Unsupervised clustering of dopamine transporter PET imaging discovers heterogeneity of parkinsonism
Funding information: National Research Foundation of Korea, Grant/Award Numbers: NRF-2019K1A3A1A14065446, NRF-2019R1F1A1061412
Abstract
Parkinsonism has heterogeneous nature, showing distinctive patterns of disease progression and prognosis. We aimed to find clusters of parkinsonism based on 18F-fluoropropyl-carbomethoxyiodophenylnortropane (FP-CIT) PET as a data-driven approach to evaluate heterogenous dopaminergic neurodegeneration patterns. Two different cohorts of patients who received FP-CIT PET were collected. A labeled cohort (n = 94) included patients with parkinsonism who underwent a clinical follow-up of at least 3 years (mean 59.0 ± 14.6 months). An unlabeled cohort (n = 813) included all FP-CIT PET data of a single-center. All PET data were clustered by a dimension reduction method followed by hierarchical clustering. Four distinct clusters were defined according to the imaging patterns. When the diagnosis of the labeled cohort of 94 patients was compared with the corresponding cluster, parkinsonism patients were mostly included in two clusters, cluster “0” and “2.” Specifically, patients with progressive supranuclear palsy were significantly more included in cluster 0. The two distinct clusters showed significantly different clinical features. Furthermore, even in PD patients, two clusters showed a trend of different clinical features. We found distinctive clusters of parkinsonism based on FP-CIT PET-derived heterogeneous neurodegeneration patterns, which were associated with different clinical features. Our results support a biological underpinning for the heterogeneity of neurodegeneration in parkinsonism.
1 INTRODUCTION
Parkinsonism is a clinical syndrome with distinctive disease entities, including idiopathic Parkinson's disease, multiple system atrophy (MSA) and progressive supranuclear palsy (PSP). By sharing dopaminergic neurodegeneration in common, parkinsonism can be characterized by a combination of clinical features such as bradykinesia, rigidity, resting tremor, and postural instability (Hughes, Daniel, & Lees, 1993; Jankovic, 2008). Despite considerable common motor symptoms, clinical presentations and prognosis of parkinsonism are heterogeneous among each of disease entities (Aleksovski, Miljkovic, Bravi, & Antonini, 2018; Fereshtehnejad et al., 2015; Foltynie, Brayne, & Barker, 2002; Marras & Chaudhuri, 2016; Marras & Lang, 2013; Thenganatt & Jankovic, 2014; Titova, Padmakumar, Lewis, & Chaudhuri, 2017; van Rooden et al., 2011). Because of broad clinical overlap, an objective and reproducible biomarker based on the neurodegeneration pattern is needed to better understand the disease progression and to predict patient's prognosis in a personalized manner, which may eventually aid establishing efficient treatment strategies of parkinsonism (Espay et al., 2017; Tolosa, Wenning, & Poewe, 2006).
The dopamine transporter (DAT) is known to be the single most important factor of extracellular dopamine concentration, reflecting the axonal dysfunction (Nutt, Carter, & Sexton, 2004; Saari et al., 2017). DAT imaging does not directly reflect dopaminergic neurons, but still, as from the previous study, showing a positive correlation of DAT binding and symptom duration, it reflects the dopaminergic neuronal functionality (Saari et al., 2017). Furthermore, the loss of dopaminergic function is associated with phenotypic changes in the nigrostriatal systems and affects clinical presentation in the early disease state (Kordower et al., 2013). DAT imaging using SPECT (Single-photon emission computed tomography) or positron emission tomography (PET) is a well-established method for evaluating dopaminergic neurodegeneration. Thus, it has now been widely used due to the clinical utility in the diagnosis of parkinsonism (Catafau, Tolosa, & Da, 2004). In particular, 18F-fluoropropyl-carbomethoxyiodophenylnortropane (FP-CIT) PET has a relatively higher resolution than SPECT, which enables us to find spatial patterns of neurodegeneration of parkinsonism(Lee et al., 2018; Oh et al., 2012). Accordingly, the heterogeneity of parkinsonism in terms of dopaminergic dysfunction and its related clinical presentation may be associated with the spatial degeneration patterns identified by FP-CIT PET.
In this study, we aimed to identify the heterogeneity of parkinsonism in terms of the spatial patterns of dopaminergic neurodegeneration. As a data-driven approach, a large dataset of FP-CIT PET was used to capture spatial patterns of FP-CIT PET and to find clusters. Since this approach is a type of work discovering different groups using only imaging patterns without clinical diagnostic labels, it is possible to propose a robust and reproducible parkinsonism cluster, which is expected to eventually be used as an imaging biomarker to objectively evaluate parkinsonism (Choi, Jin, & Initiative, 2018).
