Human Schwann cells exhibit long-term cell survival, are not tumorigenic and promote repair when transplanted into the contused spinal cord
Funding information: The Christine E. Lynn Human Clinical Trials Initiative; The John M. and Jocelyn H.K. Watkins Distinguished Chair in Cell Therapies (DDP); The Christine E. Lynn Distinguished Professor of Neuroscience (MBB); CDMRP; The Department of Defense, Grant number: Award W81XWH-10-1-0793; The Bryon Riesch Paralysis Foundation; The Gordon Family Foundation; The Miami Project to Cure Paralysis; The Buoniconti Fund.
Abstract
The transplantation of rodent Schwann cells (SCs) provides anatomical and functional restitution in a variety of spinal cord injury (SCI) models, supporting the recent translation of SCs to phase 1 clinical trials for human SCI. Whereas human (Hu)SCs have been examined experimentally in a complete SCI transection paradigm, to date the reported behavior of SCs when transplanted after a clinically relevant contusive SCI has been restricted to the use of rodent SCs. Here, in a xenotransplant, contusive SCI paradigm, the survival, biodistribution, proliferation and tumorgenicity as well as host responses to HuSCs, cultured according to a protocol analogous to that developed for clinical application, were investigated. HuSCs persisted within the contused nude rat spinal cord through 6 months after transplantation (longest time examined), exhibited low cell proliferation, displayed no evidence of tumorigenicity and showed a restricted biodistribution to the lesion. Neuropathological examination of the CNS revealed no adverse effects of HuSCs. Animals exhibiting higher numbers of surviving HuSCs within the lesion showed greater volumes of preserved white matter and host rat SC and astrocyte ingress as well as axon ingrowth and myelination. These results demonstrate the safety of HuSCs when employed in a clinically relevant experimental SCI paradigm. Further, signs of a potentially positive influence of HuSC transplants on host tissue pathology were observed. These findings show that HuSCs exhibit a favorable toxicity profile for up to 6 months after transplantation into the contused rat spinal cord, an important outcome for FDA consideration of their use in human clinical trials.
1 INTRODUCTION
Spinal cord injury (SCI) is a devastating condition that results in a permanent reduction in the quality of life of the individual. In 2014, 276,000 people were living with a SCI in the US and an estimated 12,500 new injuries occur annually (NSCISC, 2013). Currently, there are no regenerative treatments clinically available that can provide robust restoration of function. In experimental studies, cell therapeutics have been one of the approaches at the forefront of strategies aimed at promoting SCI repair. The introduction of a diversity of cell types, including Schwann cells (SCs) (Bamber et al., 2001; Barakat et al., 2005; Ghosh et al., 2012; Guest, Rao, Olson, Bunge, & Bunge, 1997b; Paino and Bunge, 1991; Pearse et al., 2004a, 2004b; Schaal et al., 2007; Takami et al., 2002; Xu, Chen, Guenard, Kleitman, & Bunge, 1997; Xu, Guenard, Kleitman, Aebischer, & Bunge, 1995a; Xu, Guenard, Kleitman, & Bunge, 1995b; Xu, Zhang, Li, Aebischer, & Bunge, 1999), neural stem cells (NSCs) (Bottai, Madaschi, Di Giulio, & Gorio 2008; Cao et al., 2001; Karimi-Abdolrezaee, Eftekharpour, Wang, Morshead, & Fehlings, 2006; Ogawa et al., 2002; Parr et al., 2008; Ziv, Avidan, Pluchino, Martino, & Schwartz, 2006), olfactory ensheathing cells (OECs) (Cao et al., 2004; Kubasak et al., 2008; Munoz-Quiles, Santos-Benito, Llamusi, & Ramon-Cueto, 2009; Ramon-Cueto, Cordero, Santos-Benito, & Avila, 2000), mesenchymal stem cells (MSCs) (Cizkova, Rosocha, Vanicky, Jergova, & Cizek 2006; Kim, Oh, Lee, & Yoon 2006; Parr, Tator, & Keating, 2007), and bone marrow stromal cells (BMSCs) (Chiba et al., 2009; Fan, Du, Cheng, Peng, & Liu 2008; Neuhuber, Timothy Himes, Shumsky, Gallo, & Fischer, 2005; Ohta et al., 2004; Parr et al., 2007; Zurita and Vaquero, 2004), among others, have been reported independently to provide varying levels of improvement in tissue preservation, axon growth support, remyelination repair and axon conduction as well as functional recovery in animal models. However, unwanted side effects including the development of neuropathic pain (Hofstetter et al., 2005; Lang et al., 2013; Macias et al., 2006), and tumorgenicity (Lee, Tang, Rao, Weissman, & Wu, 2013) have been reported with cell transplants in some experimental paradigms. Evaluating potential pain, toxicity and aberrant effects from the introduction of exogenous cells into the injured spinal cord preclinically, in addition to the reproducible demonstration of efficacy, is a critical step prior to the translation of these approaches to clinical investigation.
SC transplantation, in the form of a peripheral nerve (PN) implant or as an injected, purified cell suspension, has been examined as a therapeutic strategy in various experimental SCI paradigms for over 30 years (Wiliams and Bunge, 2012). The transplantation of SCs into the injured spinal cord has been reported to provide neuroprotection and prevent neuronal cell loss (Pearse et al., 2004a; Schaal et al., 2007; Takami et al., 2002), support axon growth (Bamber et al., 2001; Guest et al., 1997b; Xu et al., 1995a, 1997, 1999) and remyelination repair (Paino and Bunge, 1991; Pauls, Nolte, Forterre, & Brunnberg, 2004; Pearse et al., 2004a, 2004b; Takami et al., 2002; Xu et al., 1995b, 1999) as well as produce the restoration of function (Barakat et al., 2005; Ghosh et al., 2012; Schaal et al., 2007; Takami et al., 2002) in a diversity of animal SCI models. SCs can be harvested from a PN biopsy, purified and expanded to large numbers (more than 0.5 billion) for autologous transplantation into the same individual's injured spinal cord (Bunge and Wood, 2012; Levi et al., 1995; Rutkowski, Kirk, Lerner, & Tennekoon, 1995). The initial translation of SCs into clinical practice for human SCI occurred as implanted PN grafts, a practice which has recently shown indications of efficacy when used in combination with OECs in a human SCI patient in Poland (Tabakow et al., 2014). The implantation of a more purified population of human SCs (HuSCs) has progressed to clinical trials for human SCI outside of the US and to date no adverse effects have been reported (Saberi et al., 2008, 2011).
Currently, the preclinical data supporting the safe and effective use of SCs upon their transplantation into the injured spinal cord has almost exclusively employed rodent-derived cells (Bunge and Wood, 2012), with the exception of work to investigate the utility of HuSCs in a complete spinal cord transection xenograft paradigm in the nude rat. In these studies, reported by Guest and colleagues (Guest et al., 1997a, 1997b), the ability of HuSCs to support regeneration and functional recovery was investigated. HuSCs, suspended in DMEM/Matrigel and allowed to solidify into an organized SC cable within a hollow polymer guidance channel, were inserted as a bridge across a spinal cord gap after complete transection and removal of the T9-T10 spinal cord. It was reported in this model that HuSCs supported axon regeneration and provided a modest improvement in locomotor function, though the studies did not include a systematic evaluation of adverse effects associated with the HuSC transplants (Guest et al., 1997a, 1997b).
To date, the safety and behavior of HuSCs within a contusion SCI experimental paradigm, which better models the human condition, has not been undertaken. The current study sought to examine the cell behavior and host response to HuSCs when transplanted into a contusive SCI paradigm in the nude rat (Kreutziger et al., 2011; Numasawa et al., 2011). The HuSCs were obtained from donors of both genders and from a broad age range. Importantly, the cells were used as either fresh isolates or from cryopreserved preparations, compatible with cellular storage. The evaluation of safety included assessments of HuSC persistence, proliferation, tumorgenicity and biodistribution along the neural axis from 3 days to 6 months post-transplantation. Measurement of host responses to the presence of HuSCs involved the analysis of immune cell infiltration, glial scar formation, tissue preservation and neuropathology. Lastly, the ability of HuSCs to support axon growth and myelination was examined. The data presented herein formed part of the preclinical safety studies used in support of IND 14856 that sought to investigate the safety and efficacy of autologous SC transplantation in a Phase 1 Clinical Trial for sub-acute, complete human thoracic SCI (Anderson et al., 2017) (http://clinicaltrials.gov/show/NCT01739023).
2 MATERIALS AND METHODS
2.1 Preparation of HuSC cultures
Experimental procedures involving human cells were reviewed and approved by the institutional review board (IRB) of the University of Miami. HuSCs were obtained independently from six cadaveric and organ donor sural nerve biopsies following methods previously described by Casella, Bunge, & Wood (1996) with a number of modifications implemented by the Miami Project Cell Processing Group to achieve large numbers of highly pure HuSCs for clinical application (Levi et al., 2016).
In brief, sural nerve biopsies were obtained from adult donors (aged 21–62 years, donor demographic information is presented in Table 1). Donors showed negative standard serology for infectious diseases (HIV, HBV, HCV, HTLV, CMV, EVB, Syphilis, Toxoplasma) and had no known diagnosis of peripheral nerve pathology or other neurological illnesses (e.g., diabetic-related neuropathology). Sural nerves were harvested from cadaveric donors (D01, D02, D03, D04) at an interval of 9–25 hr after the time of death. For the two organ donors (D05 and D06), harvesting of nerves occurred immediately following the removal of the vital organs, which ranged between 1 and 2 hr after aortic clamping (Table 1). Sural nerve biopsies were processed under sterile conditions, during which the nerves were cut into 0.5–1 cm segments and the nerve fascicles dissected from the nerve segments. Fascicles were then placed for 1 week in HuSC growth medium (DMEM supplemented with 10% FBS, 2 µM forskolin, 10 nM heregulin and 50 µg mL−1 gentamicin). Next, the fascicles were dissociated into a single cell suspension (Passage 0, P0 cells) and plated onto laminin-coated flasks (1 µg cm−2; Sigma, St Louis, MO). HuSCs were cultured to 60-80% confluence then trypsinized and split (P1). HuSCs were further passaged to P2 before use for transplantation. The purity of HuSCs was confirmed using immunocytochemistry for S100 (1:200, DAKO, Catalog#: 2031129-2, Carpinteria, CA) a protein highly expressed in SCs (Chelyshev Iu and Saitkulov, 2000), see Figure 1b. In all cases the purity of HuSCs was higher than 80%. HuSCs were used for transplantation at P2, either from ongoing cultures (fresh) or following their thawing and expansion to confluency from cryopreserved stocks. For the cryopreservation cohort, HuSCs were cryopreserved at P2, then later thawed and cultured for 24–48 hr before use. The number of days that HuSCs were kept in cryopreservation for each donor was recorded (Figure 2o–p).
