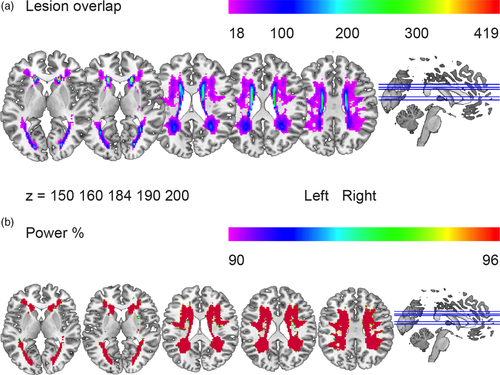
Figure 2a shows the significant voxels associated with worse scores in the Category Fluency test. A statistically significant cluster was found (p = .048), with a peak within the right cingulum, also including the forceps minor, caudate nucleus and ATR (internal capsule) in the right hemisphere. The VLSM map for SCWT Word Reading is shown in Figure 2b. A statistically significant cluster was found (p = .012), with a peak at the anterior right corpus callosum, also including the forceps minor, caudate nucleus and ATR (internal capsule) in the right hemisphere. In Figure 2c, the VLSM results of the SCWT Color-Naming task are shown. Three statistically significant clusters were found. The largest cluster (p = .012) had a peak in the right anterior corpus callosum, the second cluster (p = .013) had a peak in the left anterior corpus callosum, and the third cluster (p = .029) had a peak in the posterior segment of the arcuate fasciculus. Additional voxels extended into the forceps minor, caudate nucleus, and ATR (internal capsule) in the right and left hemispheres.
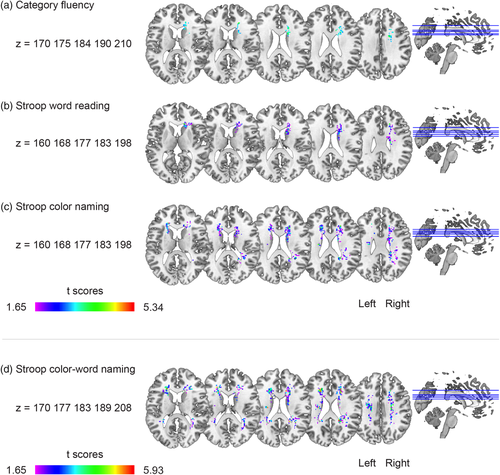
In Figure 2d, the results of SCWT Color-Word naming are shown. Two statistically significant clusters were found. The peak of the largest cluster (p = .001) was located at the posterior part of the right corpus callosum and of the second cluster (p = .001) at the anterior part of the left corpus callosum. The remaining voxels were located in the forceps minor, caudate nucleus, and ATR (internal capsule) in the right and left hemispheres.
The results of the VLSM for the SDMT were not significant after adjusting for lesion size, age, sex, and education (p = .389).
3.1 Processing speed correction and post hoc analyses
Since performance on the Category Fluency test and SCWT Color-Word Naming can also be driven by processing speed, which is known to be affected in SVD, we repeated the VLSM analyses after adjusting for processing speed (e.g., Oosterman et al., 2010). The results of these analyses were similar to the results uncorrected for processing speed, as shown in Figure 3a,b and Table 3. All reported associations of symptom-lesion location remained unchanged following additional analyses that controlled for the presence of microbleeds and lacunes and excluded participants with lacunes and microbleeds in the caudate nucleus.