2 MATERIALS AND METHODS
2.1 Subjects
Two different cohorts were included for this study: labeled and unlabeled cohorts. The labeled cohort consists of 94 patients with parkinsonism who underwent FP-CIT PET in the early stage of the disease and then followed for at least 3 years (mean 59.0 ± 14.6 months) when a diagnostic re-appraisal was made. Diagnosis of PD, MSA-P (parkinsonian type), and PSP was made according to the clinical diagnostic criteria and all patients were assessed by neurologists specialized in movement disorders (Gilman et al., 2008; Hughes, Daniel, Kilford, & Lees, 1992; Litvan et al., 1996). Among these 94 patients, 49 patients were idiopathic Parkinson's disease, 17 patients were MSA-P and 10 patients were PSP. Sixteen patients had parkinsonism with uncertain diagnosis even at re-appraisal. One patient was found not to have parkinsonism through follow-up. Corresponding clinical features such as autonomic nerve symptom (ANS), cognitive deficit, dyskinesia, and freezing of gait (FOG) were assessed at the time of the last diagnosis. All patients underwent FP-CIT PET at baseline for differentiating parkinsonism, the mean period between motor symptom onset and FP-CIT PET was 1.98 ± 1.87 years. FP-CIT PET studies were acquired from July 2009 to Sep 2013.
Another cohort, the unlabeled cohort (n = 813), included all FP-CIT PET data acquired from Jan 2015 to June 2018. This cohort included all FP-CIT PET data acquired in a single-center, which were acquired for evaluating patients with parkinsonism and other neurodegenerative disorders.
This study was approved by the Institutional Review Board of our institute, and informed consent was waived as a retrospective design. All procedures performed in this study were under the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
2.2 FP-CIT PET/CT acquisition
As a clinical routine protocol, patients underwent PET/CT imaging, 2 hr after 185 MBq (5 mCi) of 18F-FP-CIT injection. Emission scans were acquired for 10 min using dedicated PET/CT scanners (Biograph 40 or mCT, Siemens, Erlangen, Germany), followed by CT scans for attenuation correction. PET images were reconstructed by an iterative algorithm (ordered-subset expectation maximization, OSEM) with 24 subsets and 5 iterations. Images were reconstructed with the same matrix size with 256 × 256. Four millimeters of Gaussian post-reconstruction filter was applied.
2.3 Image preprocessing and calculation of binding ratio
All the PET images were spatially normalized into an in-house 18F-FP-CIT PET template (Choi et al., 2016; Kim et al., 2012). The spatial normalization was performed using statistical parametric mapping software (SPM8, University College of London, London, UK). Voxel counts were changed to binding ratio, defined as BR = Cspecific/Cnonspecific, where C represented PET counts. Mean counts of occipital cortex were regarded as nonspecific binding, Cnonspecific. Automated Anatomical Labeling (AAL) was used for predefined volume-of-interests to define the occipital cortex as well as the striatum. The voxel size of spatially normalized BR maps was 2.0 × 2.0 × 2.0 mm. The binding ratio of the putamen and caudate was obtained by the predefined AAL VOIs. BR of bilateral putamen and caudate were calculated by the volume-of-interests and mean values were used. We also included qualitative evaluation of FP-CIT PET of all data using the formal reading reports. All reports of all FP-CIT PET was made by a consensus of more than two nuclear medicine brain imaging experts. We classified all FP-CIT PET images into two classes of whether imaging patterns suggested dopaminergic neurodegeneration or not.