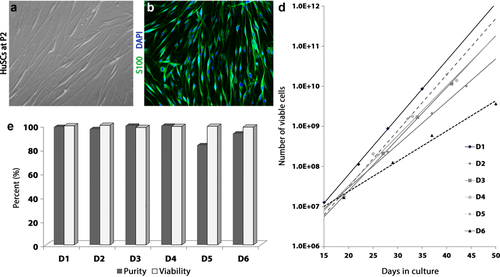
HuSCs exhibited high purity and viability with growth curves displaying more rapid expansion in younger donors. (a) In culture the morphology of HuSCs was largely spindle-shaped when grown to P2 on laminin and (b) cells almost exclusively expressed the Schwann cell marker S100. Cells were costained with DAPI to demarcate nuclei. (c) Purity and viability of HuSCs were high (>90%) among the different donors used except donor D5. (d) Examination of in vitro growth curves across donors showed expansion from P1 to P4 (represented by symbols displayed on the line for time in culture), though the growth rate and cell yield were higher in the younger donors (D1 and D5) compared with the oldest (D6) donor [Color figure can be viewed at wileyonlinelibrary.com]
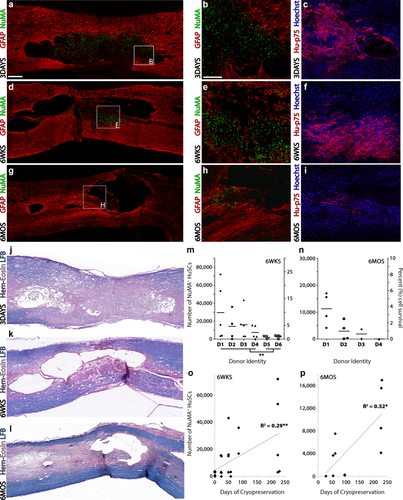
HuSC persistence was observed through 6 months post-transplantation within the injured spinal cord with improved cell survivability when cells were from cryopreserved stocks as opposed to fresh isolates. (a–c) At 3 days following implantation into the injured cord, HuSCs, as identified using human-specific (a-b) NuMA or (c) p75NTR antibodies, were present throughout the lesion site as demarcated with GFAP. (d–f) Lower numbers of (d,e) NuMA or (f) p75NTR-positive HuSCs were found within the lesion at 6 weeks post-transplantation while only scattered (g,h) NuMA or (i) p75NTR-positive cell profiles were identified at 6 months. Sections (c, f, i) were costained with DAPI to identify cell nuclei. Adjacent spinal cord sections from a, d and g are shown stained with hematoxylin, eosin and luxol fast blue in panels (j–l), respectively. Scale bar for (a,d,g, and j–l) = 200 µm and for (b,c,e,f,h, and i) = 100 µm. (m,n) Graphs show the stereological counts of NuMA+ HuSCs (left axis) and percent survival rate (right axis) across animals by donor ID at (m) 6 weeks and (n) 6 months after transplantation. At 6 weeks, the persistence of HuSCs was greater in animals receiving cells from cryopreserved stocks rather than from fresh isolates. (o–p) Linear correlation analysis shows a significant, positive correlation between the length of cell cryopreservation and the number of NuMA+ HuSCs found within the injured spinal cord at (o) 6 weeks or (p) 6 months after transplantation. Statistical significance indicated a *p < 0.05 or **p < 0.01 [Color figure can be viewed at wileyonlinelibrary.com]
| Donor ID | Age/sex | Donor type | Death/aorta clamping to harvest |
|---|---|---|---|
| D1 | 20/Male | Cadaveric | 13 hr 59 min |
| D2 | 53/Male | Cadaveric | 24 hr 4 min |
| D3 | 47/Male | Cadaveric | 24 hr 57 min |
| D4 | 25/Male | Cadaveric | 8 hr 34 min |
| D5 | 23/Female | Organ donor | 1 hr 20 min |
| D6 | 62/Male | Organ donor | 1 hr 44 min |
For comparing the growth curves of HuSCs that had been derived from different donors, some cells were maintained to P4. At each harvest, total cell counts were obtained by staining HuSCs for Syto24 (1:4, Invitrogen, Catalog# S7020, Carlsbad, CA) and for dead cells, Sytox Green (1:4, Invitrogen, Catalog# S7559).
2.2 Animal care and procedures
All animal procedures were approved by the IACUC of the University of Miami and were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals. The 73 adult female athymic nude rats (160–180 g; Charles River Laboratories, Chicago, IL) that were used in these experiments were housed in micro-isolated cages and kept in a dedicated virus antibody free (VAF) room with a 12-hr light/dark cycle. Autoclaved food and water were available ad libitum. Cages, bedding and other housing supplies were autoclaved prior to use and changed every 3 days. Cage changes, clinical observations, and drug administrations for the animals were performed within a laminar flow hood using sterile gloves and supplies. Health and mortality records were kept for the duration of the study. For animals found dead, a mortality report was completed and when appropriate, histopathological analysis was performed.
2.3 Contusive spinal cord injury
To enact a contusive SCI to the thoracic spinal cord, the MASCIS NYU impactor (Gruner, 1992), a well characterized and reproducible model, was employed. For these studies, a mild severity SCI was used to examine HuSC transplantation based upon preliminary studies that showed significantly larger lesions in immunocompromised animals compared with normal rats (unpublished results). Prior to surgery, animals were anesthetized with 4% isoflurane and 1.5 L min−1 of oxygen in a designated induction chamber. Next, ketamine (70 mg kg−1) and xylazine (5 mg kg−1) were injected intraperitoneally for long-lasting anesthesia during surgical procedures. The level of anesthesia was monitored by corneal and hind limb withdrawal reflexes. Once sufficient anesthesia was obtained, the posterior thoracic area of the animals was shaved and aseptically prepared with chlorhexidine (Phoenix Pharmaceutical, St. Joseph, MO). The eyes of the animals received the application of Lacrilube ophthalmic ointment (Allergan Pharmaceuticals, Irvine, CA) to prevent drying. To maintain body temperature at 37°C ± 0.5°C, as measured by rectal probe, animals were placed upon a homoeothermic blanket system (Harvard Apparatus, Kent, UK) during surgery. Under sterile conditions, a longitudinal incision was made along the back of the animal and a laminectomy was performed to expose the dorsal spinal cord at the thoracic (T8) vertebral level without disrupting the dura mater underneath. The exposed spinal cord was injured by dropping a 10-g rod from a height of 6.25 mm. To ensure consistency in the injury severity, the parameters of the impact were monitored and animals excluded when the impact parameters were determined to be out of range: height or velocity errors >7% from the expected value or if the compression distance was not between 0.75 and 1.25 mm (Patel et al., 2010; Pearse et al., 2004a). After SCI, the overlying musculature was sutured and the skin closed using wound clips. The rats received post-operative treatment and care that included the administration of analgesics twice a day for 3 days (buprenorphine, 0.03 mg kg−1, subcutaneous), fluids once a day for 3 days (ringer electrolyte solution, 6 cm3, subcutaneous) and antibiotics once a day for 7 days (gentamicin, 5 mg kg−1, subcutaneous). Bladder expression was performed twice a day until animals recovered spontaneous voiding. After surgery, animals were kept in a warmed cage with water and food easily accessible.
2.4 HuSC transplantation
Prior to spinal cord implantation, HuSCs at P2 underwent three washes with medium to remove mitogens, laminin, and bovine products and were trypsinized, collected for centrifugation, and then re-suspended in DMEM-F12 for counting. HuSCs were prepared in 10 µL aliquots at a density of 50,000 cell µL−1 in DMEM-F12 medium and kept on ice for a maximum of 2 hr prior to implantation. Cell injection occurred at 4 weeks after SCI, modelling the clinical protocol. Animals were anesthetized with ketamine/xylazine and the injured spinal cord exposed by removing the overlaying scar tissue while avoiding unintended injury to the underlying dura mater and spinal cord. Following spinal cord re-exposure, animals were fixed by the spinal process rostral to the injury site (T7) with a spinal clamp attached to a stereotactic device (Narishige instruments, Catalog#SR-5R/STS-B, Tokyo, Japan). Upon fixation of the spinal process, a 10 µL Hamilton syringe was loaded with 8 µL of HuSCs in suspension. The syringe was attached ahead of time to a liquid silicon pre-coated, pulled-and-beveled glass capillary needle (∼120 µm diameter). Once fixed in a stereotactic micromanipulator, the needle was lowered through the dura mater at the center of the lesion, which was visualized by a darkened discoloration of the spinal tissue. After lowering the needle to a depth of 1.0 mm into the spinal cord, an injection of 6 µL of HuSCs (300,000 cells total) was performed at a rate of 2 µL min−1 using an automatic microinjector (Quintessential Microinjector, Stoelting, Wood Dale, IL). The needle was kept in place for an additional 3 min to prevent leakage upon withdrawal. A concentration of 50,000 cells per µL was chosen for HuSC dose based upon the two to three times larger size of the cells compared with that of rat SCs. Following HuSC implantation, animals received the same postoperative care described after SCI. HuSCs from at least two different donors were prepared and used for transplantation, independently in different hosts, on any given day. HuSCs originating from cadaveric donors (D1, D2, D3, and D4) were previously frozen, prepared from cryopreserved stocks, and allocated evenly between survival cohorts (3 days, 6 weeks, and 6 months). HuSCs from organ donors (D5 and D6) were transplanted fresh and allocated to the 6 weeks survival cohort. The group allocation and survival cohorts are summarized in Table 2. This table includes only those animals that survived to study endpoint.
| Survival cohort | Cell source | Type of cell preparation | N (endpoint) | Measured outcomes |
|---|---|---|---|---|
| 3 Days | D1 (cadaveric) | Cryopreserved | 4 | Animal morbidity, HuSC distribution, implant morphology, NK cell presence, astrocyte-HuSC intermingling |
| D2 (cadaveric) | Cryopreserved | 4 | ||
| D3 (cadaveric) | Cryopreserved | 4 | ||
| D4 (cadaveric) | Cryopreserved | 4 | ||
| 6 Weeks | D1 (cadaveric) | Cryopreserved | 5 | Animal morbidity, HuSC survival, proliferation and distribution, implant morphology. NK cell presence, tumorgenicity, tissue volume analysis, astrogliosis, axon retraction, axon growth and SC myelination (P0), astrocyte-HuSC intermingling |
| D2 (cadaveric) | Cryopreserved | 4 | ||
| D3 (cadaveric) | Cryopreserved | 5 | ||
| D4 (cadaveric) | Cryopreserved | 5 | ||
| D5 (organ donor) | Fresh | 5 | ||
| D6 (organ donor) | Fresh | 5 | ||
| 6 Months | D1 (cadaveric) | Cryopreserved | 4 | Animal morbidity, HuSC survival, proliferation and distribution, implant morphology, tumorgenicity, tissue volume analysis, astrogliosis, axon retraction, axon growth and SC myelination (P0) |
| D2 (cadaveric) | Cryopreserved | 4 | ||
| D3 (cadaveric) | Cryopreserved | 3 | ||
| D4 (cadaveric) | Cryopreserved | 1 |
To improve the long term survival of HuSCs in this xenotransplant paradigm, animals received additional immunosuppression with anti-Asialo GM1 antibody (50 µL intraperitoneal; Wako, Catalog#986-10001, Richmond, VA) every 3 days starting at 3 days before transplantation to block natural killer (NK) cell activity (Drewinko, Moskwa, Lotzova, & Trujillo, 1986).
