Heterogeneity of the astrocytic AMPA-receptor transcriptome
Funding information: Deutsche Forschungsgemeinschaft (DFG), Grant/Award Number: KL 1168/7-1
Abstract
Astrocytes form the largest class of glial cells in the central nervous system. They serve plenty of diverse functions that range from supporting the formation and proper operation of synapses to controlling the blood–brain barrier. For many of them, the expression of ionotropic glutamate receptors of the AMPA subtype (AMPARs) in astrocytes is of key importance. AMPARs form as macromolecular protein complexes, whose composition of the pore-lining GluA subunits and of an extensive set of core and peripheral complex constituents defines both their trafficking and gating behavior. Although astrocytic AMPARs have been reported to exhibit heterogeneous properties, their molecular composition is largely unknown. In this study, we sought to quantify the astrocytic AMPAR transcriptome during brain development and with respect to selected brain regions. Whereas the early postnatal pattern of AMPAR mRNA expression showed minor variation over time, it did show significant heterogeneity in different brain regions. Cerebellar astrocytes express a combination of AMPAR complex constituents that is remarkably distinct from the one in neocortical or hippocampal astrocytes. Our study provides a workflow and a first reference for future investigations into the molecular and functional diversity of glial AMPARs.
1 INTRODUCTION
Astrocytes represent the largest class of glial cells in the mammalian central nervous system (CNS) and are distributed throughout the whole brain. They are crucial for CNS development including synaptogenesis, they control ion homeostasis and neurotransmitter uptake, and they are important regulators of the blood–brain barrier (Allaman, Bélanger, & Magistretti, 2011; Allen, 2014; Haim & Rowitch, 2016). Astrocytes have traditionally been viewed as a rather uniform subpopulation of glial cells. However, growing experimental evidence demonstrates that astrocytes are highly diverse, with respect to not only their morphology but also their physiology (Matyash & Kettenmann, 2010; Zhang & Barres, 2010). Recent whole transcriptome and proteome analyses provide an exciting insight into the developmental and regional heterogeneity of astrocytes (Chai et al., 2017; Zhang et al., 2014).
Astrocytes may express neurotransmitter receptors, among them ionotropic glutamate receptors of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid subtype (AMPARs), which enable them to sense and respond to neuronal signaling. Electrophysiological studies indicate substantial heterogeneity of astrocytic AMPAR properties between brain regions. Thus, cerebellar Bergmann glia cells express inwardly rectifying and calcium-permeable AMPARs, which have been demonstrated to be crucial for the formation and maintenance of synapses between climbing fibers and Purkinje cells. Their genetic deletion leads to impaired motor performance and changes in eyeblink conditioning (Saab et al., 2012). Also, in both neocortex and brainstem, astrocytes show pharmacologically identifiable AMPAR currents and/or AMPAR calcium responses, respectively (Lalo, Pankratov, Kirchhoff, North, & Verkhratsky, 2006; McDougal, Hermann, & Rogers, 2011). In addition to neuron–glia interaction, they might be involved in glia-vascular signaling and control vasodilation (Parfenova et al., 2012). Whereas in thalamic nuclei, a subpopulation of astrocytes do express functional AMPARs, hippocampal astrocytes have been reported to be devoid of them, underlining a concept of significant astrocytic heterogeneity even beyond brain regions but within neuronal circuits (Chai et al., 2017; Israel, Schipke, Ohlemeyer, Theodosis, & Kettenmann, 2003; Matthias et al., 2003). Native AMPARs exist as macromolecular protein complexes. Their core is formed by tetrameric assembly of the pore-lining subunits GluA1–4 (Hollmann & Heinemann, 1994; Seeburg, 1993; Sobolevsky, Rosconi, & Gouaux, 2009) and members of the family of transmembrane AMPAR regulatory proteins (TARPs; Milstein, Zhou, Karimzadegan, Bredt, & Nicoll, 2007; Tomita et al., 2003), the cornichon homologs (CNIHs) 2 or 3 (Schwenk et al., 2009), and the germ cell- specific gene 1 like (GSG1l) protein (Schwenk et al., 2012; Shanks et al., 2012). Peripheral constituents of native AMPARs comprise the cysteine-knot AMPAR modulating proteins (CKAMPs) 44 and 52 (von Engelhardt et al., 2010), the soluble noelins (olfactomedins) 1–3, the proline-rich transmembrane proteins (PRRTs) 1 and 2 and Leucine-rich repeat transmembrane protein 4 (LRRTM4), and four isoforms of the membrane-associated guanylate kinase (MAGUK) family (Schwenk et al., 2012). Porcupine (PORCN) and ferric-chelate reductase 1-like protein (FRRS1l) seem to be rather transient complex constituents serving a role in subcellular processing of AMPARs along the secretory pathway (Brechet et al., 2017; Erlenhardt et al., 2016). The great number of complex constituents identified in previous comprehensive proteomic analyses strongly suggests an equally great molecular diversity of AMPARs in their regional, cellular, or even subcellular composition. Regional heterogeneity and developmental dynamics have recently been addressed in a high-resolution proteomic study of native AMPARs from brain (Schwenk et al., 2014). Due to methodological constraints, however, diverse cell types were sampled. Thus, the molecular composition of glial cell-specific AMPARs is still elusive.
Here, we sought to quantify the astrocytic AMPAR transcriptome during early postnatal brain development and with respect to selected brain regions. Our data will provide a first reference for future investigations into the molecular and functional diversity of glial AMPARs.
2 MATERIALS AND METHODS
2.1 Animals
For all experiments, hGFAP-GFP transgenic mice (FVB/N-Tg(GFAPGFP)14Mes/J, Jackson Lab) were used, in which the expression of GFP is controlled by the human GFAP (hGFAP) promoter. As a negative control for FACS experiments, FVB/N mice (Janvier Labs) were used. All experiments were in compliance with German law and were approved by the local authorities of the University of Düsseldorf.
