Helper CD4 T cells expressing granzyme B cause glial fibrillary acidic protein fragmentation in astrocytes in an MHCII-independent manner
Abstract
During inflammatory processes of the central nervous system, helper T cells have the capacity to cross the blood–brain barrier and injure or kill neural cells through cytotoxic mechanisms. Glial fibrillary acidic protein (GFAP) is an intermediate filament protein that is part of the astrocyte cytoskeleton that can become fragmented in neuroinflammatory conditions. The mechanism of action by which helper T cells with cytotoxic properties injure astrocytes is not completely understood. Primary human astrocytes were obtained from fetal brain tissue. Human helper (CD4+) T cells were isolated from peripheral blood mononuclear cells and activated with the superantigen staphylococcal enterotoxin E (SEE). Granzyme B was detected by enzyme linked immunosorbent assay and intracellular flow cytometry. GFAP fragmentation was monitored by western blotting. Cell death was monitored by lactic acid dehydrogenase release and terminal biotin-dUTP nick labeling (TUNEL). Astrocyte migration was monitored by scratch assay. Adult human oligodendrocytes were cultured with sublethally injured astrocytes to determine support function. Helper T cells activated with SEE expressed granzyme B but not perforin. Helper T cells released granzyme B upon contact with astrocytes and caused GFAP fragmentation in a caspase-dependent, MHCII-independent manner. Sublethally injured astrocytes were not apoptotic; however, their processes were thin and elongated, their migration was attenuated, and their ability to support oligodendrocytes was reduced in vitro. Helper T cells can release granzyme B causing sublethal injury to astrocytes, which compromises the supportive functions of astrocytes. Blocking these pathways may lead to improved resolution of neuroinflammatory lesions.
1 INTRODUCTION
Certain immune cells have the potential to target and eliminate host cells that have become cancerous or infected. Helper (CD4+) T cells can acquire cytotoxic properties by expressing granzyme B, a serine protease that causes apoptosis in target cells (Du et al., 2016; Pardo et al., 2009). While helper T cells are known to play a role in immune-surveillance, their cytotoxic mechanism of action is not very well understood. In conventional cytotoxic cells, such as natural killer (NK) cells, and cytotoxic CD8 T cells, perforin and granzymes are stored in cytotoxic granules, which degranulate onto a target cell. Degranulation relies on acidification of the granule, which can be inhibited by the compound concanamycin A (CMA) (Kataoka et al., 1996). Caspase enzyme pathways are triggered by granzymes, which ultimately lead to proteins being cleaved and DNA damage including single stranded and double stranded DNA breaks. Perforin is not always necessary for granzyme to enter a cell target (Shi et al., 1997). The amount of granzyme B secreted by helper T cells is comparable to that secreted by cytotoxic CD8 T cells; however, perforin is not always expressed by helper T cells (Lin et al., 2014).
Cytotoxic helper T cells, including those releasing granzyme B, can cross the blood brain barrier and are toxic to neurons and oligodendrocytes (Broux et al., 2012; Giuliani, Goodyer, Antel, & Yong, 2003; Haile et al., 2015; Kebir et al., 2007; Lutz et al., 2017; Matsui, Kawahara, Kimoto, & Yoshida, 2015). Cerebral spinal fluid-derived granzyme B and cytokine IL-1β are synergistic in causing harm to neurons, which may contribute to chronic neuron loss in multiple sclerosis (MS) (Lee et al., 2017). Collateral damage to neurons has also been reported to be a result of granzyme B action (Göbel et al., 2010; Lee et al., 2017; Wang et al., 2006). The cytotoxic potential of helper T cells towards astrocytes is less understood.