2.5 Histological procedures
At endpoint (3 days, 6 weeks or 6 months post-transplantation), animals were deeply anesthetized (ketamine 140 mg kg−1; xylazine 7 mg kg−1) and then transcardially perfused with 250 mL of cold 0.9% NaCl followed by 400–500 mL of 4% Paraformaldehyde (PFA). The spinal cord was extracted and post-fixed in 4% PFA for at least 5 days. Following post-fixation, the injury site was identified and a 2 cm segment of the thoracic spinal cord (T6-T10), encompassing at its center the lesion, was blocked. The remaining cord was then cut into additional 2 cm segments corresponding approximately to the following anatomical regions: (1) cervical (C1 to T1), (2) rostral thoracic cord (T2 to T5), (3) lumbar (T11-L4) and, (4) sacral cord and cauda equina. After tissue blocking, the spinal cord segments were paraffin embedded and sagittally sectioned on a microtome at a thickness of 10 µm. Sections were collected into 10 series (100 µm interval between sections) for histological analysis. The spinal cord injury-graft site from two HuSC transplanted nude rats with a 6 week survival period that were not part of the main experiment were sagittally sectioned on a cryostat at 20 um to be used for human-specific P0 immunohistochemistry.
2.6 Immunohistochemistry
To identify the transplanted HuSCs and examine their interaction with selected host spinal cord tissue elements, immunohistochemistry with specific cell markers was employed. Tissue series from the injury-implant site were subjected to immunohistochemistry with one or more of the following primary antibodies: anti-natural killer cell activation structures, ANK61 (1:200; catalog#: ab36392, Abcam, Cambridge, MA); anti-rat CD68 (1:200; catalog#: MCA341R, Serotec, Raleigh, NC); anti-glial fibrillary acidic protein, GFAP (1:1000; catalog#: Z0334, Dako; or catalog#: GFAP, Aves, Tigard, OR); anti-human Ki-67 antigen clone MIB-1, Ki-67 (1:100; catalog#: M724029, Dako); anti-high molecular weight neurofilament, NF-200 (1:100; catalog#: N4142, Sigma–Aldrich, St. Louis, MO); anti-low molecular weight neurofilament, NF-L (1:500; catalog#: PA1-4745, Thermo Scientific; or 1:100; catalog#:C28E10, Cell Signaling technology, Danvers, MA); anti-human-specific nuclear mitotic apparatus marker, NuMA (1:500; catalog#: PA3-16829, Affinity Bioreagents, Rockford, IL); anti-myelin protein zero (multispecies), P0 (1:100,Catalog#: ab39375; Abcam); anti-myelin protein zero (human specific), hP0 (1:10, P0592; a gift of Drs. M Schachner and R. Martini); anti-low affinity neurotrophin receptor (human specific), p75NTR (1:400; catalog#: ab18877, Abcam). In preparation for immunohistochemistry, paraffin tissue sections were first de-paraffinized in xylene and rehydrated through a series of ethanol washes. Antigen retrieval was performed using Citrate buffer (pH 6.0, 20 min) within a water bath (96°C). Slides were cooled for 20 min at room temperature and then exposed to Sudan black solution (0.05%, 10 min) to quench background fluorescence. Following blocking with 5% normal goat serum for 60 min, to prevent non-specific antibody binding, sections were incubated overnight with specific primary antibodies at 4°C. Sections were then washed 5× with PBS and incubated with appropriate Alexa goat anti-rabbit, goat anti-mouse or goat anti-chicken fluorescent secondary antibodies (1:200; Molecular Probes, Eugene, OR) diluted in blocking solution, for a period of 2 hr at room temperature. The blocking buffer also contained Hoechst 33342 (1:500, Invitrogen, Catalog# H3570) to permit fluorescent labeling of cell nuclei. After washing with PBS 3×, slides were dried on a slide warmer for 10 min, cover-slipped with Vectashield mounting medium (Vector Laboratories, Burlingame, CA), and stored at 4°C until imaged.
To ensure the specificity of observed immunoreactivity, both negative and positive controls were included in each staining run. Negative controls consisted of the incubation of the primary or secondary antibodies alone. Positive control tissues were used for the detection of non-rodent or non-CNS-related proteins, which included: human Schwannoma tissue (for NuMA and human Ki-67 antibodies), rat peripheral nerve (for the multispecies P0 antibody), human cauda equina (for the human P0 antibody) and rat spinal cord tissue with a known high number of HuSCs present at 3 days post-transplantation (for the human p75NTR antibody).
2.7 Neuropathology assessment
Sequential serial sections (100 µm interval between sections) from spinal cord segments (cervical, high thoracic, low thoracic, lumbar, sacral and cauda equina) obtained from all animals allocated to the 6 week and 6 month cohorts were stained with hematoxylin, eosin and Luxol fast blue. The staining was employed to enable the gross neuropathological evaluation of the injured, HuSC implanted spinal cord for any unexpected pathological changes, evidence of aberrant cell proliferation, or signs of tumorigenicity. Sections were examined in an unbiased and blinded fashion under light microscopy by a certified Neuropathologist. In addition to solid tumor formation, the general architecture of the injury-implant was qualitatively reported. For quality control, slides were included with spinal cord sections from two positive control animals that received lesion site injections after SCI of a glioma cell line (ATCC, Catalog # CCL-107, Manassas, VA; part of a preliminary tumorgenicity experiment). Slides containing sections from an injured, nontransplanted animal at 6 months post-SCI were also included as a negative control in this evaluation
2.8 Quantification of NuMA+ HuSC profiles within the injured spinal cord
Transplanted HuSCs were identified following immunohistochemical labeling with the human-specific nuclear marker NuMA (Price and Pettijohn, 1986). NuMA+ HuSCs were quantified in sagittal tissue sections (serial, 100 µm interval between sections) using an unbiased method with computer-assisted fluorescent microscopy and Stereological software (Stereo Investigator, MicroBrightfield Inc, Williston, VT) as described previously (Ghosh et al., 2012; Pearse et al., 2007). Briefly, in serial section manager the area containing the HuSC implant was first contoured under the 10× objective and logged into Stereo Investigator. Next, using optical fractionator the area was divided into grids and sampling areas within the grids [50 × 50 µm2 counting frame] were used to mark and count positive cell profiles under a 40× objective. Algorithms for estimating the total NuMA+ HuSC profile counts within the volume of cord sampled were used according to the software. For this analysis, animals from the 6 weeks and 6 months survival cohorts were evaluated. Approximately 10–12 sagittal sections, across the entire width of the spinal cord, were analyzed for each animal.
2.9 Assessment of HuSC biodistribution
The spatial distribution of HuSCs across all spinal cord levels following their initial deposition into the injury epicenter was qualitatively assessed using fluorescent microscopy in sagittal serial sections (100-µm interval) stained with antibodies against NuMA and GFAP.
The biodistribution of NuMA+ HuSCs was initially characterized within the mid-thoracic (injury-implant) segment in sections where nuclear immunoreactivity was identified. The spatial descriptors for the localization of NuMA+ HuSCs were made according to the following anatomical regions: the injury cyst (a region devoid of healthy host tissue elements), the injury penumbra (the peri-lesional area surrounding the cyst, <1 mm in thickness and exhibiting the presence of both host cells as well as immune cell infiltration), the adjacent rostral and caudal cord tissue (1 to 5 mm from the edge of the cyst), the proximal rostral and caudal cord (6 to 10 mm from the edge of the cyst) as well as the meninges, dorsal roots and dorsal ganglia. The presence or absence of GFAP immunoreactivity permitted the demarcation of the normal host tissue-lesion site boundaries as well as allowed the identification of other tissue elements (i.e., meninges, dorsal roots, and dorsal root ganglia). Computer-assisted fluorescent microscopy and stereological software tools (Stereo Investigator, MicroBrightfield) were used to measure the migration or egress distance for NuMA+ HuSCs into tissue regions adjacent to the lesion site by microscopy using a 20× objective. The examination of cell localization was performed using a zig-zag review pattern to avoid repetitive scanning.
Beyond the immediate injured spinal cord segment and transplant site (2 cm length), HuSC biodistribution was also examined in spinal cord segments above and below the level of the lesion for the entirety of its length (cervical, lumbar, sacral, and rostral thoracic cord). The spinal cord biodistribution of HuSCs was performed in all animals from the 3 days, 6 weeks, and 6 months post-transplantation survival cohorts.
2.10 Determination of the transplanted HuSC proliferation rate
The rate of HuSC proliferation was estimated in animals following the comparative quantification of cells double-labeled with NuMA and the cell proliferation marker Ki-67 when compared against the total NuMA+ cell population. The Ki-67 antigen is a large nuclear protein preferentially expressed during all active phases of the cell cycle (G1, S, G2 and M-phases), but is absent in resting cells (G0-phase) (Scholzen and Gerdes, 2000).
The rate of proliferation for the transplanted HuSCs was obtained by counting the number of NuMA and Ki-67 immunoreactive nuclear cell profiles in tissue sections at 100 µm intervals. The analysis was performed using fluorescent microscopy and a hand held enumerator. Each tissue section was visually scanned for NuMA+ and Ki-67+ nuclear profiles at 40× using a zig-zag pattern. Single and dual immunoreactive profiles were recorded independently. A percent of double labeled cells (NuMA+ and Ki-67+) compared with single labeled (NuMA+) cells was obtained for each sample.