2.2 Fluorescence activated cell sorting (FACS)
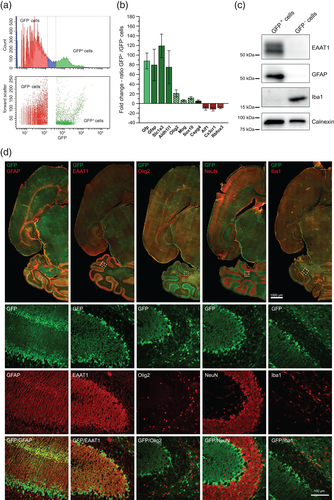
Mice were anesthetized, decapitated, and the brains were rapidly removed. For tissue dissociation, the Neural Tissue Dissociation Kit (Miltenyi Biotec) was used according to the manufacturer's instructions. Dissociated cells were harvested by centrifugation and cells of 3–4 littermates were pooled for FACS. The cells were resuspended in Hank's BSS (HBSS) supplemented with 10 mM HEPES pH 7.4 and 5% fetal bovine serum (FBS Superior, Biochrome/Merck) to warrant sufficient cell survival. Cells were then strained using a 40 μM cell strainer directly before FACS to prevent clotting. FACS was performed on a BD FACS AriaII (BD Biosciences) using a 70 μm nozzle. Dead cells and debris were first gated out by forward and side scatter, and second by high propidium iodide (PI) staining. The fluorescence threshold for the GFP-positive (GFP+) cells was set using a non-fluorescent cell suspension derived from FVB/N mice. As control, GFP-negative (GFP−) cells were also collected using a second lower threshold, which was determined considering a Gaussian distribution of fluorescence populations (Figure 1a). After sorting, cells were centrifuged for 10 min at 300g and 4°C and stored at −80°C. For the preparation of crude membrane fractions (MF), pelleted cells were pooled from different FACS experiments, for all other experiments one cell pellet of each population was used.
2.3 Quantitative real-time PCR (qPCR) and cluster analysis
For qPCR analysis of AMPAR constituent expression, sorted cells were split into different experimental groups. In one set of experiments, we compared AMPAR expression in cells sorted from whole brains of 7–9 days (p7–p9, week [w] 1) and 13–16 days old mice (p13–p16, week [w] 2). In another set of experiments, AMPAR expression was studied in three different brain regions, that are cerebellum, neocortex, and hippocampus of 13–16 days (week [w] 2) old mice. Total RNA was isolated from sorted GFP+ and GFP− cells using the RNeasy Micro kit (Qiagen). For every reverse transcription, the total RNA was first spiked with 150 pg of E. coli AraB RNA (Applied Microarray, Tempe) as an external standard. The RNA mixture was reverse-transcribed with the QuantiTect Reverse Transcription kit (Qiagen) including a genomic DNA elimination step. All kits were used according to the manufacturer's instructions. The external bacterial RNA spike was used for normalization of gene expression because several conventional house-keeping genes are differentially regulated under our experimental conditions as described before (Mauric et al., 2013; Schroeter et al., 2015). Quantitative real-time PCR was performed in an Applied Biosystems StepOne real time PCR System using SYBR green PCR Mastermix (Applied Biosystems/Thermo Fisher Scientific). Assays were run in triplicates with a total volume of 10 μl containing 200 ng cDNA and forward and reverse primers at 100 nM each in 1× SYBR green PCR Master mix and MicroAmp optical 96-well reaction plates (Applied Biosystems/Thermo Fisher Scientific). Real-time PCR parameters were: 10 min at 95°C, 40 cycles of 15 s at 95°C, 30 s at 55°C, and 60 s at 60°C each. Melting-curve analysis was performed to verify the amplification of a single product with a specific melting temperature, and the specificity of all PCR amplifications was verified by sequencing. Standard curves with sequential dilutions up to 1:128 were used to determine the primer efficiency. Primer efficiency with linear regression coefficients >0.98 was found optimal. The relative expression levels were normalized to the Ct value of the bacterial AraB RNA spike (% of AraB), and for the calculation, the corresponding primer efficiencies were taken into account. For controlling the enrichment of astrocytes in the GFP+ cells, the relative expression ratios between GFP+ cells and GFP− cells (Fold Change, log scale) were calculated using the ΔΔCt method considering the efficiencies. Table 1 shows the primer pairs that were used (sequence in 5′–3′ direction). Data are given as mean ± standard error of the mean (SEM) unless otherwise stated. Statistical differences between expression values were assessed by two-way ANOVA with a Bonferroni's multiple comparisons test or Tukey's multiple comparisons test using GraphPad Software (GraphPad Software). Cluster analysis and dendrogram calculation of normalized gene expression data was performed according to complete-linkage hierarchical clustering and using the Euclidean distance, as implemented in the heatmap.2 function of the gplots R package (version 3.3.1). Heatmap colors are based upon normalization across rows (row z-score), and scales represent standard deviations above or below mean.
| Gene/protein | Primer sequence | Amplicon size (bp) | Efficiency |
|---|---|---|---|
| AraB/Ribulokinase (E. coli) | F: ATCCCCTGATCGGTAAAGCA R: ACGCCTGAAAGGGGTGATTA |
126 | 1.93 |
| Gria1/GluA1 | F: GACCATAACCTTGGTCCGGG R: CTGGTTGTCTGGTCTCGTCC |
258 | 2.00 |
| Gria2/GluA2 | F: GAGGACTACCGCAGAAGGAGTAGC R: TCGTACCACCATTTGTTTTTCA |
251 | 1.88 |
| Gria3/GluA3 | F: GCCAGGCGTCTTTTCATTCC R: TGCGCCCAGAAAGTGATCTT |
272 | 2.00 |