Astrocytes provide vital support for neurons and oligodendrocytes and other neural cells. They play an immune-surveillance role by expressing MHCI and MHCII surface proteins (Brosnan & Raine, 2013) and can synergize with T cells through local cytokine production (Sénécal et al., 2016). Astrocytes secrete soluble factors for oligodendrocytes to support their growth and function (Ishibashi et al., 2006; Miyamoto et al., 2015). In demyelinating syndromes such as MS and encephalomyelitis, astrocytes may actually impede remyelination. Induction of encephalomyelitis in glial fibrillary acidic protein (GFAP)-null mice showed an unusual pathology marked by a lack of membrane specializations at the glia limitans. Despite the worsened clinical course, there was a marked increase in remyelination (Liedtke, Edelmann, Chiu, Kucherlapati, & Raine, 1998). Astrocytes, through formation of glia limitans, act as a barrier to Schwann cell-remyelination in rodent progenitor cell-transplantation models (Blakemore, 1992). High levels of covalently bound (nonreducible) GFAP polymers were present, suggesting a dynamic equilibrium between fragmentation and polymerization that favors retention of GFAP fibrous structures (Darlington et al., 2008). The presence of such structures in glial scars may represent a barrier to remyelination.
Astrocytes can be sensitive to helper T cells in neuroinflammatory contexts. For example, they caused apoptosis of astrocytes in Theiler's virus model of demyelination (Palma, Yauch, Lang, & Kim, 1999), and helper T cells with encephalitogenic capacity injured astrocytes by cytotoxicity or by detaching them from their substrate (Halachmi et al., 1992; Halachmi et al., 1992). In a previous study, we showed that a mix of helper CD4 T cells and cytotoxic CD8 T cells caused caspase-dependent fragmentation of glial fibrillary acidic protein (GFAP) without killing the astrocytes (Darlington et al., 2008). GFAP is an intermediate filament protein found exclusively in astrocytes. Similar fragmentation of GFAP was found in MS (Darlington et al., 2008; Newcombe, Woodroofe, & Cuzner, 1986), Alzheimer's disease (Mouser, Head, Ha, & Rohn, 2006), prion infection (Gray, Skipp, O'Connor, & Perry, 2006), excitotoxic damage (Acarin et al., 2007), aged monkey brains (Sloane et al., 2000), Alexander disease murine model (Tanaka et al., 2007), and degenerative motor neuron disease (Fujita et al., 1998). These diverse findings indicate that GFAP is commonly altered in neuroinflammatory conditions.
In this article, we demonstrate that activated helper T cells expressing granzyme B induce GFAP fragmentation in primary astrocytes in a caspase-dependent manner. Sublethally injured astrocytes had a morphology change, were not apoptotic, and had impaired migration and oligodendrocyte support function in vitro.
2 MATERIALS AND METHODS
2.1 Immune cell preparation and flow cytometry analysis
Peripheral blood mononuclear cells (PBMCs) were prepared from venous blood of healthy adult donors using Ficoll density-gradient techniques (Tabatabaei Shafiei et al., 2014). PBMCs were cultured for 3 days with 0.5 ng/mL of staphylococcal enterotoxin E (SEE) (Toxin Technology, Sarasota, FL) or 0.5 μg/mL of anti-CD3 antibody (OKT3 eBioscience, San Diego, CA). The bacterial-derived SEE binds to T cell receptor (TCR) causing polyclonal T cell activation (Bueno et al., 2006). Helper (CD4+CD3+) T cells were obtained by magnetic activated cell sorting (MACS) using CD4-negative selection followed by CD4-positive selection (Miltenylil Biotech, Bergisch Gladbach Germany). The CD4-negative selection removes CD8, CD14, CD16, CD19, CD56, CD36, CD123, anti-TCRγ/δ, and CD235a (glycophorin A). Surface and intracellular staining for flow cytometry analysis of immune cells was performed on a BD FACSCalibur. All flow cytometry antibodies were from BD Bioscience (Mississauga, ON, Canada).