2.4 Unsupervised clustering of FP-CIT PET data
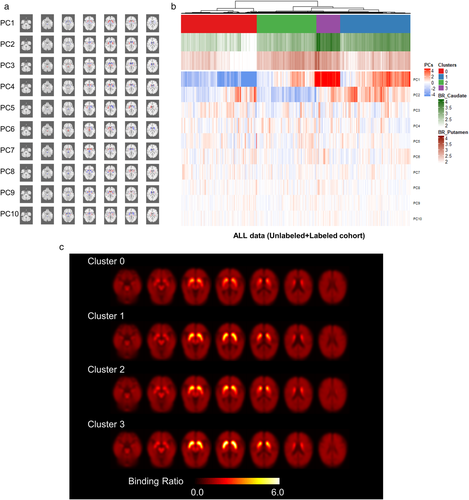
All FP-CIT PET data (n = 907) were clustered according to the brain BR of FP-CIT patterns. Firstly, principal component analyses (PCA) were applied for data dimension reduction. PCA determines a set of linearly uncorrelated features that account for variability in a dataset. All spatially normalized FP-CIT PET data were changed to a matrix Mi,j, where i is the number of subjects and j is the number of voxels of the brain. Principal components (PCs) were computed from the SVD (singular value decomposition) of the matrix. The matrix was centered by subtracting the mean image and then decomposed to n components: M = USVT. PCs are given by SVT and the contribution of the kth PC to the ith subject is given by the U(i,k). We extracted 10 PCs from FP-CIT PET data.
Samples were clustered by a hierarchical clustering method. Using PCs of all FP-CIT PET data, the similarity was determined by the average distance between elements of each hierarchical cluster. A given cutoff of the hierarchical dendrogram provides a number of clusters, resulting in four clusters that reflect FP-CIT PET patterns.
2.5 Data visualization by 2-dimensional projection
To intuitively visualize FP-CIT PET patterns, another dimension reduction method, t-distributed stochastic neighborhood embedding (t-SNE), was employed (Maaten & Hinton, 2008). t-SNE retains local similarities between data in a way that similar FP-CIT PET are modeled by nearby points and dissimilar samples are modeled by distant points. The similarity between data is modeled as Gaussian with a given number of neighbors, perplexity. Here, we set the perplexity to 30 and we reduced dimension to two axes.
2.6 Statistics
As all data including the labeled and unlabeled cohorts were clustered by FP-CIT PET patterns, final clinical diagnosis, and clinical symptoms were compared for patients with different clusters in the labeled cohort. In particular, clinical symptoms including the freezing of gait, autonomic nerve symptoms, and dyskinesia were compared for patients with different clusters. Student's t-test was used to compare continuous variables including BR of two different clusters. The differences between groups were considered statistically significant at p-value < .05. Pearson's chi-square test was performed to analyze the categorical variables including clinical symptoms.
3 RESULTS
3.1 Four clusters identified from FP-CIT PET patterns
Brief demographics of labeled and unlabeled cohorts were summarized in Table 1. PCs, the sets of linearly uncorrelated image-based patterns, were extracted from all FP-CIT PET data (Figure 1a). The anatomical location of peak voxels is summarized in Table S1. Notably, these patterns were extracted from all subjects using a data-driven unsupervised manner without labels such as clinical diagnosis. Imaging patterns were divided into four clusters by hierarchical clustering. A heatmap for PCs of all cohorts and conventional BR quantification results is presented in Figure 1b. Averaged FP-CIT PET images across the patients with the same cluster was represented in Figure 1c.
| Labeled cohort (n = 94) | Unlabeled cohort (n = 813) | |
|---|---|---|
| Age | 61.9 ± 10.3 (range: 30.4–80.5) | 67.5 ± 10.4 (range: 12.9–95.9) |
| Sex | M:F = 41:53 | M:F = 357:456 |
| Clusters | 0–41 1–5 2–47 3–1 |
0–258 1–272 2–189 3–94 |
| Diagnosis | Parkinson's disease – 49 Progressive Supranuclear palsy – 10 Multiple system atrophy (Parkinsonian type) – 17 Diffuse Lewy body disease – 1 Other parkinsonism – 16 Non-parkinsonian disorder – 1 |
N/A |
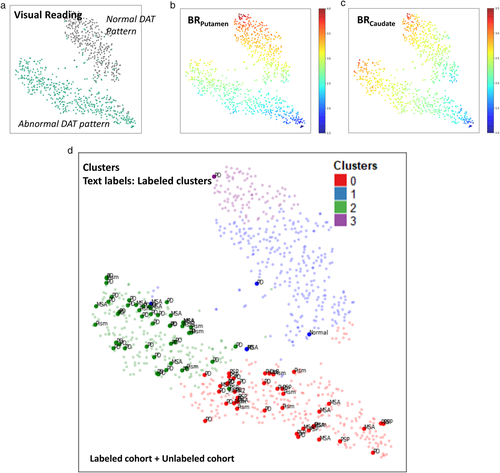
All data were intuitively visualized by 2-dimensional embedding using t-SNE plots (Figure 2, Figure S1). All images were divided into visually normal and abnormal patterns according to the formal report which includes visual interpretation of FP-CIT PET (Figure 2a). By mapping with the quantification of caudate and putamen BR, Putamen and caudate BR were differently associated with data distribution patterns on the t-SNE plot (Figure 2b,c). The t-SNE map was also plotted with the four clusters (Figure 2d). The cluster 0 and cluster 2 were mostly overlapped with visually abnormal patterns and cluster 1 and cluster 3 were mostly overlapped with visually normal patterns.