| Gria4/GluA4 | F: TCTTGGCAATGACACAGCAG R: TGCGTCCCTTGCTCCATATTT |
220 | 1.99 |
| Cnih2/CNIH2 | F: TGGCACATCATAGCCTTTGA R: GGACGGTGGAAGTACCTCC |
150 | 1.97 |
| Cnih3/CHNIH3 | F: GAGGAACATCGAACGCATCT R: GGCATTCATGACAACTGGTG |
214 | 2.00 |
| Cacng2/TARP γ-2 | F: GGCTGACACCGCAGAGTATT R: ACTTAGACCTGCAGACACGA |
175 | 1.99 |
| Cacng3/TARP γ-3 | F: CCGCAGTAGGCACAGTGTTA R: AGGACCAGCCGTAGGAGTAG |
152 | 1.93 |
| Cacng4/TARP γ-4 | F: TTTATTGTGGCGGAGACCGT R: CCTGTAACTCGGCATCCTGG |
138 | 1.86 |
| Cacng5/TARP γ-5 | F: GTGATGCCCATGAACTCCCA R: AAAGCCAGTATGGTCCGGTG |
164 | 2.00 |
| Cacng7/TARP γ-7 | F: ACTACTCGGGCCAGTTTCTG R: AGGTGGTCCGGGTACTTGAT |
127 | 1.90 |
| Cacng8/TARP γ-8 | F: GCTGCCTGGAAGGGTTGAA R: TTTGTAGACGCGAGAGGCAG |
188 | 1.89 |
| Gsg1l/GSG1l | F: CATTTACCTGCTGCATGGCGG R: GGAAGTATTTGATGGCCTCAGGA |
147 | 1.97 |
| Shisa9/CKAMP44 | F: CACCAAGGACAAGACCAACC R: AGGTCTCTCTCCATGTGGTCA |
192 | 1.95 |
| Shisa6/CKAMP52 | F: GCAGACTCCAGGTGATCGTC R: GTTCTGGTAAGAGCGCGAGA |
145 | 2.00 |
| Prrt1/PRRT1 | F: ACACGACTACATGCCCATCG R: CGATCTCGGCAGACACCAAA |
131 | 1.91 |
| Prrt2/PRRT2 | F: GGTAGCCTAAGCCGTCATCC R: CCACAATGTTGACAGGCCAC |
139 | 1.86 |
| Olfm1/Noelin-1 | F: GGCCATGATCACCAACTGGA R: CTGTACACCTGCCAGCTCTC |
147 | 1.92 |
| Olfm2/Noelin-2 | F: ATCTCCAGTATGTTCGCAGC R: GCTCTGTCATCCTGTCCTTC |
120 | 2.00 |
| Olfm3/Noelin-3 | F: CAAAACCGACGCAAAGCTCA R: TTCATGCAGTCACGAAGCCT |
156 | 2.00 |
| Nrn1/Neuritin | F: CGCGGTGCAAATAGCTTACC R: TGTTCGTCTTGTCGTCCAGG |
143 | 2.00 |
| Frrs1l/FRRS1l | F: TACCTGTTTGCTTGGGGTCC R: GAAGGTCAGGGCGACAATGA |
180 | 1.92 |
| Vwc2/Brorin | F: CCGATCTGCAAAAACGGTCC R: CGTTCAATTCTCCACGTGCC |
128 | 1.92 |
| Vwc2l/Brorin-2-like | F: AAGTTGCGCCTTTGCTTCAC R: CACAGTGTTTGCTACGGCAG |
187 | 1.87 |
| Lrrtm4/LRRTM4 | F: AAACCGGATGCAGTCCCAG R: CGCTCCCTGCGATGATTTTG |
118 | 1.95 |
| Porcn/PORCN | F: TCCTTCCACAGCTACCTACA R: CTCAGACAGAAAGCCCACAA |
300 | 1.87 |
| Abhd6/ABHD6 | F: GGATTCTCCGCACACAAGGA R: CTTGCCCCACTATGGACAGG |
139 | 2.00 |
| Abhd12/ABHD12 | F: GCGCTGGGCAGACGAAA R: TCAGTTTGGCCTGTATCCCAG |
156 | 2.00 |
| Gfp/GFP | F: GCGGATCTTGAAGTTCACCTTGATGCC R: GCACGACTTCTTCAAGTCCGCCATGCC |
280 | 1.65 |
| Gfap/GFAP | F: ACCAGCTTACGGCCAACAGTG R: TGTCTATACGCAGCCAGGTTGTTC |
138 | 1.84 |
| Slc1a3/EAAT1 | F: AACAACGTGTCAGAGGCCAT R: ACGAAACCGAAGCACATGGA |
125 | 1.87 |
| Aldh1l1/ALDH1L1 | F: GCCCAATGTCCCAGAGGTAG R: GGATGAAGTCCCCGAAGGTG |
161 | 1.90 |
| Olig2/OLIG2 | F: ATCTTCCTCCAGCACCTCCT R: GTTCGCGGCTGTTGATCTTC |
122 | 1.95 |
| Mog/Mog | F: TCCCATCCGGGCTTTAGTTG R: GGTGCTTGCTCTGCATCTTG |
159 | 1.96 |
| Sox10/Sox10 | F: TACAAGTACCAACCTCGGCG R: GACATGGGGGAGCCTTCTTC |
161 | 1.90 |
| Cspg4/NG2 | F: ACCCAGGCTGAGGTAAATGC R: ACAGGCAGCATCGAAAGACA |
162 | 1.98 |
| Aif1/Iba1 | F: GCTTTTGGACTGCTGAAGGC R: GGGAACCCCAAGTTTCTCCA |
207 | 2.00 |
| Cx3cr1/CX3CR1 | F: CATGTGCAAGCTCACGACTG R: CCCAGACGCCCAGACTAATG |
164 | 2.00 |
| Rbfox3, Fox-3/NeuN | F: GGCTGGAAGCTAAACCCTGT R: ACACGACCGCTCCATAAGTT |
196 | 1.90 |
2.4 Heterologous expression in Xenopus laevis oocytes
The following cDNAs were used for in vitro transcription: Gria1 (M38060.1), Gria4 (M36421.1), Cacng5 (NM_001199301.1), and Shisa9 (NM_028277.2). A tandem FLAG-tag was inserted into the Shisa9 cDNA between codons 29 (CAC, histidine) and 30 (GGG, glycine) according to von Engelhardt et al. (2010), and a V5-tag was fused at the C-terminal end of Cacng5. cRNA was synthesized from 1 μg of linearized plasmid DNA (backbone pBF) using the mMESSAGE mMACHINE® SP6 in-vitro transcription kit (Thermo Fisher Scientific). Xenopus laevis oocytes were purchased from EcoCyte Bioscience (Germany). Within 24 hr after surgery, oocytes were injected with 2 ng Gria1 (GluA1) and 2 ng Gria4 (GluA4) cRNA (= 4 ng total Gria cRNA), 4 ng Shisa9 (CKAMP44) and 0.4 ng Cacng5 cRNA (TARP γ-5) per oocyte using a Micro4 nanoliter injector (World Precision Instruments).
2.5 Cell lysis and preparation of membrane fractions
For cell lysis, pellets of GFP+ and GFP− cells (from p12 mice) were resuspended in cell homogenization buffer (20 mM Tris–HCl pH 7.4, 1 mM iodoacetamide, 1 mM EDTA, 150 mM NaCl, protease inhibitors: aprotinin, leupeptin, pepstatin A [at 1 μg/ml each], and 1 mM PMSF) supplemented with 1% dodecanoyl d-sucrose and incubated for 30 min on ice. After ultracentrifugation (131,000g, 25 min, 4°C) 5× modified Laemmli and 0.1 M DTT were added to the supernatant and incubated for 10 min at 37°C for SDS-Page. Crude membrane fractions were prepared from whole mouse brain and from pooled GFP+ cell pellets (from p7–14 old mice), respectively. Mouse brains were lysed with the gentleMACS Dissociator (Miltenyi Biotec), and the sorted GFP+ cells were lysed by sonication in homogenization buffer and then centrifuged at 1,000g for 5 min at 4°C. The supernatant was ultracentrifuged at 145,000g for 60 min at 4°C. Pellets containing the crude membrane fraction were resuspended in homogenization buffer, and the final protein concentration was determined in a BCA Assay using bovine serum albumin as a standard (Pierce/Thermo Fisher Scientific). For SDS-PAGE, 5× modified Laemmli and 0.1 M DTT were added to 20 μg of protein from the sorted GFP+ cells and 7 μg of protein from whole brain MF fraction as a positive control and then incubated for 10 min at 37°C.