2.11 Evaluation of activated NK cell counts as a measure of GM1-mediated immunosuppression
The ANK61 antibody was used to recognize the NK cell activation structures antigen, which is highly expressed by IL-2 activated NK cells (Luo et al., 2000). To evaluate the efficacy of the GM1 antibody to suppress NK cell activity, we quantified the number of immunoreactive NK cells within the lesion and perilesional areas in a selected cohort of transplanted animals from the 3 days and 6 weeks survival groups. Spinal cord tissue sections containing the lesion-implant area were selected from two animals (one each with the highest and lowest NuMA+ cell counts) per donor cohort. Therefore, a total of eight samples in the 3 days cohort (D01, D02, D03, and D04) and 12 samples in the 6 weeks cohort (D01, D02, D03, D04, D05, and D06) were selected for evaluation. Quantification of ANK61+ immunoreactive cell profiles was performed using Stereo Investigator software. Three contiguous sections containing the highest numbers of NK cells were selected. The spinal cord tissue area containing ANK61 immunoreactive cells was identified, contoured under a 10× magnification and logged into Stereo Investigator. Using optical fractionator, the contoured area was divided into grids (50 × 50 µm2 counting frame) to mark and count positive cell profiles under a 40× objective. Algorithms for estimating the total NK profile counts within region of cord sampled were obtained according to the Stereo Investigator software.
2.12 Measurement of preserved tissue
To examine the effect of HuSC implantation on secondary tissue injury following SCI, stereological quantification of preserved (white and grey matter) tissue volumes was performed. For this analysis, three sequential serial sections (10 µm thickness, 200 µm interval between sections), stained with hematoxylin, eosin and Luxol fast blue, were analyzed using computer-assisted microscopy and volumetric image analysis software (Neurolucida, MicroBrightfield) as described previously (Pearse et al., 2004a). The three spinal cord sections chosen for the analysis included the section closest to the midline (by the identification of the central canal) and the two sections immediately either side. Next, the center of the lesion was marked and a region of spinal cord encompassing lengths of 5 mm rostral and caudal was selected to be included in the analysis. For this outcome 11/11 animals from the 6 months cohort and 27/29 animals from the 6 weeks group were included. Two samples from the 6 weeks group were excluded due to tissue sectioning issues which did not permit an accurate tissue volume estimation. For each section evaluated, the perimeter (contour) of the total spinal cord (using the 10× objective) was first outlined followed by separate contours for each of the normal-appearing gray and white matter (using the 20× objective). Normal-appearing preserved gray matter was distinguished from damaged tissue by the presence of healthy neurons and normal cellular density (without the presence of numerous nuclei that would be indicative of immune cell infiltration). Normal-appearing preserved white matter was defined by dark blue (not pale blue) filamentous staining without the signs of immune cell infiltration or evidence of myelin fragmentation. Luxol fast blue filamentous staining within the lesion-implant region, which could represent SC myelination, was not measured as preserved white matter. Each traced section was logged into the Neurolucida software using serial section manager, which tracked the positions of each section within the 3D Z-stack. Algorithms for the computation of Z-stack volumes of the injured spinal cord segments from areas measured on each of the three serial section were performed according to NeuroExplorer 3.0 (MicroBrightfield) and the 3D enclosed volume contour calculation.
2.13 Imaging
Micrographs of spinal cord sections in their entirety were taken using the meander scan function of Stereo Investigator (MicroBrightfield Inc) on a fluorescent microscope with camera (Olympus BX51; MBF-CX9000 camera) under a 20× objective. To maintain imaging consistency, the same exposure, gain and filter channel color settings were used for all samples stained with the same marker. For GFAP, ED1, and NF-200, three spinal cord sections closest to the midline (defined by the visualization of the central canal) were imaged. For the NF-L marker the three consecutive tissue sections containing the HuSC implant center were selected. Immunostained preparations for the P0 marker were imaged using a confocal microscope (LSM710, Carl Zeiss Microscopy) under 40× magnification. For these micrographs, Z-stack images (1 µm thick × 5 slices) of a 400-μm length, encompassing the HuSC implant center, were obtained from three consecutive tissue sections at a laser line of 630 nm. Then, a maximum intensity projection composite was obtained. For other markers, representative images of the immunostained preparations were obtained using confocal microscopy (LSM710, Carl Zeiss Microscopy, Jena, Germany) under either 40× or 60× magnification. Z-stack images (1 µm thick × 5 slices) at the region of interest at laser lines of 405, 488, 594, and 630 nm based on the specific Alexa-flour secondary antibodies used. To maintain consistency, the same image settings (laser intensities, gain, contrast) were used for all samples in each staining combination. The images were processed using the Zeiss microscope software with a median filter to reduce background. Finally, a maximum intensity projection composite was obtained.
2.14 Measurement of GFAP, NF-200, ED1, NF-60 and P0 immunoreactivity
To quantify the immunoreactivity density of the markers used to measure host cellular responses, images of immunostained spinal cord tissue sections were first converted to black and white images and thresholded to demarcate the area of positive immunostaining in Adobe Photoshop CS3. This area was then measured using Image J (Image Processing and Analysis in Java) (Collins, 2007). The analysis was performed by observers that were blind to the experimental design.
For GFAP, NF-200, and ED1, the meander scan images were separated in half, along the epicenter of the lesion, into rostral and caudal parts that were then cropped to a length of 500 µm distally to contain both regions of the lesion-implant as well as the surrounding host spinal cord tissue. In images of GFAP and NF-200, the area of immunoreactivity was expressed relative to the total area of tissue for each region (as a percent of pixels). To estimate the number of ED1+ cells in the rostral and caudal segments, the area of ED1 immunoreactivity measured was divided by the average area of an ED1+ cell profile (this was independently recorded and averaged from 50 randomly identified, individual ED1+ cells). To determine the degree to which the HuSC implants were supportive for axon growth and myelination, the immunoreactive densities of NF-L or P0, respectively, within the lesion-implant were measured. Meander scans of spinal cord sections were cropped to include only the lesion-implant area by overlaying a contour from an adjacent imaged section stained with hematoxylin, eosin and luxol fast blue. The area of immunoreactivity for NF-L or P0 was measured (as pixels) and averaged across the three meander scans.
2.15 Statistical analysis
Statistical analysis was performed using Graphpad Version 4.0 (Graphpad Software, La Jolla, CA). For comparison between groups, an unpaired student t test with unequal variance (two groups comparison, i.e., fresh vs. cryopreserved cells) or a one-way-ANOVA (i.e., multiple groups analysis) was performed followed by a Bonferroni post-hoc test. Differences were accepted to be statistically significant at p < .05 and the confidence interval of significance was expressed using asterisks; *p < .05, **p < .01, or ***p < .001. All errors given are expressed as the standard error of the mean (SEM). For independent linear correlation using Pearson correlation coefficients (R2), standard R tables were employed for the calculation of statistical significance from the degrees of freedom (n − 2).
3 RESULTS
3.1 Animal morbidity and mortality
For the duration of the study 25 health concerns were reported and investigated. Morbidity and mortality cases are summarized in Table 3. In 23/25 reports, animals were identified as having an opportunistic bacterial infection. All of the cases were identified after the initiation of the additional immunosuppression regimen with the GM1 antibody. The most common infection, identified in 15/25 cases, was a pink macular rash on the skin that presented over part of, or across, the entire body and which during the course of 3–4 days became small, crusted lesions. Pathological examination of these cases showed that the bacterial infection extended to the subcutis and bacterial cultures of lesion swabs identified the growth of Staphylococcus spp. Animals exhibiting this skin rash were treated promptly with gentamicin (35 mg/oz.) in the drinking water for 14 days. In animals treated in this fashion 11/15 showed a gradual improvement, with a reduction in the number and severity of the skin lesions, while the remaining animals deteriorated and were either euthanized or found dead. In 5/25 cases, animals were found dead without a previous health concern reported. Necropsy and histopathological findings in these animals were suggestive of peritonitis, likely associated with the repetitive I.P. injections of the GM1 antibody. In 2/25 cases a bacterial eye infection was diagnosed and in 1/25 cases an infected leg ulcer was reported. In 2/25 cases pathological investigations were inconclusive. One animal was euthanized pre-study endpoint due to the presence of a soft skin mass on the right lower back. Although histopathological analysis of this lesion was not performed, neuropathological analysis of the CNS in its entirety and organs showed no signs of abnormal cell proliferation or tumorgenicity. Overall, pathological evaluation of the tissues from animals included in the study that reached endpoint or exhibited morbidity pre-endpoint did not identify any adverse changes related to the HuSC therapy.
| Diagnosis | Description | No. cases | Mortality/early euthanasia |
|---|---|---|---|
| Bacterial skin infection | Skin lesions (started as a pink macular rash that became scabrous, identified as Staphiloccoco spp.) | 15 | 4 |
| Peritonitis | Animals found dead without a health concern reported. Necropsy and microscopy findings suggested peritonitis | 5 | 5 |
| Eye infection | Red eyes with purulent secretion | 2 | 1 |
| Pressure ulcer | Skin irritation and lesion on leg | 1 | 1 |
| Inconclusive | Euthanized early due to the presence of a skin mass on lower back | 1 | 1 |
| Inconclusive | Found dead without a health concern reported. Inconclusive pathology | 1 | 1 |
3.2 Purity, viability, and growth curves of donor HuSCs
HuSCs were obtained in high numbers from the six cadaveric and organ donors (male and female, aged 20–62; Table 1 and Figure 1). Cultured HuSCs presented a classic spindle shaped cell morphology (Figure 1a,b), S100 immunoreactivity and developed a swirling pattern of cell orientation when grown on laminin-coated flasks. At the time of transplantation, HuSC viability was >98% for all donors, while purity (measured as the percentage of cells immunoreactive for S100) was >90% for all donors except D5, which exhibited a purity of 83% (Figure 1c). After sural nerve harvest, similar initial numbers of HuSCs were obtained from all six donors and the HuSC growth curves from each donor showed exponential expansion of cells, with growth rates highest in younger donors (Figure 1d). The growth rate for HuSCs was greatest in donor D1 (male, 20 years old), with cells reaching P4 confluence at 35 days of culture, whereas donor D6 (male, 62 years old) exhibited the slowest growth rate, reaching P4 confluence at 50 days of culture. The average time taken for the HuSCs to reach P4 confluency was 42 ± 2 days.