3.2 FP-CIT PET-based clusters associated with different diagnosis of parkinsonism
We then applied the clusters to the labeled cohort, which included 94 patients who were clinically diagnosed as Parkinson's disease, PSP, MSA-P, or parkinsonism with uncertain diagnosis (Figure 3a). Cluster 0 and cluster 2 showed different patterns particularly in PC1 and PC2, while PC3 of cluster 0 and cluster 2 were not significantly different (Figure S2). Notably, PC1 was associated with BR of the whole putamen, while PC2 was associated with BR of posterior putamen. The frequency of clinical diagnosis was significantly different according to the FP-CIT PET-based clusters (chi-square = 35.4, p = .0004) (Figure 3b,c). Among 94 patients, 88 patients with parkinsonism were classified as either cluster 0 or cluster 2. PSP was significantly more classified as the cluster 0 compared with other clusters (chi-square = 9.7, p = .002). In cluster 2, only one patient was PSP, while nine patients with PSP were classified as cluster 0 (Figure 3b). The deviation of diagnosis in different clusters were presented by an association plot (Figure 3c). The colored bar indicated more frequent diagnosis in the cluster.
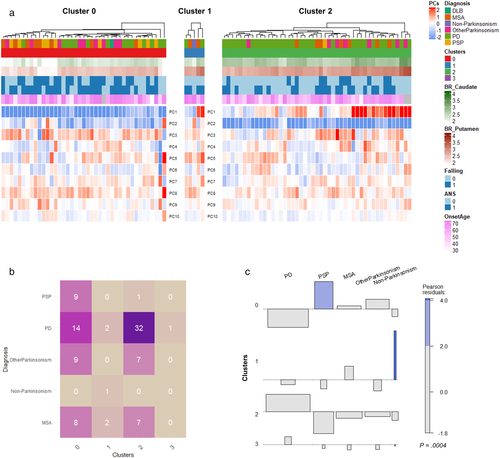
Onset age and disease duration of both clusters were not significantly different (cluster 0 vs. cluster 2:60.4 ± 11.6 vs. 58.1 ± 9.0 and 2.3 ± 1.8 vs. 2.3 ± 1.7 for onset age and duration between onset to PET scan, respectively). Patients in the cluster 0 showed poor response to levodopa treatment compared with cluster 2 (73.2% and 89.3% in cluster 0 and cluster 2, respectively, p = .049). FOG (cluster 0 vs. cluster 2; 53.6% vs. 23.4%, p = .003), ANS (70.7% vs. 46.8%, p = .023) and cognitive deficit (39.0% vs. 14.9%, p = .010) were significantly more common in cluster 0 (Table 2).
| Features | Cluster 2 (n = 47) | Cluster 0 (n = 41) | p-Value |
|---|---|---|---|
| Onset age | 58.1 ± 9.0 | 60.4 ± 11.6 | N.S. |
| Duration (onset to scan) | 2.3 ± 1.8 | 2.3 ± 1.7 | N.S. |
| Symptoms | |||
| DOPA response | 42 (89.3%) | 30 (73.2%) | .049 |
| Freezing of gait | 11 (23.4%) | 22 (53.6%) | .003 |
| Autonomic nerve symptoms | 22 (46.8%) | 29 (70.7%) | .023 |
| Cognitive deficit | 7 (14.9%) | 16 (39.0%) | .010 |
| Dyskinesia | 12 (25.5%) | 7 (17.1%) | N.S. |
3.3 Difference of clinical presentation in FP-CIT PET-based clusters of Parkinson's disease patient
Among 49 Parkinson's disease patients, 32 were included in the cluster 2 and 14 were included in the cluster 0. The Parkinson's disease patients with the different clusters show different patterns of clinical features as well as FP-CIT BR. Both BR of putamen and caudate were significantly lower in Parkinson's disease patients of the cluster 0 than those of the cluster 2 (2.44 ± 0.29 vs. 2.85 ± 0.21, p = .0001 for putamen; 2.33 ± 0.25 vs. 2.75 ± 0.19, p < .0001 for caudate) (Figure S3). Onset age and disease duration were not significantly different between two clusters (cluster 0 vs. cluster 2; 56.2 ± 12.6 vs. 55.4 ± 9.0 and 1.71 ± 1.38 vs. 2.16 ± 1.69 years, onset age and disease duration, respectively) (Figure S3). FOG was significantly more common in the cluster 0 (35.7% and 9.4% of Parkinson's disease patients in cluster 0 and cluster 2, respectively, p = .03). On the other hand, dyskinesia appeared more common in the Parkinson's disease patients in the cluster 2, though it did not reach a statistical significance (34.4% for the cluster 2 vs. 14.3% for the cluster 0, p = .16) (Table 3).