2.6 Immunoprecipitation
2.6.1 GFP+ cells
Crude membrane fractions of sorted cell pellets were solubilized in solubilization buffer (cell homogenization buffer supplemented with 1% dodecanoyl d-sucrose), incubated for 30 min on ice and cleared by ultracentrifugation for 25 min at 131,000g and 4°C. The supernatant was then incubated with respective immobilized antibodies for 2 hr at 4°C. A 1:10 ratio of immobilized antibodies to solubilized protein was used. For immunoprecipitation, rabbit anti-GluA1 (AB1504, Millipore) alone as well as in combination with mouse anti-GluA2 (75-002, NeuroMab) were coupled to Protein G Dynabeads™ (Invitrogen/Thermo Fisher Scientific). After brief washing with solubilization buffer containing 0.1% dodecanoyl d-sucrose, bound proteins were eluted with 1× modified Laemmli buffer at 37°C for 10 min. 0.1 M DTT was added after elution.
2.6.2 Xenopus laevis oocytes
Immunoprecipitations were performed 3 days after cRNA injection. After two washing steps with oocyte homogenization buffer (83 mM NaCl, 1.5 mM MgCl2, 10 mM HEPES, 1 mM EGTA, pH 7.4, protease inhibitors: aprotinin, leupeptin, pepstatin A [at 1 μg/ml each], and 1 mM PMSF), the oocytes were homogenized in 20 μL buffer per oocyte. The homogenate was cleared by centrifugation, and the membranes were sedimented at 125,000g for 10 min at 4°C. Membranes were then solubilized at 4°C for 30 min in solubilization buffer (20 mM Tris–HCl pH 7.4, 1 mM iodoacetamide, 1 mM EDTA, 150 mM NaCl, protease inhibitors: aprotinin, leupeptin, pepstatin A [at 1 μg/mL each], and 1 mM PMSF, 1% dodecanoyl d-sucrose). The solubilizate was cleared by ultracentrifugation at 125,000g for 5 min at 4°C and incubated with 5 μg Protein G Dynabead™-coupled mouse monoclonal anti-V5-antibody (Invitrogen/Thermo Fisher Scientific, R96025) per 10 oocytes for 2 hr at 4°C. The beads were washed twice with washing buffer (20 mM Tris–HCl pH 7.4, 1 mM EDTA, 150 mM NaCl, 1 mM IAA, 0.1% dodecanoyl d-sucrose) and bound proteins were eluted by heating the washed beads in 2 μl modified Laemmli buffer per oocyte for 10 min at 37°C. For SDS-PAGE, 0.1 M DTT was added, and the proteins were denatured for 10 min at 60°C.
2.7 SDS-PAGE and immunoblotting
Protein samples were separated by 10% or 12% SDS-PAGE, electro-blotted on PVDF membrane (Millipore/Merck, Germany), and detected by immunoblot analysis. If necessary, the blot membrane was cut horizontally at different molecular weight ranges. The membranes were blocked with 5% BSA in TBS-T (20 mM Tris, 140 mM NaCl, 0.1% Tween 20, pH 7.6) and incubated overnight at 4°C with the primary antibodies. After incubation with the secondary antibodies, the blots were developed using ECL plus reagent (GE Healthcare, UK) or Westar ɲc Ultra 2.0 reagent (Cyanagen).
The following antibodies were used for immunoblotting: mouse anti-GLAST (1:250, 130-095-822, Miltenyi BioTec), rabbit anti-GFAP (1:1000, Z0334, DAKO/Agilent technologies), rabbit anti-Iba1 (1:1000, 016-20001, Wako), rabbit anti-Calnexin (1:1000, ab13504, Abcam), rabbit anti-GluA1 (1:1000, AB1504, Millipore/Merck), mouse anti-GluA2 (1:1000, 75-002, UC Davis/NIH NeuroMab Facility), rabbit anti-GluA4 (1:1000, AB1508, Millipore/Merck), mouse anti-V5 (1:5000, R96025, Invitrogen/Thermo Fisher Scientific), mouse anti-FLAG M2 (1:2000, F3165, Sigma-Aldrich), and goat anti-rabbit and anti-mouse secondary antibodies conjugated to horseradish peroxidase (1:10,000–15,000, Santa Cruz Biotechnologies).
2.8 Immunohistochemistry
Mice (p14–15 old mice) were anesthetized, decapitated, and the brains were rapidly removed. After brief washing with phosphate-buffered saline (PBS), the brain tissue was subjected to immersion fixation in 4% paraformaldehyde in PBS at 4°C for 2 days. The brains were cut at 35–40 μM thickness using a vibratome (Microm HM650V, Thermo Fisher Scientific). The brain slices were first permeabilized in 2% Triton X100/PBS, unspecific antibody binding was blocked by 10% normal goat serum (NGS) in PBS for 2 hr and then incubated overnight with the respective primary antibody in the staining solution (2% NGS, 0.1% Triton X100 in PBS) at room temperature (RT) or 4°C. After repeated washing with PBS, the slices were incubated with the secondary antibody in the staining solution for 2 hr at RT. Subsequently, the slices were thoroughly washed with PBS including nuclei staining with the NucBlue Fixed Cell Stain DAPI Solution (Molecular Probes Thermo Fisher Scientific). Brain slices were mounted and imaged using an AxioScan and a confocal LSM810 (Zeiss).
The following antibodies were used for immunohistochemistry: anti-GLAST (1:250, 130-095-822, Miltenyi BioTec), rabbit anti-GFAP (1:1000, Z0334, DAKO/Agilent technologies), rabbit anti-Olig2 (1:500, AB9610, Millipore/Merck), mouse anti-NeuN (1:100, MAB377, Millipore/Merck), rabbit anti-Iba1 (1:1000, 016-20001, Wako), chicken anti-GFP (1:500, ab13970, Abcam), and as secondary antibodies goat anti-rabbit conjugated Cy3 (1:500, A10520, Thermo Fisher Scientific), goat anti-mouse conjugated Cy3 (1:500, A10521, Thermo Fisher Scientific), and goat anti-chicken conjugated Alexa Fluor 488 (1:500, A11039, Thermo Fisher Scientific). Primary antibodies were omitted in negative controls.