2.2 Primary human astrocyte culture and scratch assay
Human brain tissue from between 20 and 24 gestational weeks was provided by the Albert Einstein College of Medicine, Bronx, NY, human fetal tissue repository in accordance with Albert Einstein College of Medicine and McGill University institutional review boards. Astrocyte-enriched cultures were prepared as previously described (Darlington et al., 2008). In brief, a dispersed cell suspension was obtained from fetal brain tissue by trypsinization and mechanical disruption, and then cultured in Dulbecco Modified Eagle media (DMEM) containing 10% fetal bovine serum (FBS), pen/strep, l-glutamine, with at least three passages before use. Astrocytes were seeded in 24-well plates and used upon confluency. For experiments requiring fluorescent staining for microscopy, astrocytes were seeded onto thermanox coverslips in 24-well plates which were later removed for staining procedures (Thermo-Fisher Scientific, Mississauga, ON, Canada). Cultures were >95% astrocytes, with the remainder being primarily neurons. For scratch assay, four scratches were carefully made on each well of confluent astrocytes using the pointed side of a 24 G needle. Light micrographs were obtained of each scratch using a Leica microscope. After the culture period, the same scratches were imaged again. Six linear measurement lines were drawn across each of the four scratches per well, and then averaged to determine the distance traveled after culture period using Image J software (National Institute of Health, Bethesda, MD). Adult mature oligodendrocyte fraction was obtained from resected temporal lobe brain tissue with informed consent and protocols described previously (Cui et al., 2010).
2.3 T cell, inhibitors, and iDC culture
Helper T cells (0.5 × 106) were added to confluent astrocytes in Roswell parks memorial institute (RPMI) media with 10% FBS, pen/strep, l-glutamine, at a 1:1 ratio of helper T cells to astrocytes for 18 hr. Where indicated, inhibitors dissolved in DMSO vehicle were added to the culture at final concentrations of 50 μM for ZVAD-fmk, or 10 μM for Ac-IETD-CHO. Anti-MHC II (DR DP DQ) and functional grade IgG1 isotype controls were used at 5 μg/mL (eBioscience, San Diego, CA). The lectin phytohemaglutinin (PHA; Sigma-Aldrich, St. Louis, MO) was used at a final concentration of 2 μg/mL in the T cell astrocyte culture. In some experiments transwell (0.4 μm pore; Thermo-Fisher Scientific) were placed in the 24-well plates containing the astrocytes cultures, and T cells were added to the upper portion. In some experiments, SEE-activated helper T cells were pretreated with CMA (Sigma-Aldrich) for 2 hr with 10 nM or a DMSO at equivalent amounts to control for the vehicle used to dissolve CMA (Sigma-Aldrich). Induced dendritic cells (iDC) were created from CD14+ monocytes obtained from PBMC by MACS positive selection, then incubated for 6 days with GMCSF and IL-4, with 1 day of LPS according the previously published protocol (Levtova et al., 2017). CFSE labeling to measure proliferation was performed on isolated T cells using a published protocol (Carvajal Gonczi et al., 2017).
2.4 GFAP fragmentation analyses by western blot, immunocytochemistry, enumeration, and granzyme measures
Cultures were washed gently to remove excess T cells, and lysates were prepared with triton-X containing lysis buffer that contained protease inhibitors (Darlington et al., 2008). Reducing SDS-PAGE gels were run with equal amounts of protein loaded for each condition and western blotting with anti-human GFAP (1:4000 dilution; clone G-A-5, Sigma-Aldrich). For terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling (TUNEL) staining, which indicates double stranded DNA breaks, cultures were fixed with 4% paraformaldehyde for 10 min at room temperature, permeabilized with 0.25% Triton X-100, and antibodies diluted in HHG (1 mM HEPES, 2% horse serum, 10% goat serum, in Hank's balanced salt solution) were applied for 1 hr. The terminal TUNEL assay was performed according to manufacturer instruction (Promega, Madison, WI). Images were obtained on epi-fluorescent microscope (Leica) and enumerated using Fiji (NIH). Significance was determined with student t test and one-way ANOVA with a Tukey's multiple comparison test where p < .05 or less was considered to be significant. Lactic dehydrogenase (LDH) assays were performed on cell-free supernatants according to manufacturer's instructions (Roche, Indianapolis, IN). Intracellular granzyme analysis was done by gently scraping astrocyte cultures from the culture plate, and intracellular staining with directly labeled GFAP-Cy3 antibody (Sigma-Aldrich) and granzyme B-APC antibody (BD Bioscience). Human granzyme B in the cell culture supernatants was measured with ELISA (R&D Systems/Cedarlane, kit DY2906-05).