| Symptoms | Cluster 2 (n = 32) | Cluster 0 (n = 14) | p-Value |
|---|---|---|---|
| DOPA response | 32 (100%) | 14 (100%) | N.S. |
| Freezing of gait | 3 (9.4%) | 5 (35.7%) | .03 |
| Autonomic nerve symptoms | 12 (37.5%) | 6 (42.9%) | N.S. |
| Cognitive deficit | 2 (6.3%) | 1 (7.1%) | N.S. |
| Dyskinesia | 11 (34.4%) | 2 (14.3%) | .16 |
4 DISCUSSION
In the present study, four distinct clusters were defined according to the spatial patterns of dopaminergic degeneration on DAT imaging. Two clusters, cluster 0 and cluster 2, mainly comprised DAT images with a visually abnormal pattern, which has been a clinically routine and conventional qualitative interpretation. When the labeled cohort, which included patients with parkinsonism according to the clinical diagnosis, was analyzed with corresponding clusters, Parkinson's disease and other atypical parkinsonism mostly occupied cluster 0 and cluster 2. These two clusters showed a different distribution of disease entities, diagnosis based on 3 years follow-up, and distinctive clinical features. Furthermore, even among patients with the diagnosis of Parkinson's disease, different clinical features were observed between two clusters. Our finding suggested the heterogeneity of parkinsonism in terms of the various dopaminergic neurodegeneration patterns.
The image patterns used for the clustering were in a similar vein with previous studies regarding different sub-regional DAT loss patterns according to each entity of parkinsonism. Kahraman et al. revealed visually distinct DAT patterns between atypical parkinsonism and idiopathic Parkinson's disease using 123I-FP-CIT SPECT (Kahraman, Eggers, Schicha, Timmermann, & Schmidt, 2012). Diffuse and severe DAT loss pattern was a predictive marker for atypical parkinsonism. More recently, PSP patients show extensive and symmetric DAT loss across the whole striatum, while Parkinson's disease and MSA-P patients show relatively preserved binding in the caudate nucleus and anterior putamen (Antonini et al., 2003; Filippi et al., 2006; Oh et al., 2012). These DAT image studies were supported by the Post-mortem study revealing excessive neuronal loss in PSP compared with Parkinson's disease (Murphy, Karaconji, Hardman, & Halliday, 2008). In our results, cluster 0 mainly showed an extensive DAT loss in the whole striatum associated with PC1, and atypical parkinsonism patients, specifically PSP, were mainly included in this cluster. Cluster 2, which was more relevant to typical Parkinson's disease, showed relatively preserved DAT binding in the anterior portion of the putamen and relatively decreased DAT binding in the posterior putamen, related to reduced PC2 (Figure 1c).
The heterogeneous neurodegeneration patterns of FP-CIT PET proved its clinical implication by showing distinctive clinical presentations among the labeled cohort of parkinsonism patients. Cluster 0, which mainly represents atypical parkinsonism, showed clinical characteristics of significantly poor treatment response, high FOG, high frequency of ANS, and more cognitive deficit. Parkinson's disease patients who were allocated in the cluster 0 also showed significantly high FOG presentation, but relatively low dyskinesia. Previous subtype studies reported that, in addition to the cognitive deficit, FOG and lack of dyskinesia, which implies poor dopamine response, were relevant to Parkinson's disease subtypes defined as diffuse/malignant (Fereshtehnejad et al., 2015) and Postural Instability/gait disturbance (Aleksovski et al., 2018), and also, it is known to be a commonly observed feature of atypical parkinsonism, including PSP, MSA-P, and dementia with Lewy bodies (Giladi, Kao, & Fahn, 1997). ANS is a key configuration for Parkinson's disease subtyping and is a characteristic feature of MSA-P. These clinical features, all in common, are related to severe disease manifestation and poor prognosis, which might be the clinical characteristic of cluster 0. Poor levodopa response observed in our study further refine the characteristics of this cluster. On the other hand, although not significant, patients allocated in the cluster 2 show a high prevalence of dyskinesia, which is associated with levodopa responsiveness, and thus, favors a diagnosis of Parkinson's disease over atypical parkinsonism (Postuma et al., 2015). As future work, further in-depth investigation regarding the clinical outcome of these different two clusters in parkinsonism patients could clarify the association of heterogeneous neurodegeneration patterns and prognosis.