2.9 Recombinant electrophysiology in Xenopus laevis oocytes
AMPAR current responses were detected by two-electrode voltage clamp recordings in oocytes 3–4 days after cRNA injection at −70 mV holding potential using a Turbo Tec-03X amplifier (npi electronic) controlled by Pulse software (HEKA). Electrodes were filled with 3 M KCl and had resistances of 0.5–1.5 MΩ. Oocytes were superfused with calcium-free Mg2+-Ringer's solution (in mM: 115 NaCl, 2.5 KCl, 1.8 MgCl2, and 10 HEPES, pH 7.2) to prevent the activation of endogenous Ca2+-gated chloride channels. Agonists (300 μM glutamate (Glu) with and without 50 μM cyclothiazide (CTZ), 150 μM kainate (KA)) were applied for 20 s. Current–voltage relationships were determined by ramping the holding potential (Vh) from −140 mV to 50 mV corrected for background conductivities. Data are given as mean ± SEM. The rectification index (RI) was defined as the ratio of current response at Vh = 50 mV to the response at Vh = −80 mV (I50mV/I−80mV). Statistical differences were assessed by a one-way ANOVA with a Tukey's multiple comparisons test using GraphPad Software.
3 RESULTS
3.1 Isolation of astrocytes
First, we established a FACS protocol to isolate astrocytes from dissociated brain tissue of hGFAP/GFP transgenic mice (Zhuo et al., 1997). As plotted in Figure 1a, the chosen FACS gating parameters separated a GFP+ from a GFP− cell population. In GFP+ cells, mRNA expression of the astrocytic marker genes Gfap, Slc1a3 (EAAT1), and Aldh1l1 was enriched 75- to 120-fold compared to the GFP− cell population (Figure 1b). In good agreement, we were able to detect GFAP and EAAT1 protein expression only in GFP+ cells (Figure 1c). By contrast, expression of the microglial marker genes Aif1 (Iba1) and Cx3cr1, and also of the neuronal marker gene Rbfox3 was depleted from GFP+ cells. The enriched Aif1 mRNA expression in GFP− cells was paralleled by selective Iba1 protein detection in these cells (Figure 1b,c). We found little higher expression of Olig2, Mog, Sox10, and Cspg4 mRNAs in GFP+ compared to GFP− cells, indicating limited contamination of the targeted astrocytes with cells of the oligodendrocyte lineage. Routine qPCR of marker mRNAs provided standard quality control in all FACS experiments (Supporting Information, Table S1).
To further ascertain the predominantly astrocytic origin of sorted GFP+ cells, we performed immunohistochemical analysis in hGFAP/GFP mice (Figure 1d). As exemplified in cerebellar stainings (insets), immunoreactivities of GFAP and EAAT1 were found to co-localize with GFP, particularly in Bergmann glia processes as expected (Sofroniew & Vinters, 2010), whereas the ones of oligodendrocytic Olig2, neuronal NeuN, and microglial Iba1 did not show any overlap with GFP localization. Respective immunostainings of hippocampus and neocortex at higher magnification are depicted in Supporting Information, Figure 1. In summary, the chosen experimental approach enabled us to isolate cells for mRNA and protein analysis, which are predominantly of astrocytic origin.
3.2 AMPAR expression in astrocytes during early postnatal development
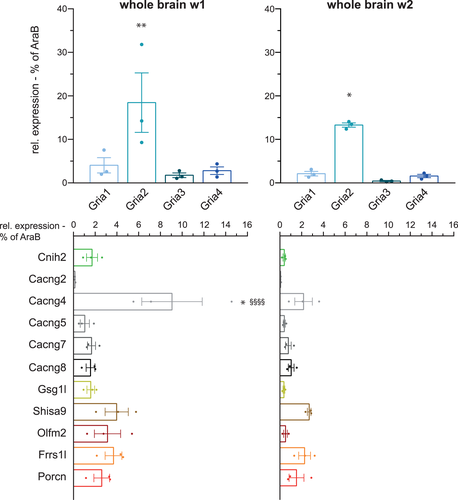
We next quantified mRNA expression of AMPARs in astrocytes sorted from whole brains. As astrocytogenesis and synaptic growth and maturation takes place within the first two postnatal weeks (Reemst, Noctor, Lucassen, & Hol, 2016; Wang & Bordey, 2008), the expression profiles of the pore-lining GluA subunits, Gria1–4, and other AMPAR constituents as defined in our previous proteomics study (Schwenk et al., 2012) were analyzed for this period of time. As depicted in Figure 2 (upper panels), a distinct mRNA expression pattern of the four Gria genes was observed, with Gria2 being the most abundant subunit and Gria1, 3, and 4 summing up to not even half of Gria2 expression (Supporting Information, Table S2). This differential pattern did not change within the first two postnatal weeks; however, overall expression of total Gria1–4 decreased by ~36% from w1 to w2. Similarly, we found rather moderate changes in the differential expression pattern of examined AMPAR constituents in whole brain astrocytes during the same period of development (Figure 2 lower panels and Supporting Information, Table S2). A total of 22 from 24 selected AMPAR constituent mRNAs could be detected; neither Cacng3 (TARP γ-3) nor Vwc2l (Brorin-2-like) expression was above background and hence excluded from further analysis. In the first postnatal week, Cacng4 mRNA (TARP γ-4) was the most abundant AMPAR constituent. Despite a strong decrease in expression by >75% during the second postnatal week, Cacng4 still remained the predominant TARP mRNA expressed in astrocytes. Surprisingly, we found appreciable expression of Shisa9 (CKAMP44), which had formerly been described as a neuronal auxiliary subunit (von Engelhardt et al., 2010). In parallel with the developmental reduction of mRNAs coding for the pore-lining GluA subunits, also the ones coding for other AMPAR constituents decreased in expression from w1 to w2. However, the reduction in mRNA expression of non-pore-lining AMPAR constituents was more pronounced amounting up to 59%.