The cytotoxicity of recombinant activated granzyme B was tested at 10 nM according to Lee et al., 2017. To activate granzyme B, the recombinant human pro-granzyme B (R&D systems/Cedarlane) was resuspended at 100 μg/mL in activation buffer (50 mM MES sodium salt with 50 mM NaCl at pH 5.5 from R&D systems/Cedarlane). Samples were incubated in solution at 37°C for 4 hr, with 10 μg of recombinant mouse active cathepsin C (R&D systems/Cedarlane), or the equivalent amount of activation buffer. After incubation, the cysteine protease inhibitor E64 (SIGMA) was added to both tubes at 10 μM to inhibit cathepsin C. For granzyme exposure, media was changed to F12 media plus N1 supplement (SIGMA) to avoid FBS. Astrocytes were incubated for 24 hr with 10 nM active granzyme B, no compound added, 10 nM of nonactivated granzyme B (where cathepsin had been omitted), or staurosporine at 1 μM as a cytotoxicity control. Each test group was performed in quadruplicate. GFAP and TUNEL were imaged for at least five pictures per coverslip (Leica DM16000B).
3 RESULTS
3.1 Helper T cells activated with SEE cause GFAP fragmentation in a granzyme B-dependent, MHC II-independent manner
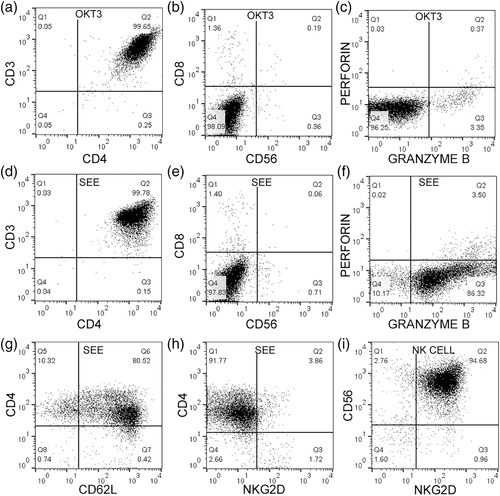
To create helper T cells with cytotoxic potential, a potent superantigen (SEE) was used that stimulates TCR. It was compared to monoclonal OKT3, which cross-links CD3. PBMCs were activated with each stimulus and helper T cells were purified from the samples. Over 99% of the sample had a CD3+ CD4+ profile and were devoid of CD8, a conventional cytotoxic T cell marker. There was also no presence of NK cells, using CD56 as a marker (Figure 1a,b,d,e). Helper T cells activated with OKT3 were perforin negative and less than 4% positive for granzyme B (Figure 1c). When activated with SEE, they were 3.5% positive for perforin and over 85% positive for granzyme B (Figure 1f). Helper T cells expressed CD62L, a marker of central memory T cells (Figure 1g) but did not express cytotoxic receptor NKG2D (Figure 1h). As a control stain, NK cells were positive for CD56 and NKG2D (Figure 1i). The helper T cells did not express FOXP3, a regulatory T cell transcription factor (Figure 4i,j).