Recently, many attempts using cluster analysis, based on diverse clinical features, have been made to identify distinct subtypes of Parkinson's disease (De Pablo-Fernandez, Lees, Holton, & Warner, 2019; Erro et al., 2016; Faghri et al., 2018; Fereshtehnejad et al., 2015; Lawton et al., 2018). Longitudinal follow-up studies revealed the relevance of subtype classification at an early stage with treatment response (Lawton et al., 2018), disease course, and survival (De Pablo-Fernandez et al., 2019). However, the lack of a valid and robust method for subtyping Parkinson's disease only based on clinical features has made it fail to reproduce in a clinical trial cohort (Mestre et al., 2018). More specifically, previous subtyping systems used a combination of diverse phenotypes of the disease, which was hard to be supported by a plausible explanation of the pathophysiologic process such as neurodegeneration of Parkinson's disease. Such a lack of reproducibility and consideration for pathophysiology have limited widely acceptable subtyping system in the clinic. On the other hand, our data-driven approach based on unsupervised clustering of imaging employing a relatively large dataset is robust, since there is little room for ambiguous and subjective elements to intervene. Thus, FP-CIT PET-based clustering methods could be objective and reproducible. Furthermore, as FP-CIT PET directly reflects the DAT expression and dopaminergic function (Ba & Martin, 2015; Saari et al., 2017), our approach could lead to suitable biomarker development for subtyping of Parkinson's disease by explaining the heterogeneous neurodegeneration patterns.
Some limitations should be noted. Firstly, validated motor scales, such as UPDRS (Unified Parkinson's Disease Rating Scale) for PD, UMSARS (Unified Multiple System Atrophy Rating Scale) for MSA, were not performed in the labeled cohort. Despite the absence of disease-specific symptom scales, patients were regularly examined for a sufficiently long-term period, and at every visit reevaluated to consolidate the clinical diagnosis. Secondly, as a single institute retrospective study, the number of patients with MSA-P and PSP was relatively limited. However, we speculate that, since comprehensive diagnosis was made through long term clinical assessment, each group would well reflect the disease entity. Nonetheless, prospective cohort studies with a larger number of patients and comprehensive neurological symptom evaluation will be necessary to solidify the clinical significance of our data-driven approach. Furthermore, a longitudinal follow-up study will corroborate whether the FP-CIT PET-based clusters have a clinical value to predict disease progression and outcome.
The unsupervised clustering of FP-CIT PET showed a heterogeneous pattern of dopaminergic neurodegeneration in patients with parkinsonism. These clusters were associated with different diagnosis of parkinsonism. Furthermore, the results of distinctive clinical features observed between different clusters in Parkinson's disease as well as all patients with parkinsonism, support the clinical heterogeneity of parkinsonism in terms of dopaminergic neurodegeneration. Furthermore, we expect that our approach of a quantitative assessment of degeneration patterns using PCs and FP-CIT PET PET-based clusters can be used at each patient level. Although further clinical validation studies are needed, this approach may be applied to predicting the course of clinical symptoms particularly in patients with early parkinsonism as well as Supporting Information on differential diagnosis of parkinsonism. Our data-driven approach to identify the heterogeneity will lead to a better understanding of the disease and personalized management of the patient.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
AUTHOR CONTRIBUTIONS
Hongyoon Choi and Han-Joon Kim designed the study. Minseok Suh and Hongyoon Choi analyzed imaging data. Jin Hee Im and Han-Joon Kim collected and analyzed clinical data. Gi Jeong Cheon and Beomseok Jeon interpreted results and supported the analysis. All authors wrote and edited the manuscript. All authors approved the manuscript.
ETHICS STATEMENT
This study was approved by the Institutional Review Board of our institute, and informed consent was waived as a retrospective design. All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Open Research
DATA AVAILABILITY STATEMENT
Our PET data with anonymized personal information will be accessible to readers upon reasonable request and following the completion of our ongoing projects.