3.3 The persistence of HuSCs after transplantation
Though stereological assessment of HuSC numbers in the 3 day cohort was not performed, NuMA or p75NTR and GFAP or Hoechst stained sections from the injured, transplanted spinal cord segment showed a dense implant within the lesion at this time (Figure 2a,b). Hematoxylin, eosin- and luxol fast blue-stained sections revealed that the lesion site was largely filled by the HuSC implant with little cavitation (Figure 2j)
The quantitative evaluation of NuMA+ HuSC profiles was performed in 29/30 rats from the 6 weeks cohort (1 animal was euthanized pre-endpoint due to a severe skin infection that did not respond to treatment). These animals received HuSCs originating from one of the six donors (D1, D3, D4, D5, and D6, five animals each and D2, four animals). The HuSCs prepared from donors D1, D2, D3, and D4 were grown to P2, cryopreserved and then thawed for transplantation while for donors D5 and D6, the HuSCs were harvested, grown to P2 and used for transplantation without cryopreservation (fresh). The presence of NuMA+ HuSC profiles within the injured spinal cord segment was confirmed in 25 out of 29 immunostained preparations. When expressed against the original number of transplanted HuSCs, the percent cell survival post-implantation for the 6 week cohort ranged from 0 to 23.9%, with the average survival rate across all donors and animals being 3.9% ± 1.1% (Figure 2m). Stained sections from the injured, transplanted spinal cord segment for NuMA and GFAP from an animal with a high HuSC survival rate at 6 weeks shows a compact implant, with scattered NuMA+ nuclei in a pocket of lesioned tissue that is surrounded by several small cysts (Figure 2d,k). The survival rate of HuSCs within the injured spinal cord was significantly higher in those animals receiving cryopreserved cells (5.9% ± 1.1%, n = 19) as opposed to those implanted with freshly prepared HuSCs (0.8% ± 0.1%, n = 10; p < 0.001). The number of days that HuSCs were maintained in cryopreservation before their preparation for transplantation presented a positive and significant correlation with the HuSC survival rate (Figure 2o; p < 0.01, R2 = 0.29). The donor with the longest period of HuSC cryopreservation prior to use (D1, 224 days) exhibited the highest average survival rate for transplanted HuSCs, 9.9% ± 4.6%.
The presence of NuMA+ HuSCs cell profiles was examined in 11/23 animals from the 6 months post-implantation cohort (12 animals were either euthanized or found dead pre-endpoint, Table 3). These animals received HuSCs originating from one of the four cryopreserved-only donors (D1 and D2, four animals; D3, two animals; D4, one animal). In comparison to 6 weeks post-implantation, fewer NuMA+ HuSCs were detected within the injured spinal cord at 6 months. An average survival rate for HuSCs of 1.8% ± 0.6% was obtained for the animals that reached endpoint, with HuSCs absent in one sample (Figure 2n). NuMA and GFAP stained tissue sections (Figure 2g) of the injured, implanted spinal cord segment from an animal with the highest HuSC survival rate at 6 months shows a few scattered NuMA+ nuclei in groupings that were interspersed within the lesion and perilesional areas. The number of days that HuSCs were cryopreserved before their preparation for transplantation also presented a positive and significant correlation with the HuSC survival rate at 6 months post-implantation (p < 0.05, R2 = 0.53; Figure 2p). The donor with the longest period of HuSC cryopreservation prior to use (D1, 228 days) presented an average survival rate of 3.8% ± 1.0% for transplanted HuSCs among the animals that reached the 6-month endpoint.
3.4 Effectiveness of NK cell depletion following GM1 antibody administration
Examination of the HuSC transplanted spinal cord at 3 days and 6 weeks after grafting revealed the presence of NK cells in all samples as identified by the cell specific marker ANK61 (data not shown). NK cells were found predominantly within the lesion-implant, though a few cells were present within the penumbra region and adjacent tissue. The numbers of NK cells did not significantly differ from 3 days (lesion; 485 ± 74 cells mm−2 and the penumbra; 97 ± 16 cells mm−2) to 6 weeks (lesion; 591 ± 181 cells mm−2 and the penumbra; 87 ± 15 cells mm−2) post-transplantation. Linear correlation analysis between the number of NuMA+ HuSCs within the injured spinal cord and ANK61+ NK cell counts showed no correlation between the putative depletion of NK cell numbers and NuMA+ HuSC persistence (data not shown).
3.5 Low proliferative rate of HuSCs following transplantation with no evidence of tumorgenicity
Assessment of NuMA+ HuSC proliferation was obtained by quantification of their coimmunoreactivity with human-specific Ki-67 (Figure 3a,b; NuMA, green; Ki-67, red). Examination of the implant after double staining revealed very few transplanted HuSCs that were actively undergoing proliferation at 6 weeks (0.09% ± 0.05%) or 6 months (0.35% ± 0.25%) after transplantation (Figure 3c,d). Those isolated HuSCs co-labelled for Ki-67 and NuMA+ were found primarily at the boundary of the implant (Figure 3a). An absence of human-specific Ki-67+ cell profiles was observed in 23/29 and 7/11 of the animals from the 6 week and 6 month cohorts, respectively. In contrast, significant Ki-67/NuMA co-immunoreactivity was obtained in the positive control sample, a section of tissue from a human Schwannoma biopsy (Figure 3b, arrows). No significant differences in Ki-67+ cell counts were observed in transplanted animals from the different donors or across times post-transplantation. Further, there was no correlation between the degree of HuSC persistence and the recorded proliferation rates.
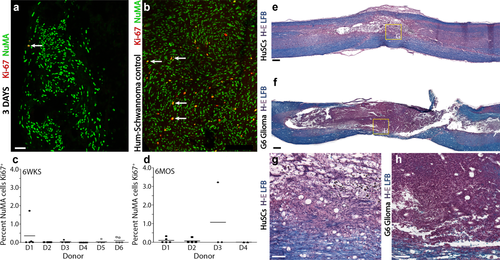
Transplanted HuSCs exhibited low proliferation rates and an absence of tumorigenicity after SCI. (a,b) Costaining for human-specific Ki-67 and NuMA of (a) a spinal cord specimen containing HuSCs at 3 days after transplantation and (b) a biopsy of a human Schwannoma shows the marked difference in the number of Ki-67-positive nuclei between the two samples. Scale bar for (a and b) = 200 µm. (c,d) Stereological quantification of colabeled Ki-67/NuMA+ nuclei at (c) 6 weeks and (d) 6 months after transplantation shows very low proliferation indices (0–3.5%) across all samples as displayed by donor ID. (e–f) Comparison of the injured spinal cord segment from animals receiving (e, g) HuSCs or (f, h) G6 glioma cells at 6 weeks following transplantation. Whereas glioma cell implants exhibit high cell mitosis, volume expansion and poor host cord integration, HuSCs do not show these features. Scale bar for (e and f) = 200 µm and for (g and h) = 50 µm [Color figure can be viewed at wileyonlinelibrary.com]
The low proliferative index of HuSCs post-implantation was confirmed by blinded, neuropathological examination of hematoxylin, eosin- and luxol fast blue-stained tissue sections, where the presence of solid tumors or active mitosis was not observed within the spinal cord along the entirety of the neural axis. In addition, no unexpected pathological findings were found along the neural axis in animals analyzed from all post-transplantation time points. In contrast, after intraspinal implantation of the rat G6 glioma cell line, a positive control for tumorgenicity, tissue sections presented an extremely high cellular density, indicative of active mitosis, with putative neoplasm formation (Figure 3f,h).
3.6 Limited HuSC biodistribution along the neural axis
The distribution of NuMA+ HuSC profiles within the spinal cord was examined relative to their initial site of deposition within the lesion epicenter. Whereas the migration of SCs across adjacent segments of damaged nerve is critical to their reparative actions following peripheral nerve injury, it has been previously demonstrated that the distribution of rat SCs is highly restricted to lesion implant sites when they are injected into the injury epicenter after contusive SCI (Pearse et al., 2007; Barakat et al., 2005; Wang and Xu, 2014). The biodistribution of NuMA+ HuSCs was similar to that of rat SCs with cells being restricted to the injury cyst and immediate lesion penumbra across all time points following intraspinal injection into the injury epicenter. The biodistribution of HuSCs was confirmed with a human-specific P75NTR antibody, which also displayed a similar restricted localization of immunoreactive profiles within the lesion.
Temporal evaluation of the biodistribution of NuMA+ HuSCs revealed that at 3 days post-transplantation immunoreactive cells were localized to the lesion (Figures 2a, 4c,d, 5a and 6a,b) and injury penumbra (Figure 4h), with a few scattered cells present along the meninges near the entry site of the injection (Figure 4i). HuSCs were neither found within the dorsal roots or ganglia nor outside the injured (2cm) spinal cord segment. NuMA+ HuSC implants existed as either a single, densely packed deposition of cells (8/16; Figures 2a, 4g, 5a, and 6a,b) or as multiple foci of cell collections (8/16; Figure 4a). At 6 weeks and 6 months, NuMA+ HuSCs were not found outside the lesion site and were fewer in number and more dispersed within the lesion. HuSC implants at 6 weeks showed cell groupings within the lesion (15/25; Figures 2d, 5f, and 6g,h) or were scattered without implant foci (10/22). At 6 months post-transplantation, only a few scattered NuMA+ HuSCs were identified within the lesion (Figures 2g, 6m, and 7e). SC implant morphology and the extent of lesion filling was also visualized on hematoxylin, eosin and luxol fast blue-stained preparations (Fig 8a,b,d,e). No donor-dependent differences in NuMA+ HuSC biodistribution were found.