3.3 AMPAR expression in astrocytes from different brain regions
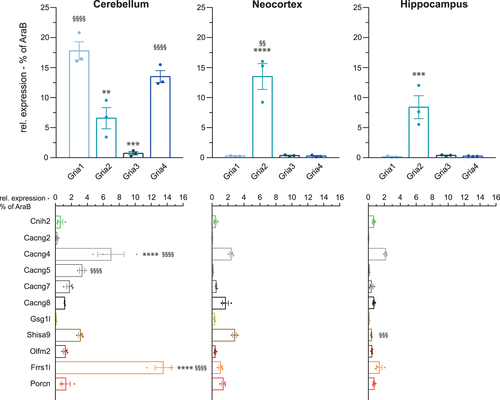
Functional properties of astrocytic AMPARs vary considerably between brain regions. Cerebellar Bergmann glia express Ca2+-permeable and inwardly rectifying AMPARs (Burnashev et al., 1992; Muller, Möller, Berger, Schnitzer, & Kettenmann, 1992). In contrast, hippocampal astrocytes exhibit no AMPAR currents at all, and astrocytes in the neocortex show only small AMPA-mediated currents (Lalo et al., 2006; Matthias et al., 2003). To address the question, whether the reported functional diversity is reflected by specific expression patterns of AMPAR constituents, we sorted astrocytes from cerebellum, neocortex, and hippocampus from the second postnatal week for further qPCR analysis. Indeed, we found distinct expression levels and patterns of AMPAR complex constituents in the selected brain regions (Figure 3). Whereas in astrocytes sorted from cerebellum, Gria1 and Gria4 represented the predominant pore-forming subunits (17.8% ± 1.5% and 13.5% ± 1%, respectively), Gria2 was the predominant one in neocortex (13.5% ± 2.2%) and hippocampus (8.4% ± 1.9%). In the latter two regions, the other Gria genes were barely expressed (Figure 3, upper panels). Brain region-specific differences were also found in the expression patterns of the other AMPAR complex constituents (Figure 3, lower panels). Astrocytes from cerebellum expressed strikingly high levels of mRNA coding for Frrs1l (13.5% ± 1.0%) compared to neocortex (1.1% ± 0.2%) or hippocampus (1.4% ± 0.4%). Among the TARPs, Cacng4 mRNA was predominant in astrocytes from all brain regions; Cacng5 and Cacng7 mRNAs were rather selectively expressed in cerebellum. In contrast, Shisa9 mRNA showed appreciable expression in both cerebellum and neocortex. In general, however, we observed that total mRNA expression levels of AMPARs in astrocytes sorted from neocortex and hippocampus were lower than in astrocytes from cerebellum. Overall, the observed regional AMPAR expression profiles showed prominent differences and more heterogeneity than the temporal AMPAR expression profiles within the first two postnatal weeks when averaged throughout whole brain.
3.4 Hierarchical clustering of AMPAR expression levels reveals appreciable heterogeneity between brain regions
To assess the relationship between the mRNA expression profiles of the different brain regions, we performed hierarchical clustering analysis. As shown in Figure 4, the resulting heat map and dendrogram depicts unequivocal heterogeneity among cerebellar, neocortical, and hippocampal astrocytic AMPARs. Overall, AMPARs in cerebellar astrocytes are most distinct from neocortical and hippocampal ones with distances of 27.32 and 26.13, respectively, whereas AMPARs in neocortical and hippocampal astrocytes are much more alike with a distance of only 7.96. Exceptions exist at the level of specific genes, including Shisa and Abhd, for which astrocytic AMPARs from either neocortex or hippocampus and cerebellum share more similarity, respectively.
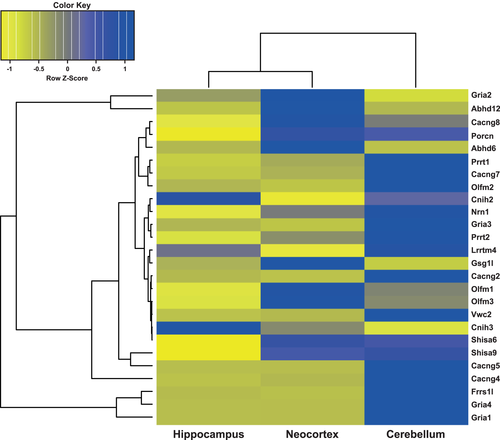
In summary, our qPCR analysis of astrocytic AMPAR expression disclosed appreciable heterogeneity among brain regions, which may give rise to region-specific molecular compositions and hence the reported functional heterogeneity of glial AMPARs.
3.5 Native AMPAR protein complexes in sorted astrocytes
To check whether mRNA expression translates into protein, immunoblot analysis was performed on crude membrane fractions of sorted GFP+ cells. As shown in Figure 5a, the expression of the pore-lining subunits GluA1, GluA2, and GluA4 could be confirmed. However, their level of expression was by far lower than in crude membrane fractions from whole brain containing neuronal AMPARs as well. We also sought to investigate whether intact native AMPAR complexes may be affinity-purified from sorted astrocytes. Figure 5b shows the isolation of native AMPAR pore-forming complexes. Affinity-purification of GluA1-containing complexes (IP GluA1) from whole brain astrocytic membrane fractions co-purified both GluA2 and GluA4. While GluA1 was depleted in these experiments, significant amounts of GluA2 and GluA4 remained unbound. Affinity-purifications virtually depleting GluA2 as well (IP GluA1/2) co-depleted GluA4. GluA3 was not further pursued because of negligible expression in astrocytes (Figures 2 and 3) and the lack of available antibodies specifically purifying native GluA3.
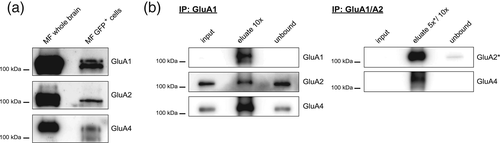
Given a preferential heteromeric assembly of the pore-lining AMPAR subunits (Greger, Watson, & Cull-Candy, 2017), we conclude that astrocytes express mainly GluA1/2, GluA1/4, and GluA2/4 heteromers.
3.6 Functional reconstitution of selected astrocytic AMPARs
Intrigued by the novel finding of astrocytic Shisa9 expression, we sought to reconstitute AMPAR complexes, which are prototypic for cerebellar astrocytes. To this end, GluA1/4 heteromeric receptors were co-expressed in Xenopus laevis oocytes with CKAMP44 and TARP γ-5. Functional properties of resulting receptor complexes were probed by two-electrode voltage-clamp (TEVC) bath-applying the ligands glutamate or kainate (Figure 6a).