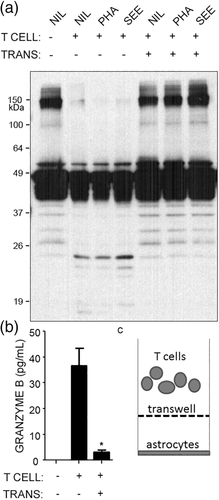
To further explore the impact of helper T cells with cytotoxic properties, SEE activation was used to induce strong granzyme B expression. Primary human astrocytes were cultured with SEE-activated helper T cells, and GFAP fragments were first measured as an indicator of cytoskeletal injury. Helper T cells caused GFAP fragments (~20 kDa) to appear on western blots, along with loss of high molecular weight forms of GFAP (~150 kDa) (Figure 2a). GFAP fragmentation was prevented by separating helper T cells and astrocytes with transwells (Figure 2a). We also added a lectin PHA to facilitate cell–cell interactions or SEE directly into the culture with astrocytes and T cells, but these additives did not appear to have effects beyond what was seen by just adding the SEE-activated T cells to astrocytes (Figure 2a). Upon contact with astrocytes, SEE-activated helper T cells released high levels of granzyme B into the supernatants, which was blocked by transwells (Figure 2b). These transwells prevent cell–cell interactions from occurring, but still allow small molecules to pass (Figure 2c). The requirement for contact between helper T cells and astrocytes suggested that allogeneic TCR and MHC II interactions may have occurred. Primary human fetal astrocytes expressed low but detectable levels of MHC II, and this level remained similar after culturing astrocytes with SEE-activated helper T cells (Figure 3a,b). GFAP fragmentation still occurred when a neutralizing antibody against MHC II was added to the cultures of T cells and astrocytes (Figure 3c,d). The effectiveness of this neutralizing antibody was confirmed in a mixed lymphocyte reaction where allogeneic T cells were cultured with induced-dendritic cells (iDC) expressing high levels of MHC II (Figure 3e,f). Primary human astrocytes express the adhesion molecule CD54 (ICAM-1) (Darlington et al., 2008); however, neutralizing antibodies against CD54 did not prevent helper T cell-mediated GFAP fragmentation (data not shown). Thus, SEE-activated helper T cells cause MHC-II independent, CD54-independent GFAP fragmentation in primary human astrocytes.
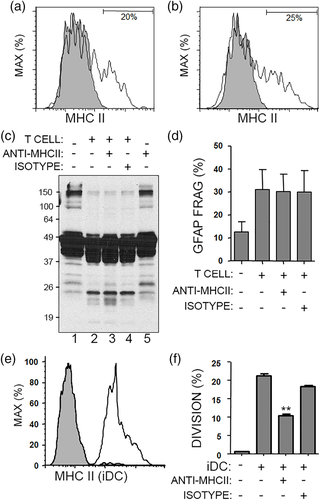
To track the mechanism of action by which SEE-activated helper T cells fragmented GFAP, the amount of granzyme B was measured inside of the astrocytes using intracellular flow cytometry and a gating strategy that allowed for analysis on GFAP+ astrocytes (Figure 4a–c). To test the involvement of granzyme B release, SEE-activated helper T cells were pretreated with CMA which inhibits cells from degranulating and releasing cytotoxic molecules (Kataoka et al., 1996). Astrocytes cultured alone did not contain intracellular granzyme B, while astrocytes cultured with SEE-activated helper T cells contained high levels of intracellular granzyme B (Figure 4d,e). Pretreatment of T cells with CMA attenuated the transfer of granzyme B into astrocytes, confirming that degranulation is required for granzyme release (Figure 4f). Moreover, CMA-pretreated T cells caused less GFAP-fragmentation in astrocytes as compared to vehicle-T cells (Figure 4g,h). As a control, we verified that CMA pretreatment did not alter the level of granzyme B within the T cells (Figure 4i,j).
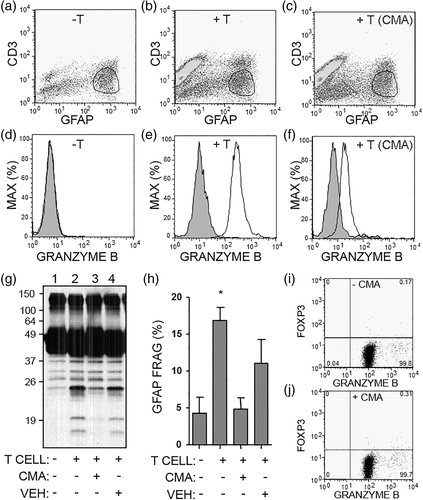
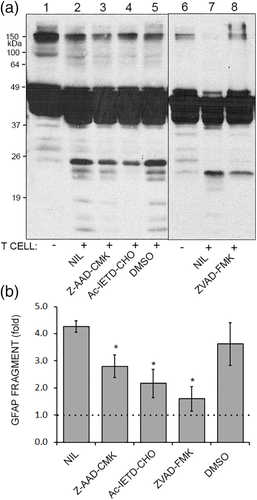
Next, chemical inhibitors were used to probe the pathway of GFAP fragmentation. Primary human astrocytes were cultured with SEE-activated helper T cells along with Z-AAD-CMK (a weak granzyme B inhibitor), Ac-IETD-CHO (a strong granzyme B and caspase-8 inhibitor), ZVAD-FMK (a pan-caspase inhibitor), and a DMSO vehicle control. GFAP fragmentation was attenuated by Z-AAD-CMK or Ac-IETD-CHO, and almost completely inhibited by ZVAD-fmk (Figure 5a,b). In summary, SEE-activated helper T cell release granzyme B upon contact with astrocytes in an MHC-independent manner. Granzyme B enters astrocytes, and GFAP fragmentation occurs due to granzyme and caspase activity.