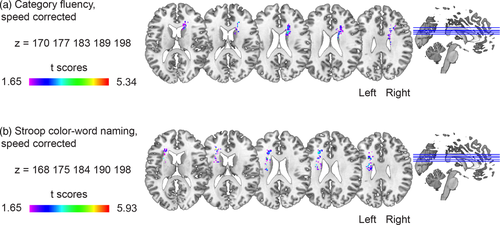
| Task | N voxels in cluster | Peak t value | MNI coordinates at peak | Anatomical labels (Catani & Thiebaut de Schotten, 2012; Rorden & Brett, 2000) |
|---|---|---|---|---|
| Category Fluency | 232 | 3.7 | 16, 2, 34 | Right cingulum |
| Category Fluency, corrected for processing speed | 281 | 3.6 | 18, 8, 22 | Right corpus callosum |
| SCWT Word Reading | 433 | 4.0 | 14, 26, 8 | Right corpus callosum |
| SCWT Color Naming | ||||
| Cluster 1 | 426 | 5.1 | 20, 24, 22 | Right corpus callosum |
| Cluster 2 | 421 | 5.3 | −18, 32, 14 | Left corpus callosum |
| Cluster 3 | 345 | 4.2 | 34, −44, 18 | Right arcuate posterior segment |
| SCWT Color-Word Naming | ||||
| Cluster 1 | 857 | 5.0 | 18, −42, 24 | Right corpus callosum |
| Cluster 2 | 822 | 5.0 | −22, 28, 16 | Left corpus callosum |
| SCWT Color-Word Naming, corrected for processing speed | 502 | 5.0 | −26, 30, 18a | Left corpus callosum |
- Abbreviations: MNI, Montreal Neurological Institute 152; SCWT, Stroop Color Word Test; VLSM, voxel-based lesion–symptom mapping.
- a Label for MNI coordinates: −22, 30, 18 from natbrainlab atlas.
4 DISCUSSION
In the present study, our first aim was to assess the relation between the location of white matter lesions and performance on language and executive tasks in patients with SVD without dementia. Our second aim was to investigate whether executive and language measures are associated with the same location of WMH. The results of our study provide the first evidence that WMH in the thalamic radiations, caudate nuclei, and corpus callosum are associated with lower performance in tasks with language and executive components in SVD without dementia. The tests involving language abilities showed the strongest relationship with the WMH, whereas the SDMT, which required no language use for its execution, showed no such relationship. The effects were found either bilaterally or in the right hemisphere for Category Fluency and Word Reading. The locations identified for the tasks tapping more heavily into language components were similar to those identified for the more executive measures. Taken together, our results suggest that damage to the white matter in subcortical networks, as found in SVD, might lead to more generalized cognitive changes that influence both verbal and executive abilities. The findings are only partly in line with our hypotheses. Although our hypothesis was confirmed that similar regions would be identified for all tasks on the language-executive continuum, our hypothesis that tasks tapping more into the language domain would be associated with lesioned voxels in left anterior white matter tracts was not supported by our data. We discuss these findings in more detail below.
We ran each VLSM analysis on individual tests tapping into a language-executive continuum. This allowed us to test the hypothesis of whether executive and language measures are associated with WMH in the same or different white matter locations. Our finding that WMH in the thalamic radiations and corpus callosum are associated with lower executive functioning in SVD is in line with previous findings (Biesbroek et al., 2017). We did not find an association between WMH location and processing speed (SDMT), while in previous studies (Biesbroek et al., 2016; Duering et al., 2014), such an association was found. The lack of association in the present study might be due to insufficient power. Statistical power varies as a function of sample size and effect size. Given that we kept the sample size constant (i.e., the same group for all tasks), not finding an effect for SDMT would entail that the effect size is lower than for the other tasks we used (i.e., the relationship between SDMT and WMH is weak). Previous studies that found an association between processing speed and WMH location had either a cohort with mixed forms of dementia and Alzheimer's disease and, thus, likely more severe deficits (Biesbroek et al., 2016), or found only weak associations once statistical correction for lesion size was used (Biesbroek et al., 2016; Duering et al., 2014). Since these previous studies did not investigate the association between WMH location and language versus processing speed tasks in the same cohort, they do not provide a comparison of effect sizes. Our results suggest that tasks requiring language use have a stronger association with WMH location than processing speed tasks.
Although language functioning is classically associated with perisylvian areas, recent evidence indicates that other cortical and subcortical areas are also critical for language (Chenery, Copland, & Murdoch, 2002; Hope, Seghier, Leff, & Price, 2013). Moreover, current theories on language widely converge on the idea that language production is strongly dependent on executive functioning (Roelofs & Piai, 2011). For example, the model of Crosson et al. (2007) proposes that the presupplementary motor area-basal ganglia loop is involved in word generation (Bohsali Anastasia, 2012). The subcortical circuitry we identified, and its overlap for both language production and executive tasks, are well in line with these most recent lines of research. Among the structures of the basal ganglia, the caudate nucleus has a well-established contribution to language tasks (Barbas et al., 2013; Chouiter et al., 2016; Da Cunha et al., 2015; Duffau, Moritz-Gasser, & Mandonnet, 2014). Executive-language functions may not be impaired by disruption of a single brain region but are likely to depend on a more distributed network of regions (Baldassarre, Metcalf, Shulman, & Corbetta, 2019; Fridriksson et al., 2018) that include the caudate nucleus.