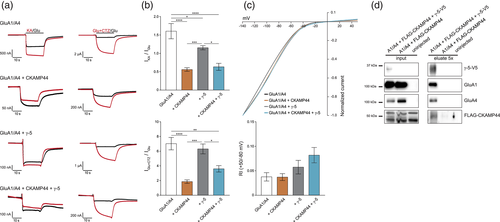
As quantified in Figure 6b, GluA1/4 heteromers alone show a kainate/glutamate (KA/Glu) ratio of 1.60 ± 0.2 (n = 12) and strong desensitization, which is inhibited by application of cyclothiazide (CTZ). The current/voltage (I/V) relationship of GluA1/4 receptors is characterized by inward rectification typical of GluA2-lacking receptor complexes (Boulter et al., 1990; Bowie & Mayer, 1995; Figure 6c). Co-expression of CKAMP44 significantly reduced the KA/Glu ratio to 0.56 ± 0.05 (n = 20; p < .0001) and also receptor desensitization, which is reflected by a significantly smaller effect of CTZ. Rectification properties of GluA1/4 receptors did not change upon co-expression of CKAMP44. Co-expression of TARP γ-5 also reduced the KA/Glu ratio in GluA1/4 heteromers (1.15 ± 0.06; n = 13; p = .03), but to a significantly smaller extent than did CKAMP44 (p = .0005). Desensitization of GluA1/4 heteromers was not affected, nor was the strong effect of CTZ diminished. The rectification index (RI) showed only a tendency to increase upon co-expression of TARP γ-5, but did not significantly differ from GluA1/4 heteromers alone (n = 11; p = .6642). Co-expression of both CKAMP44 and TARP γ-5 resulted in receptors with functional properties predominantly determined by CKAMP44. Thus, the KA/Glu ratio decreased to 0.63 ± 0.1 (n = 11; p < .0001) and the effect of CTZ was significantly smaller than in GluA1/4 heteromers alone or upon co-expression of TARP γ-5 (3.60 ± 0.43 (n = 11; p = .001 (vs GluA1/4), p = .011 (vs GluA1/4 + γ-5)). Only the RI further increased, although still not significantly (n = 11; p = .0681). As TEVC recordings would not distinguish between receptor subpopulations, the virtual co-assembly of GluA1/4 with both CKAMP44 and TARP γ-5 was probed by immunoprecipitation experiments. As shown in Figure 6d, precipitating TARP γ-5 co-purified CKAMP44 in the presence of GluA1/4 heteromers. Thus, the heterologously expressed constituents integrate into common receptor complexes. Whether those complexes, however, may show different stoichiometries, remains elusive at this point.
4 DISCUSSION
Here, we have quantified the astrocytic transcriptome of AMPARs in early postnatal development. Whereas the pattern of AMPAR mRNA expression showed minor variation over time, it did exhibit significant heterogeneity with respect to different brain regions. Cerebellar astrocytes express a combination of AMPAR complex constituents that is remarkably distinct from the one in neocortical or hippocampal astrocytes. Altogether, we provide both a workflow and a first reference for future investigations into the molecular and functional diversity of astrocytic AMPARs.
4.1 Experimental design
We used a transgenic mouse line expressing an optimized GFP variant (hGFP-S65T) under the human GFAP promoter to isolate astrocytes from brain tissue by FACS (Zhuo et al., 1997). GFAP was the first molecular marker used to designate astrocytic identity (Eng, Ghirnikar, & Lee, 2000). However, two observations advice caution: GFAP expression level varies among astrocytes from different brain regions (Sofroniew & Vinters, 2010) and GFAP may be expressed by other cell types than mature astrocytes, for example, progenitor cells affording constitutive neurogenesis in the adult CNS (Garcia, Doan, Imura, Bush, & Sofroniew, 2004; Molofsky & Deneen, 2015; Platel, Gordon, Heintz, & Bordey, 2009). For routine quality control, we have therefore performed extensive characterization of GFP+ cells isolated in our experimental model. The enrichment of GFAP, Slc1a3 (EAAT1), and Aldh1l1 mRNAs by two orders of magnitude and the depletion of both microglial and neuronal marker genes, confirmed by immunoblot analysis, validated the predominantly astrocytic origin of sorted GFP+ cells. A limited contamination by cells of the oligodendrocytic lineage or by the above mentioned progenitor cells may be indicated by the elevated detection of Olig2, Mog, Sox10, and Cspg4. The qPCR and immunoblot results were finally confirmed by whole brain immunohistochemistry showing exclusive co-localization of GFP-immunoreactivity with astrocytic but neither with microglial nor with neuronal markers.
4.2 Temporal profile of the early postnatal astrocytic AMPAR transcriptome
As a start, we quantified mRNA expression of AMPAR constituents in astrocytes isolated from whole brain after the first postnatal week (w1). At this developmental stage, astrocytes grow steadily and promote the formation of neuronal synapses. Among the pore-forming subunits, we found Gria2 to be the predominant isoform. This observation is in good agreement with a recent proteomic analysis of rat whole brain membrane fractions, which identified GluA2-containing AMPAR complexes to be the most abundant ones from birth until adulthood (Schwenk et al., 2014). Among the inner core complex constituents, Cacng4 (TARP γ-4) mRNA was most abundant of the Cacng (TARP) family, but also compared to CNIH2 and GSG1l. In line with these data, TARP γ-4 has been reported to be expressed in glial cells, particularly during embryonic and early postnatal development (Tomita et al., 2003). Also, proteomic analysis quantified TARP γ-4 as the most abundant TARP assembling into native AMPARs isolated from whole brain tissue in early postnatal stages (Schwenk et al., 2014). The accordance of our results obtained from isolated astrocytes with the proteomic data from whole brain tissue sampling diverse cell types, including neurons and other glial cells, was somewhat unexpected. Astrocytic AMPARs may outnumber neuronal ones dominating the whole brain proteomic results; alternatively, the temporal profile of the early postnatal AMPAR composition may rather be independent of cell type. Of note were the significant amounts of Shisa9 (CKAMP44) mRNA detected in astrocytes, which had so far been reported as a neuronal AMPAR constituent (Khodosevich et al., 2014; von Engelhardt et al., 2010). In contrast to the high mRNA and protein levels of CNIH2 and its high abundance in whole brain AMPAR complexes, astrocytic CNIH2 mRNA expression was relatively low (Mauric et al., 2013; Schwenk et al., 2014). Interestingly, we observed also low levels of TARP γ-8 mRNA in astrocytes and no developmental increase, which somewhat contrasts proteomic data showing that both CNIH2 and TARP γ-8 compete for the most abundant inner core constituent of AMPARs during development (Schwenk et al., 2014; Tomita et al., 2003).