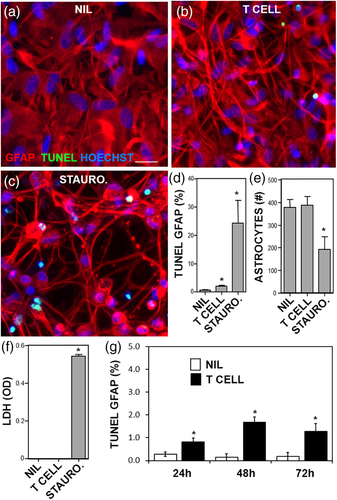
3.2 Sublethal injury of astrocytes by SEE-activated T cells impairs repair capacity of astrocytes in scratch assays and reduces oligodendrocyte support
Caspase activity in astrocytes suggested that apoptosis may have occurred. TUNEL staining was performed to measure double stranded DNA breaks that are typical of apoptosis. SEE-activated helper T cells caused a significant increase in TUNEL+ astrocytes; however, the percentage of TUNEL+ (<2%) was low compared to the positive control staurosporine (more than 25%) (Figure 6a–d). The overall number of astrocytes in the cultures was unaffected by SEE-activated helper T cells but was reduced by staurosporine (Figure 6e). The morphology of astrocytes was clearly altered by SEE-activated helper T cells; the astrocyte projections were thinner and more condensed, consistent in their appearance with an active or ramified astrocyte (compare Figure 6b with a). To determine if necrosis had occurred, LDH release assay was performed. No increase in LDH was observed in astrocytes treated with the T cells as compared to staurosporine, which significantly increased LDH (Figure 6f). As apoptosis can take several days to show DNA breakage, a longer time course was performed for the TUNEL assay. Apoptosis in astrocytes exposed to T cells remained below 2% throughout 72 hr (Figure 6g).
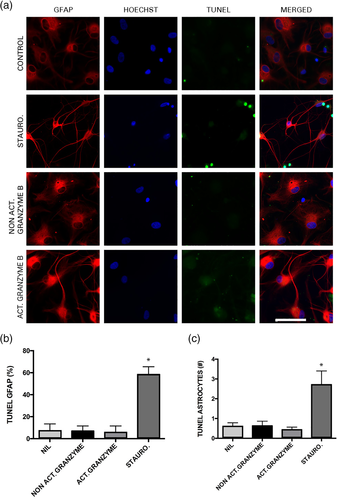
Studies have demonstrated that high concentrations of granzyme B can injure neurons (Wang et al., 2006). To determine if this is also the case in astrocytes, TUNEL staining was performed after treatments for 24 hr with staurosporine (as the positive control), no treatment (negative control), activated granzyme B, and nonactivated (pro form) granzyme B (Figure 7a). Staurosporine treatment increased the total percentage and number of TUNEL+ astrocytes (Figure 7b,c). There was no significant difference between the percent and number of TUNEL+ astrocytes treated with activated or nonactivated recombinant granzyme B (Figure 7b,c). There was, however, a difference in morphology between conditions. Astrocytes treated with activated granzyme B (bottom row of Figure 7a) had different morphology in comparison to astrocytes treated with nonactivated granzyme B or the control (no treatment) as their projections were thin and condensed, resembling more the morphology in the staurosporine condition. Thus, recombinant granzyme B induces a sublethal change in the astrocyte morphology that appears to be similar to the morphological changes induced by SEE activated helper T cells.