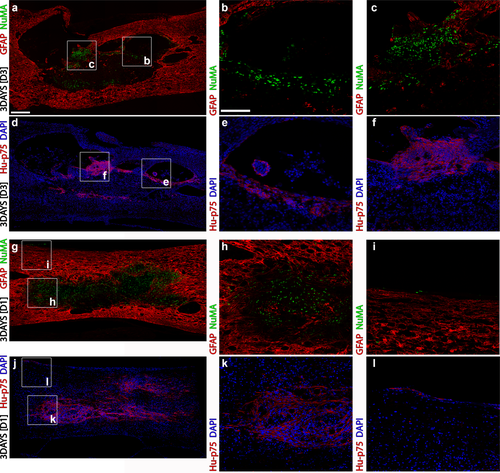
The biodistribution of HuSCs acutely following transplantation was largely restricted to the lesion site with some cells found within the injury penumbra or along the pia. Sections from the injured cord segment immunostained for (a–c) GFAP and NuMA or (d–f) human-specific p75NTR and DAPI show that HuSCs were present within tissue trabeculae within the lesion and in the surrounding injury penumbra at 3 days after transplantation. In another animal receiving HuSCs from a different donor, nearly complete filling of the lesion by HuSCs was observed with staining for (g–i) GFAP and NuMA or (j–l) human-specific p75NTR and DAPI, while (i, l) a few scattered cells were identified along the pia at the entry location of the injection needle. Box inserts in panels (a, d, g, and j) show locations of corresponding regions in higher magnification to the right. Scale bar for (a, d, g, and j) = 200 µm and for (b, c, e, f, h, i, k, and l) = 50 µm [Color figure can be viewed at wileyonlinelibrary.com]

HuSC implants are highly supportive of neurofilament-positive axon ingrowth by 6 weeks after transplantation. (a–e) At 3 days following intraspinal implantation, HuSCs are found to fill the lesion, though they are surrounded by host astrocytes and show little ingrowth of axons at this time. Preparations are immunostained for GFAP, neurofilaments low molecular weight (NF-L, 60 kDa), human-specific p75NTR and DAPI. (f–j) At 6 weeks after transplantation, host astrocytes and their processes are found throughout the HuSC implant along with numerous neurofilament-positive axons. Box inserts in panels (a and f) show locations of corresponding regions in higher magnification to the right with separated channels. Scale bar for (a and f) = 200 µm and for (b–e and g–j) = 50 µm [Color figure can be viewed at wileyonlinelibrary.com]
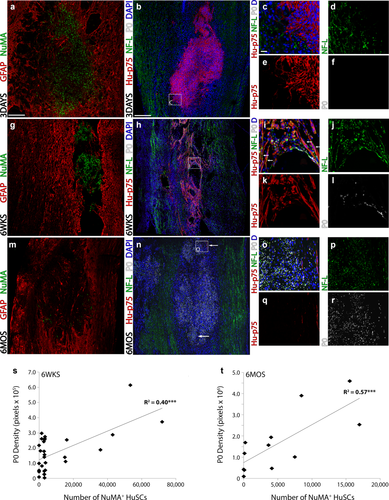
HuSC implants are highly supportive for axon growth and endogenous SC myelination. (a–f) In preparations stained with (a) GFAP and NuMA or (b–f) human-specific p75NTR, NF-L and multi-species P0 from animals at 3 days following HuSC transplantation, (a,b) a large implant was evident though (c,d) little axon ingrowth and (c, f) no peripheral myelination were observed. (g–l) At 6 weeks after implantation, (g,h) lower numbers of HuSCs were present within the lesion compared with 3 days, though the cells exhibited an elongated, spindle-shaped morphology that paralleled (h–j) the growth of neurofilament-positive axons. (i,j) Peripheral myelin was found sparsely throughout the implant. (m–r) At 6 months after implantation, (m,n) only a few NuMA- or p75NTR-positive HuSCs were identified within the lesion, though the lesion cyst was densely packed with cells, (n–p) neurofilament-positive axons and (n–p) large amounts of peripheral myelin, indicative of a pronounced endogenous SC response. (s–t) Linear correlation analysis showed that at both (s) 6 weeks and (t) 6 months after transplantation, a significant positive correlation existed between the number of NuMA+ HuSCs present and the density of P0 immunoreactivity within the lesion. White arrows indicate associations of (i) p75NTR-positive HuSCs and NF-L+ axons or (n) the location of p75NTR-positive HuSCs. Box inserts in panels (b, h, and n) show locations of corresponding regions in higher magnification to the right with separated channels. Statistical significance indicated a ***p < .001. Scale bar for (a and f) = 200 µm and for (b–e) and (g–j) = 50 µm [Color figure can be viewed at wileyonlinelibrary.com]
3.7 Host glial cell response to HuSC implants
Co-staining with the astrocyte marker, GFAP, and NuMA or human P75NTR for HuSCs, revealed a large number of astrocytic processes as well as cell bodies intermingling with HuSCs within the lesion at 6 weeks and 6 months post-implantation, but not at 3 days (Figures 2e,h, 4h, 5a,f, 6m, and 7e,f). This interaction is in contrast to syngeneic rat SC implants where implanted SCs and host astrocytes usually form distinct interfaces with very limited interaction between them (Flora et al., 2013; Ghosh et al., 2012; Patel et al., 2010; Pearse et al., 2007). Subsequent quantification of the amount of GFAP immunoreactivity within the rostral and caudal host spinal cord, immediately adjacent to the HuSC implant, revealed a temporal reduction in the average density of astroglial reactivity from 6 weeks (39.9% ± 3% and 38.2% ± 2.5% of total pixels for rostral and caudal segments, respectively) to 6 months (25% ± 2.6% and 23.4% ± 2.8% of the total pixels for rostral and caudal, respectively; p < 0.001) post-transplantation. There was no correlation between the number of NuMA+ HuSCs present and the density of GFAP immunoreactivity rostral or caudal to the implant at either post-transplantation time point (Figure 9c,d).
Next, the cellular immune reaction to the implanted HuSCs after SCI was measured by performing estimated counts of ED1+ cells within the rostral and caudal halves of the injured-implanted spinal cord segment. At 6 weeks after HuSC transplantation, an estimated average of 1,890 ± 260 and 1,920 ± 380 ED1+ cells per section was recorded for the rostral and caudal 500 µm segments, respectively. At 6 months following HuSC implantation, less ED1 immunoreactivity was observed, with estimated counts of 270 ± 50 and 330 ± 50 ED1+ cells in the rostral and caudal regions, respectively. Though there was a significant, temporal reduction in the cellular immune response post-implantation, no correlation existed between the number of NuMA+ HuSCs within the injured spinal cord and the estimated ED1+ cell counts at either time point (Figure 9g,h).
3.8 Axon and endogenous SC responses to HuSC transplantation
At 3 days after injection into the injured spinal cord, HuSC implants exhibited a dense cellular immunoreactivity for human-specific P75NTR within the lesion-implant site in an analogous deposition to that of NuMA. Stained cells showed the characteristic spindle-shaped morphology of SCs (Figure 6a,b). Costaining with NF-L revealed numerous host axons within the penumbra of the lesion, surrounding P75NTR+ HuSCs, though at this time there was no penetration of these axons into the lesion-implant proper (Figures 5a–e and 6b–d). Immunoreactivity for either multi-species or human-specific P0 was not observed within the injured spinal cord at 3 days after HuSC transplantation (Figure 6c–f).
At 6 weeks after HuSC transplantation, cellular immunoreactivity for human P75NTR was found more dispersed through the lesion in a patterning that mirrored NuMA, and numerous NF-L+ axons were found to have penetrated into the implant, intermingling with human P75NTR+ HuSCs (Figures 5f–j and 6h–j). A parallel alignment of human P75NTR+ HuSCs with NF-L+ axons was observed (Figure 6i, arrows). Staining for multispecies P0 at 6 weeks showed myelin profiles at the lesion-implant interface (Figure 6i,l), but far fewer immunoreactive profiles for myelin within the lesion-implant. Density measurements of multi-species P0 immunoreactivity within the lesion-implant, as measured by the total number of immunoreactive pixels, provided an average measurement of 1.68 ± 2.4 × 105 pixels. To evaluate HuSC myelination, tissue sections stained with the human specific P0 antibody and NF-L were evaluated. Whereas positive control human nerve samples showed human-specific P0 staining, immunoreactivity for human-specific P0 was negative in all HuSC implanted spinal cord samples. Confirmatory experiments employing cryosectioned tissue sections rather than those paraffin embedded for two animals with a 6-week survival period post-transplantation also showed an absence of human-specific P0 staining within the injured spinal cord segment. Density measurements of NF-L, expressed as immunoreactive pixels, provided an average measurement of 1.79 ± 2.7 × 105 pixels with the HuSC implant at 6 weeks post-transplantation. Correlation analysis of NF-L and multispecies P0 with the number of NuMA+ HuSC within the lesion-implant at 6 weeks post-transplantation showed a significant, positive correlation for both NF-L (Figure 7i; R2 = 0.21, p < 0.05) and multi-species P0 (Figure 6s; R2 = 0.40, p < 0.001). While there is a correlation between NF-L+ axons and P0+ profiles with NuMA counts, the numbers of myelinated axons within the HuSC implant are low at 6 weeks.
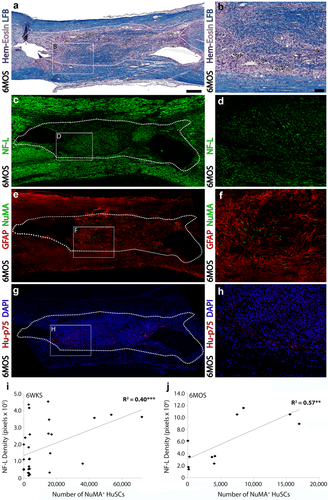
At 6 months post-transplantation, HuSC implants support significant axon ingrowth and astrocyte ingress, though numbers of HuSCs are lower than earlier time points. (a,b) A section of injured, transplanted spinal cord at 6 months stained with hematoxylin, eosin and luxol fast blue shows cellular filling of the lesion and the presence of a significant amount of myelin within the implant. Consecutive sections show that the lesion-implant contains (c,d) a large number of NF-L-positive axons, though low numbers of (e,f) NuMA+ or (g,h) human-specific p75NTR immunoreactive profiles at 6 months. Sections are costained with (e,f) GFAP for spinal cord architecture or (g,h) DAPI to identify cell nuclei. (i,j) Linear correlation analysis shows a significant positive correlation between the number of NuMA+ HuSCs and NF-L density within the lesion-implant at both (i) 6 weeks and (j) 6 months. The lesion-implant is identified by a dotted outline in panels (a, c, e, and g). Box inserts in panels (a, c, e, and g) show locations of corresponding regions in higher magnification to the right. Statistical significance indicated a ***p < .001 or **p < .01. Scale bar for (a, c, e, and g) = 200 µm and for (b, d, f, and h) = 50 µm [Color figure can be viewed at wileyonlinelibrary.com]
At 6 months after HuSC transplantation, scattered human P75NTR+ cell profiles were identified throughout the lesion that approximated the localization of NuMA+ HuSCs (Figures 6m,n and 7e–h). In contrast to 6 weeks post-transplantation, the lesion-implant site contained a large number of NF-L+ axons and P0+ myelin rings in all animals (11/11, Figure 6n–r). The considerable disparity in the number of NuMA+/human P75NTR+ HuSC profiles and the extent of immunoreactivity for multi-species P0 implied that large numbers of host rat SCs had breached the lesion to myelinate the NF-L+ axons present within. Density measurements of P0 immunoreactivity within the lesion-implant, as measured by the total number of immunoreactive pixels, presented average measurements of 1.8 ± 0.4 × 106 pixels. However, like the 6 weeks cohort, immunoreactivity for human-specific P0 was negative in all samples from the 6 month post-transplantation group. Density measurements of NF-L, expressed as immunoreactive pixels, provided an average measurement of 5.8 ± 1.2 × 106 pixels. The immunoreactive density values for NF-L and multispecies P0 within the lesion-implant at 6 months after HuSCs transplantation were ∼10-fold greater than those at 6 weeks. However, similar to 6 weeks, there remained a significant, positive correlation between the density of NF-L (Figure 7j; R2 = 0.57, p < .01) or multispecies P0 (Figure 6t; R2 = 0.57, p < .01) immunoreactivity and the number of NuMA+ HuSCs present within the lesion at 6 months post-transplantation.