In all likelihood, however, the transcriptome will not be linearly translated into its proteome challenging more detailed quantitative comparisons. The pattern of astrocytic constituent expression remained basically constant during later postnatal development, confirming earlier microarray data, which claimed the genetic profile of p7 astrocytes to closely resemble already that of mature astrocytes (Cahoy et al., 2008). We noted, however, a marked decrease in overall mRNA levels of both pore-forming subunits and other complex constituents after the second postnatal week [w2]. Such distinct dip in AMPAR expression was similarly observed in the whole brain proteomic study around p14 (Schwenk et al., 2014). It may reflect maturation of glutamatergic neurotransmission in the developing rodent brain.
4.3 Regional profile of the early postnatal astrocytic AMPAR transcriptome
In the next series of experiments, we quantified astrocytic AMPAR transcriptomes derived from cerebellum, neocortex, and hippocampus after the second postnatal week. The expression patterns of both pore-lining and other AMPAR constituents showed appreciable heterogeneity over brain region. Hierarchical clustering revealed cerebellar AMPARs most distant from neocortical and hippocampal ones. The predominant expression of Gria1 and Gria4 at the expense of Gria2 in cerebellum may well explain the prototypical Ca2+-permeable inwardly rectifying AMPAR currents recorded from Bergmann glia (Burnashev et al., 1992; Muller et al., 1992), which represent most likely the largest population of GFAP expressing cells in the cerebellum. The far lower expression of the majority of AMPAR constituents except for Gria2 in neocortex and hippocampus compared to cerebellum may account for the rather small or nondetectable currents in neocortical and hippocampal astrocytes, respectively (Lalo et al., 2006; Matthias et al., 2003). RNA editing of Gria2, translated into GluA2, has been described as a crucial determinant of AMPAR pore assembly by regulating channel tetramerization and its export from the endoplasmic reticulum (ER) (Greger & Esteban, 2007). Thus, Q/R edited GluA2 does not homotetramerize but instead favors heteromerization with the other pore-lining subunits for efficient ER export (Greger, Khatri, Kong, & Ziff, 2003; Greger, Khatri, & Ziff, 2002). However, if expression levels of the latter are rather low as suggested by our qPCR data for neocortex and hippocampus, also low numbers of functional channels are expected on the cell surface, with most GluA2 being retained within the ER. Such discrepancy of AMPAR surface expression between brain regions may well be increased by another conspicuous finding of our qPCR analysis: contrasting neocortical and hippocampal astrocytes, cerebellar astrocytes express strikingly high levels of FRRS1l, which has recently been reported as a priming catalyst for early AMPAR biogenesis in the ER (Brechet et al., 2017). High levels of FRRS1l may hence support efficient assembly and export of AMPARs from the ER, eventually resulting in robust surface expression and respective current amplitudes.
Deep sequencing of RNA (RNA-Seq) isolated from astrocytes has recently allowed establishing the first comprehensive databases of their transcriptome with respect to brain region and developmental stage (Chai et al., 2017; Srinivasan et al., 2016; Zhang et al., 2014). Our qPCR results on postnatal expression of AMPAR complex constituents in astrocytes, even though generated in independent sample preparations, may validate respective transcriptome databases. In good agreement with our study, RNA-Seq of p7 astrocytes from mouse neocortex as well as single cell expression data from neocortical astrocytes identified Gria2 as the by far predominant pore-lining subunit of AMPARs (Dzamba et al., 2015; Rusnakova et al., 2013; Zhang et al., 2014). During further development, the dominance in expression of Gria2 over the other three isoforms fades when averaged over whole brain; still, Gria2 exhibits highest transcript expression in (adult) neocortical and hippocampal astrocytes among the AMPAR pore-forming subunits (Chai et al., 2017; Rusnakova et al., 2013; Srinivasan et al., 2016; Zhang et al., 2014). For astrocytic mRNA expression of inner core constituents, we also find some discrepancies between our qPCR and published RNA-Seq datasets. While we find Cacng4 as the dominant TARP isoform in neocortical and hippocampal astrocytes after the second postnatal week, RNA-Seq data identified Cacng7 dominant in both p7 and adult astrocytes from the same brain regions (Chai et al., 2017; Srinivasan et al., 2016; Zhang et al., 2014). Such discrepancies, in general, might derive from heterogeneity among astrocytes from the same brain region but specializing within different neuronal circuits, and from different marker genes used in respective purification protocols (Chai et al., 2017; Zhang et al., 2014). However, fully consistent with our data are the similar levels of neocortical Cacng4 and Shisa9 expression throughout postnatal development (Chai et al., 2017; Srinivasan et al., 2016; Zhang et al., 2014).
4.4 Limitations
Our study provides a reference for further investigations into the molecular and functional heterogeneity of astrocytic AMPARs. As demonstrated, the cell type-specific transcriptomic profiles may further be probed by cell type-specific analysis of protein expression or even protein assembly. Recombinant electrophysiology of reconstituted AMPARs may eventually guide experiments defining the molecular basis of AMPAR currents in native cells. Finally, the herein presented experimental approach may be applied to other cell types provided the respective animal models for cell isolation.
However, certain limitations have to be taken into account when interpreting the data or seeking for further methodological improvement. Regional heterogeneity of a specific cell type is underestimated when sample preparation is solely based on anatomical borders without considering specific integration into neuronal circuitry. Functional testing prior to cell isolation and single cell profiling will help improve resolution (Chai et al., 2017; Dzamba et al., 2015; Rusnakova et al., 2013). Also, local mRNA expression might be underestimated, as cell processes will most likely be lost during cell sampling by FACS (Sakers et al., 2017). Finally, an unbiased analysis of glial AMPAR composition would require a cell type specific proteomic analysis (Schwenk et al., 2012). For making the claim of being comprehensive, such approach will always be limited by the amounts of surface membrane protein available for affinity purification of AMPARs, their enzymatic digest, and eventual high-resolution mass spectrometry.
ACKNOWLEDGMENTS
This study was supported by a grant from the Deutsche Forschungsgemeinschaft (DFG) to NK (KL 1168/7-1) within the Priority Program SPP1757. AM was an associated member of iBrain graduate program at Heinrich-Heine University of Düsseldorf. The authors would particularly like to thank Klaus Meyer (Institute of Functional Genome Research of Microorganisms, HHU Düsseldorf) for his help and support with FACS, Rafael Dellen (Center for Bioinformatics and Biostatistics, Biological Medical Research Center, HHU Düsseldorf) for support with the hierarchical clustering analysis, and René Hübbers (Cécile and Oskar Vogt Institute of Brain Research, HHU Düsseldorf) for his help with the AxioScan imaging.