Astrocytes migration can be modeled in culture by physically scratching the surface of astrocyte cultures with a high-gauge needle to create a gap (Figure 8a,b). Using this scratch migration assay, primary human astrocytes were exposed to SEE-activated helper T cells and the extent of astrocyte migration into the gap was determined (Figure 8c,d). Fluorescent staining for GFAP showed more clearly that T cells were impairing astrocyte migration (Figure 8e,f). Pooled data from fluorescent micrographs demonstrated that staurosporine completely prevented astrocyte migration, while netrin-1, an axon guidance cue (Kennedy, Serafini, de la Torre, & Tessier-Lavigne, 1994), significantly increased astrocyte migration (Figure 8g), SEE-activated helper T cells significantly inhibited astrocyte migration, and this was blocked by ZVAD-FMK the pan-caspase inhibitor (Figure 8g). Thus, helper T cells caused sublethal injury in astrocytes, which impaired migration of astrocytes in a scratch assay, in a caspase-dependent manner.
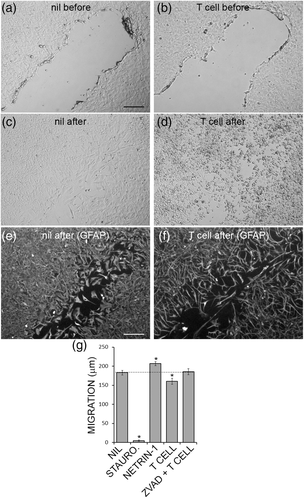
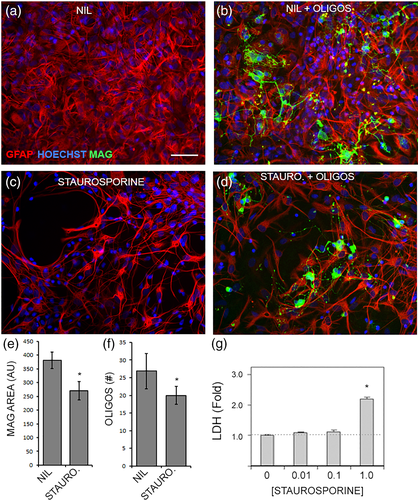
To determine the impact of sublethal injury on astrocyte support for oligodendrocytes, astrocytes were pulsed with a sublethal dose of staurosporine, washed extensively, and then used as a substrate for primary adult oligodendrocytes. The healthy astrocyte cultures had good coverage of the growth surface and supported numerous oligodendrocytes with typically branched processes (Figure 9a,b). Sublethally injured astrocytes had thinner, more compact structures with less coverage of the growth surface, and although the oligodendrocyte process branching looked normal, there were less oligodendrocytes remaining (Figure 9c,d). The overall area of MAG staining was lower in the sublethally injured astrocyte co-cultures compared to healthy astrocytes, as was the number of oligodendrocytes (Figure 9e,f). Sublethal injury of astrocytes had been confirmed with an LDH release assay where the 0.1 μM dose of staurosporine was chosen as it was the highest dose we tested that did not cause cell death (Figure 9g). In summary, T cells activated with SEE can degranulate, release granzyme B into the environment, which then gets inside of astrocytes and causes GFAP fragmentation in a caspase-dependent manner. Astrocytes that are sublethally injured display reduced migration and impaired oligodendrocyte support function.