Axon presence rostral from the lesion-implant site was quantified using measurements of NF-200 density in a 500-µm spinal cord segment encompassing the lesion-implant interface. The area of NF-200 immunoreactivity (in pixels) was expressed as a percent of total pixels measured within the 500 µm spinal cord segment. An increase in axon density was observed, with an average of 30.6% ± 2.4% and 44.4% ± 3.2% pixel coverage at 6 weeks and 6 months post-transplantation, respectively (p < 0.05). Linear correlation analysis revealed that no correlation existed between the density of NF-200+ axons in the rostral spinal cord and the number of NuMA+ HuSCs present within the lesion at 6 weeks and 6 months post-transplantation (Figure 9k,l)
3.9 Higher NuMA+ HuSC counts showed a positive correlation with the degree of white matter preservation
To evaluate the effects of HuSC transplantation on secondary tissue pathology following SCI, including cyst expansion and host tissue damage, stereological quantification of preserved white and grey matter volumes was performed. A one centimeter length of the spinal cord encompassing the injury-implant at its center was analyzed from animals in the 6 week (27/29) and 6 month (11/11) cohorts. Two samples from the 6 week group were excluded due to tissue sectioning issues (the samples included in this analysis by donor were as follows: D1 (5), D2 (3), D3 (5), D4 (5), D5 (5), and D6 (4)).
The average volumes of preserved white matter (6 weeks, 1.05 ± 0.02 mm3; 6 months, 1.15 ± 0.02 mm3) and healthy appearing grey matter (6 weeks, 0.59 ± 0.02 mm3; 6 months, 0.73 ± 0.05) were measured following HuSC transplantation. Linear correlation analysis (Figure 8c,f) showed a positive correlation between the number of NuMA+ HuSCs present within the injured spinal cord and the volume of preserved white matter at both 6 weeks (R2 = 0.4758, p < .0001) and 6 months (R2 = 0.41, p < .05) post-implantation. Micrographs show spinal cord sections from an animal with a low HuSC survival rate (Figure 8a) and an animal with a high HuSC survival rate (Figure 8b) at 6 weeks post-transplantation. Similarly, at 6 months after grafting, HuSC survival showed a positive correlation with white matter preservation (Figure 8e); in animals with fewer HuSCs, less white matter preservation and more numerous cysts were observed (Figure 8d).
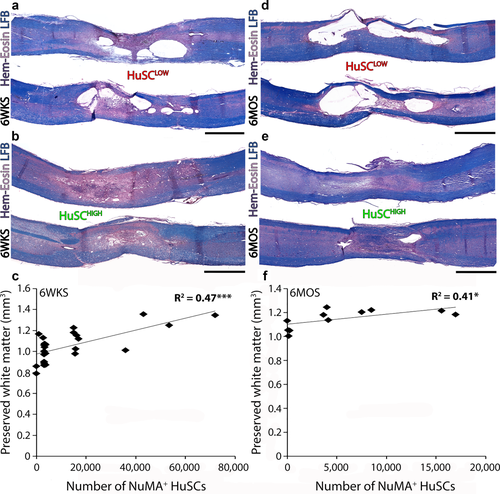
The presence of HuSCs correlates with the degree of host cord white matter preservation after SCI. (a–e) Sections of injured, HuSC transplanted spinal cords stained with hematoxylin, eosin and luxol fast blue from two animals at each of (a,b) 6 weeks and (d,e) 6 months after implantation in which either low (<5% survival) or high (>10%) numbers of cells were counted. Linear correlation analysis shows a significant positive correlation between the number of NuMA+ HuSCs and the volume of preserved host white matter within the injured-implanted spinal cord segment at both (i) 6 weeks and (j) 6 months. Statistical significance indicated a ***p <.001 or *p < .05. Scale bar for (a–e) = 800 µm [Color figure can be viewed at wileyonlinelibrary.com]
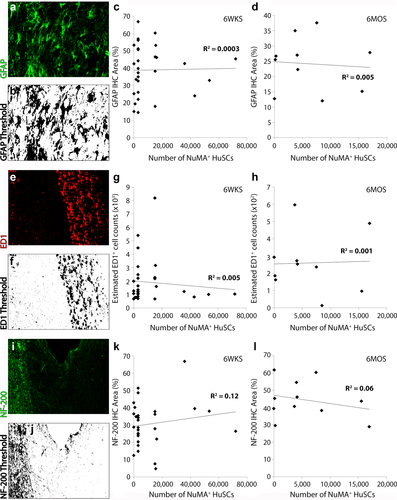
Quantification of host responses after SCI and cell implantation revealed no relationship between the presence of HuSCs and astrocyte reactivity, immune cell activation and axonal injury. Preparations immunostained, imaged and thresholded for immunoreactive density measurements were evaluated for (a,b) GFAP, (e,f) ED1 or (i,j) NF-200 kDa. Linear correlation analysis of data from (c,g,k) 6 weeks and (d,h,l) 6 months post-transplantation showed no correlations between the numbers of NuMA+ HuSCs present within the lesion-implant and the density of immunoreactivity for (c-,d) GFAP, (g,h) ED1, or (k,l) NF-200 within the injured cord segment at either time point. Scale bar for (a, b, e, f, i, and j) = 50 µm [Color figure can be viewed at wileyonlinelibrary.com]
4 DISCUSSION
The evaluation of safety is an essential component of the pre-clinical evaluation of a therapy, which when coupled with studies of efficacy, contributes to successful translation of the approach to initial investigation in man. For the use of a cell therapeutic in SCI, critical safety and toxicity endpoints include: (1) the migration or distribution of the cells, within the CNS and periphery from their original site of deposition, (2) the proliferation index of the cells and their tumorgenicity potential, (3) pathological changes along the neural axis as well as within peripheral organs caused by the transplanted cells and, (4) whether cell transplantation is associated with abnormalities in motor or sensory function, including pain and spasticity. To address the question whether HuSCs were safe when transplanted after SCI, these studies sought to best emulate the clinical use of HuSCs by employing unlabeled (virus-free) cells that were generated using the same protocol employed clinically and the comparative examination of HuSCs from multiple donors. Here, it is demonstrated that HuSCs persisted in the contused nude rat spinal cord for up to 6 months after transplantation and, analogous to rodent SCs, they presented limited migration, a low proliferation rate and no tumorgenicity potential. Although no donor differences were seen in temporal HuSC persistence after spinal cord transplantation, significantly greater HuSC survival was obtained when the cells were used from cryopreserved stocks rather than employed as fresh isolates, a finding that could have implications for other cell-based therapies and warrants further investigation. Numbers of surviving HuSCs within the lesion site positively correlated with the extent of preserved white matter of the injured cord segment and no adverse effects of the transplants on tissue pathology were observed at 6 weeks and 6 months. Furthermore, HuSCs were supportive of significant NF+ axon growth into the lesion-implant and were able to intermingle with host astrocytes, both at the implant interfaces and within the lesion-implant proper. The transplantation of HuSCs into the injured rat spinal cord also led to significant host SC migration into the lesion-implant areas, which then resulted in extensive myelination of axons that had entered the lesion-implant. The current work with HuSC transplantation extends the findings of Guest et al. (1997a, 1997b) to a contusion SCI model and coupled with a wealth of published reports on the effectiveness of SCs for the repair of the injured rodent spinal cord (Barakat et al., 2005; Ghosh et al., 2012; Guest et al., 1997a, 1997b; Kanno et al., 2014; Pearse et al., 2004a,b, 2007; Schaal et al., 2007; Wang and Xu, 2014; Xu et al., 1995a,b,1997, 1999), was used in support of the translation of autologous SCs to Phase 1 clinical trials for evaluating their safety in human SCI.
No significant differences in SC morphology, purity or viability were observed across donors, however, those of younger age exhibited a higher SC proliferation rate, a finding that has been described previously (Boyer et al., 1994; Fansa, Keilhoff, Wolf, & Schneider, 2000). Unlike the studies reported by Guest et al. (1997a, 1997b), HuSCs were transplanted in minimal culture medium, rather than Matrigel, and were obtained from both cryopreserved and fresh isolates. Previously we reported that Matrigel can significantly enhance transplanted rat SC survival as well as improve revascularization and axon growth within the lesion-implant compared with medium (Patel et al., 2010). Additionally, in the current study HuSCs were injected into the lesion epicenter following contusive SCI, an experimental SCI paradigm that differs significantly from the transection injury model used previously to examine HuSC transplantation (Guest et al., 1997a, 1997b). Transection SCI presents limited focal white matter pathology and a reduced macrophage response (Siegenthaler, Tu, & Keirstead, 2007) as well as dissimilarities in cerebrospinal flow disruption (Franco-Bourland et al., 2015; Iannotti et al., 2006), the extent of vascular injury (Mautes, Weinzierl, Donovan, & Noble, 2000; Noble and Maxwell, 1983) and the mechanisms of axonal damage (Blight, 1988) compared with the majority of non-penetrating human SCI injuries. These later injuries would be the target of a Phase 1 safety study of HuSCs in man.
In an unexpected finding, the use of HuSCs as freshly prepared cell isolates rather than from cryopreserved stocks, led to the lowest survival rates following transplantation. Furthermore, the percent HuSC survival rate exhibited a positive correlation with the length in days that HuSCs were maintained in cryopreservation. A possible explanation for this finding is that the cryopreservation protocol, which produces some degree of cell loss, may act as a selection method. In this way, HuSCs expressing higher levels of anti-apoptotic factors or molecules that make them more resistant to oxidative stress or preserve membrane stability (Baust, Corwin, VanBuskirk, & Baust, 2015; Bissoyi, Nayak, Pramanik, & Sarangi, 2014) would thus be selected during the cryopreservation and recovery process. In studies with myocardial cells, prior cryopreservation has been shown to increase the proliferation capacity of the cells as well as reduce their immunogenicity (Yokomuro et al., 2010). Identifying the factor(s) involved in enhancing cell survival following their cryopreservation, or perturbation, in the case of fresh preparations may provide a new avenue for enhancing transplant survival.