The finding of a right-hemisphere effect for Category Fluency and Word Reading was unexpected given the predominance of left-hemisphere language dominance in the general population. This finding could reflect a bias in our inclusion, as patients with more left-hemisphere WMH would potentially present with worse language abilities. However, since the RUN DMC study protocol was designed independently from the goal of the present study, patients with severe language problems were excluded. Moreover, the lesioned voxels associated with Category Fluency could be found bilaterally given the involvement of general domain-general networks in certain language tasks (e.g., Obler et al., 2010). It would be important for future studies to include SVD patients with more severe communication impairments. Nonetheless, this issue does not undermine our finding that damage to the white matter in subcortical networks leads to more general cognitive effects affecting language and executive abilities.
This study has a number of strengths. First of all, to the best of our knowledge, this is the largest lesion-symptom mapping study on SVD applying correction for lesion volume, age, sex and education while examining the effect of WMH location on cognitive functions at the level of individual neuropsychological tests. Second, participants in this study were a homogeneous group of sporadic SVD without dementia or severe cognitive impairment. This is a suitable way to study SVD because once severe cognitive impairment arises, other pathologies possibly underlying the impairment may be interacting with SVD pathology, acting as a confound.
An important limitation of the present study is that we used a limited set of language tests. More comprehensive language testing, and in particular tests more sensitive to detecting subtle deficits, for example using the efficiency of lexical access (Moritz-Gasser, Herbet, Maldonado, & Duffau, 2012), should be used for a better understanding of the role of subcortical networks for language functioning in SVD. Another limitation is due to the anisotropic size of the FLAIR image, which might have contributed to an underestimation of the presence of WMH. Finally, microbleeds and lacunes should be investigated in future studies as well but these lesions were not the focus of the present investigation.
Our results suggest that the presence of WMH in the corpus callosum, the ATR and caudate nucleus might be an element of special clinical relevance in SVD. With respect to the corpus callosum and ATR in particular, they may form an important crossroad of fibers that, when lesioned, may have a negative effect on cognitive function (Corbetta et al., 2015; Griffis, Nenert, Allendorfer, & Szaflarski, 2017). This intersection might be part of an important area associated with broader executive-language functions that is vulnerable to the damage of SVD. Therefore, sensible tests able to capture subtle language changes could be performed in SVD patients with white matter lesions in this crossroad.
5 CONCLUSIONS
To conclude, our study provides evidence that worse language and executive abilities are associated with WMH in common areas, that is, the bilateral thalamic radiations, caudate nuclei and forceps minor in SVD without dementia. Our findings, together with previous literature (Biesbroek et al., 2016; Duering et al., 2014), suggest that these areas may be part of a crossroad that is important for general cognitive functioning contributing to performance in executive-language tasks (Humphreys, Hoffman, Visser, Binney, & Lambon Ralph, 2015). Future studies should take into consideration the location of the WMH while investigating the cognitive profile of SVD as this could contribute to improving the precision of the prognosis of this disease. In addition, the role of the right hemisphere in language functioning in SVD needs to be further elucidated. Finally, more detailed language testing is required in patients with SVD to understand the precise basis of these language production difficulties and their association with the progression of the disease.
ACKNOWLEDGMENTS
This research was supported by Gravitation grant of the Language in Interaction Consortium (024.001.006) and by a Veni grant (451-17-003) to V. P. from the Netherlands Organization for Scientific Research, as well as by the Innovational Research Incentive grant (016-126-351) and the Clinical established investigator Dutch Heart Foundation grant (2014 T060) to F. E. L. and by a junior staff member grant (2016 T044) to A. M. T. from the Dutch Heart Foundation.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
The Medical Review Ethics Committee region Arnhem-Nijmegen approved the study.
PATIENT CONSENT STATEMENT
All participants signed an informed consent form.
Open Research
DATA AVAILABILITY STATEMENT
Anonymized data can be made available to qualified investigators on request to the corresponding author.