4 DISCUSSION
The capacity for cytotoxic helper T cells to injure neural tissue is not completely understood. We demonstrated that SEE activated helper (CD4+) T cells expressed and released high levels of granzyme B as a result of contacting astrocytes. Granzyme B is a serine protease released by conventional cytotoxic (CD8+) T cells and NK cells along with perforin whose purpose is to induce apoptosis and the activation of caspases within virus-infected cells or tumors (Bots & Medema, 2006). The helper T cells used in this study did not express perforin, indicating that the granzyme B entry into astrocytes is perforin-independent. Granzyme B can cross cell membranes independently of perforin (Knickelbein et al., 2008; Shi et al., 1997). Soluble granzyme B is also elevated in cerebral spinal fluid of relapsing MS patients (Malmeström et al., 2008), and in vitro studies show that neurons can be sensitive to high levels of granzyme B (Knickelbein et al., 2008; Wang et al., 2006). Thus, granzyme B can be neurotoxic even without perforin. The effects of granzyme B on astrocytes is less studied. Our data are consistent with a model whereby contact between T cells and astrocytes induces a granule-dependent secretion of granzyme B that enters the target cell and contributes to caspase activation. Recombinant active granzyme B did not cause toxicity to astrocytes but altered the morphology as seen by thin and elongated processes that appeared to be similar to what we observed when the SEE-activated helper T cells were added. A previous study added recombinant active granzyme B to fetal brain cultures and reported neuron toxicity but astrocyte toxicity was not observed (Wang et al., 2006). That finding is consistent with our observations that astrocytes are not particularly sensitive to granzyme B toxicity, although their processes appeared to be morphologically altered.
Fragmentation of GFAP was observed in astrocytes upon exposure to SEE-activated helper T cells in an MHCII-independent and CD54-independent manner. Such MHC-unrestricted granzyme B release differs from conventional cytotoxic T cells, which involves MHC/antigen restricted degranulation and perforin-dependent entry into the target. Other reports have documented MHC-unrestricted lethal or sublethal effects of helper T cells on astrocytes (Halachmi, Ben-Nun, et al., 1992). In our study, the involvement of caspases suggested apoptosis was occurring. However, there were very few apoptotic astrocytes in response to SEE-activated helper T cells as compared to staurosporine, which triggers apoptosis when used at high concentrations. There was a notable change in the morphology of astrocytes that were sublethally injured. As seen in the GFAP immunofluorescence images, their processes were thinner and elongated. From a functional standpoint, sublethal astrocyte injury by helper T cells or staurosporine impeded astrocyte migration. The scratch assay showed less migration into a model scar and thin process morphology after addition of SEE-activated helper T cells. Sublethal injury to astrocytes also affected their ability to support primary oligodendrocytes. Less oligodendrocytes could be supported by the injured astrocytes. Branching and morphology appeared to be unaffected in MAG-stained oligodendrocytes. The injured GFAP cytoskeleton was thinner, more compact and occupied less of the growth surface which may account for the loss in support for oligodendrocytes. Similar findings were made with primary neurons which lost their substrate support and detached when astrocytes were injured (Darlington et al., 2008). In a neuroinflammatory condition, loss of the ability to adapt to injury could affect surrounding cells and in turn impact the central nervous system as a whole. In the wake of astrocyte injury, these cells may become slow to adapt to changes and thus impair neuroplasticity.
In conclusion, cytotoxic helper T cells caused GFAP fragmentation in astrocytes independently of MHC II interactions. Fragmentation was mediated in part by the upstream activation of the granzyme B and caspases causing injury to astrocytes. The sublethal injury impaired cell migration of astrocytes in scratch assays and reduced their ability to support oligodendrocytes. This demonstrates that astrocytes are relatively resistant to cytotoxic insult from T cells and granzymes but display functional impairments. Sublethal astrocyte injury by cytotoxic helper T cells is evidently involved in many neuroinflammatory diseases. Lymphocyte suppression and astrocyte regeneration would be a novel target for future therapy.
ACKNOWLEDGMENTS
This work was supported by grants from the Canadian Institutes of Health Research (CIHR) and the Multiple Sclerosis Society of Canada. PJD was supported by a Multiple Sclerosis Society of Canada fellowship. BS was supported by Natural Sciences and Engineering Research Council. The authors thank Dr Chris Law and the Centre for Microscopy and Cellular Imaging at Concordia University for their equipment, support, and expertise. They thank Zoë Stopnicki for graphical layout of figures.
CONFLICT OF INTEREST STATEMENT
There are no conflicts of interest to report for any and all of the authors listed.