In the current investigation we have demonstrated that HuSCs persist within the contused spinal cord for up to 6 months after transplantation in almost all animals, though the survival rates of HuSCs in these experiments were lower and more variable than those of previously published reports employing syngeneic rat SC transplantation after contusive SCI. At 6 weeks after transplantation, the survival of rate of HuSCs was significantly lower (3.9% vs. 15–20%) than that reported for syngeneic rat SCs (Barakat et al., 2005; Hill et al., 2007; Pearse et al., 2004b, 2007). Furthermore, the numbers of HuSCs decreased further from 6 weeks to 6 months after transplantation, which contrasts with that of rat SCs where the number of surviving cells post-transplantation show little change from 6 weeks to 3 (Barakat et al., 2005) or 6 months (Wang and Xu, 2014). The lower survival rates for HuSCs after SCI compared with rat SCs could be due to host immune-rejection, principally by NK cells and macrophages that remain present in the athymic nude rat (Lin, Vandeputte, & Waer, 1997; Xia, Ji, Rutgeerts, & Waer, 2000). The variable numbers of NK cells detected around the HuSC implants at 3 days and 6 weeks after transplantation, despite the use of immunosuppression with GM1, indicate that their activity may have impacted HuSC survival. In addition, athymic nude rats are known to have two to three times higher levels of blood monocytes compared with heterozygous littermates (Vos, Kreeftenberg, Kruijt, Kruizinga, & Steerenberg, 1980), which could have resulted in greater numbers of infiltrating macrophages within the spinal cord after SCI and contributed to lower HuSC survival rates.
Similar to previous work with rat SCs, where proliferation rates have been measured in the <5% range (Patel et al., 2010; Wang and Xu, 2014), HuSCs exhibited very low cell proliferation temporally after transplantation. Furthermore, upon neuropathology examination, no abnormal cell mitosis or presence of solid tumors was observed within the lesion-implant as well as along the entirety of the spinal cord, demonstrating the very low tumorgenicity potential of HuSCs. Analogous to the distribution of rat SCs (Barakat et al., 2005; Pearse et al., 2007), HuSCs remained largely confined to the lesion site across time points, with a few scattered cells observed either along the meninges at the location of spinal penetration of the injection syringe or within the central canal at only 3 days post-implantation. SCs appear to gain access to the central canal (Guest et al., 1997b; Pearse et al., 2007; Wang and Xu, 2014) as a result of the pressure of the injection on a structurally compromised or dilated central canal (Shields, Zhang, & Shields, 2012), and their presence along the meninges can result from some cell leakage at the entry point of the injection syringe upon its withdrawal from the spinal cord. Interestingly, the HuSC implants exhibited a greater invasion of astrocytes and/or their processes than previously observed with rat SCs. Similar findings regarding the host-graft interface were reported by Guest and colleagues after grafting HuSCs in a transection SCI model in athymic rats (Guest et al., 1997b). The co-mingling of rat SCs and astrocyte processes at the rostral interface has been found to increase the regeneration of supraspinal axons into SC implants in a rat transection model (Williams, Henao, Pearse, & Bunge, 2015) and after polysialic acid engineering of rat SCs in a contusive SCI paradigm (Ghosh et al., 2012). The greater HuSC-astrocyte intermingling may be explained by the altered immunological status of the host, which could influence glial scar formation and/or extracellular matrix molecules such as: aggrecan (Afshari, Kwok, White, Fawcett, 2010b), Eph/ephrins (Afshari, Kwok, Fawcett, 2010a) or N-cadherin (Fairless, Frame, & Barnett, 2005), to facilitate improved SC integration at the host interfaces. Alternatively, HuSCs may express adhesion molecules that are not expressed in rat SCs after transplantation, thereby altering their interactions with host CNS glia. Currently, there are no studies that have investigated whether such differences exist between HuSCs and rat SCs, though work has shown that some dissimilarities are present between SCs of different species (Krudewig, Deschl, & Wewetzer, 2006; Rubio, Munoz-Quiles, & Ramon-Cueto, 2008; Wewetzer, Radtke, Kocsis, & Baumgartner, 2011).
Previous studies employing syngeneic use of rat SCs injected intra-spinally into the lesion epicenter have demonstrated that SC distribution and their ensuing migration is restricted to their original site of deposition, for up to 12 weeks post-transplantation, in both sub-acute and chronic SCI paradigms. Despite the improved intermingling observed between HuSCs and host rat astrocytes, no significant changes in HuSC migration or biodistribution compared with rat SCs were observed after SCI.
The numbers of HuSCs found within the lesion-implant site positively correlated with the amount of host preserved white matter, axon growth (NF density) and endogenous SC myelination at both 6 weeks and 6 months after transplantation, responses similar to those reported previously for rat SCs (Bamber et al., 2001; Guest et al., 1997b; Xu et al., 1995a, 1997, 1999). While density measures of fluorescent-conjugated antibody immunoreactivity is not a decisive method for quantifying protein presence, as the amount of staining may not correlate with the amount of antigen in the tissue and it exhibits a significant non-linear behavior (Taylor and Levenson, 2006), substantial structural changes within the implant-lesion comprising an increased number of stained profiles indicated that marked alterations in cell and axon profiles occurred in the lesion-transplant with time. Strong P0 immunoreactivity observed specifically at 6 months after transplantation showed that HuSCs are able to attract endogenous SCs in a similar fashion to that found in rat SC experiments (Hill, Moon, Wood, & Bunge, 2006; Pearse et al., 2007). Although it has been reported that HuSCs are able to myelinate axons when transplanted into a demyelinated spinal cord lesion (Brierley et al., 2001) or a peripheral nerve (Levi and Bunge, 1994; Levi, Guenard, Aebischer, & Bunge, 1994), we could not confirm in the injured spinal cord with multi-species or human-specific P0 antibody that HuSCs could myelinate axons in our experiments. Differences in the environment, whether the x-irradiated spinal cord or peripheral nerve, or the preparation of HuSCs, which may have produced selection of a cell population, in these studies may explain this discrepancy. It is also possible that our investigation at 6 weeks may not have allowed sufficient time for myelination. Robust rat SC myelination in a contusion SCI paradigm has been reported at 3 months after transplantation (Kanno et al., 2014; Pearse et al., 2007; Wang and Xu, 2014). In contrast, at 6 months after transplantation, only a few scattered HuSCs were detected in our samples, which potentially limited the ability of detecting myelinating cells. Examination of intermediate time points or the use of larger numbers of HuSCs may be needed to better answer the question as to whether culture expanded HuSCs can myelinate axons within the lesion-implant following SCI and transplantation in a xenotransplant paradigm.
Whereas a detrimental effect of HuSCs on injury evolution after SCI was not observed, a positive correlation was found between the number of HuSCs present within the injured spinal cord and the volume of host preserved white matter. This correlation does not imply a direct causative relationship between these outcomes and the result should be viewed in light of limitations associated with the methods of tissue sectioning and staining. Whereas sagittal sectioning provides favorable visualization of the rostro-caudal extent of the HuSC transplant in its entirety and measurement of cell biodistribution, axial sectioning along the longitudinal plane is challenging and often not exact compared with coronal sectioning (Ek et al., 2010). Further, histological staining of these preparations with hematoxylin, eosin and Luxol fast blue is not as conclusive as employing cell-specific markers for measuring cell loss and demyelination. Though HuSCs were transplanted at 1 month after SCI they could have still mediated protective effects on the host as ongoing changes in the evolution of injury have been reported at this time, including processes of Wallerian degeneration, demyelination and inflammation (Hagg and Oudega, 2006; Iizuka, Yamamoto, Iwasaki, Yamamoto, & Konno, 1987; Schwab, Zhang, Kopp, Brommer, & Popovich, 2014). In addition, the altered immunological status of the nude rat may have produced a delay in the timing of these pathological processes with the absence of T-cell-mediated inflammation as previously reported (Satzer et al., 2015). Observed differences in white matter volume could have been produced not only from a reduction in demyelination but also through an enhancement of endogenous remyelination. The absence of significant peripheral myelin within host white matter adjacent to the lesion suggests that the axons in these regions were myelinated by host oligodendrocytes rather than by SCs, either host or transplanted. To conclusively identify whether HuSC transplants were efficacious in the contusive paradigm, however, would require rigorous behavioral and neuroanatomical assessments as well as control groups that were not employed in this preclinical safety study.
While the current investigations sought to employ a SCI paradigm that best mimics the human condition and the clinical methodologies used for HuSC transplantation, there are a number of limitations of the nude rat SCI model when making interpretations regarding the behavior of autologous HuSCs in the clinical scenario. These differences include variances between species in anatomical size and scale, axon tract localization and features of pedal locomotion as well as pathological disparities related to the SCI modality, including open versus closed injuries and the types of forces enacted upon the spinal column. The inability to perform an autologous transplantation of a human cell product into a non-human host also introduces differences associated with immunosuppression or a severely immune compromised host system. The xenotransplant paradigm employed exhibits characteristics of cell-mediated immunity and rejection that would limit human cell persistence and would not be expected to be present in the more favorable immunological conditions present clinically in human SCI when HuSCs would be used autologously. Thus, while the current paradigm provides a relevant model to the clinical setting for evaluating the intrinsic properties of the human cell product, the complex immunological responses of the nude rat, particularly the absence of acquired immunity and the compensatory increase of natural immunity elements, makes the present findings more relative to a xenotransplantation and immunosuppression paradigm as opposed to when autologous grafting under clinical conditions is performed.
In conclusion, by investigating temporally the persistence and behavior of HuSCs when transplanted after contusive SCI, we have demonstrated that HuSCs can persist long-term after transplantation (up to 6 months) and have a favorable toxicity profile that is characterized by a limited biodistribution, a low proliferation rate and an absence of tumor formation or abnormal pathological changes. We have also demonstrated that HuSCs, in combination with a robust endogenous host SC response, lead to white matter protection, axon growth support, and myelination within the injured cord segment. These studies provide evidence that HuSCs do not display histological changes indicative of toxicity while presenting signs of a positive influence of transplanted HuSCs on host tissue pathology that are similar to those found in previous work that employed rodent SCs in an analogous SCI paradigm.
ACKNOWLEDGMENT
The authors gratefully acknowledge the staff in The Miami Project Animal Surgery Core, especially P. Diaz for producing spinal cord contusions and I. Oropesa and D. Koivisto for postoperative animal care. The Miami Project Histology Core is acknowledged for their supportive role in tissue sectioning as well as L. Conner and V. Aguirre for imaging assistance. Pamela Chiriboga-Yerovi is thanked for her help with immunohistochemistry. They acknowledge funding support from The Christine E. Lynn Human Clinical Trials Initiative, The John M. and Jocelyn H.K. Watkins Distinguished Chair in Cell Therapies (DDP), The Christine E. Lynn Distinguished Professor of Neuroscience (MBB), CDMRP Award W81XWH-10-1-0793 from The Department of Defense, The Bryon Riesch Paralysis Foundation, The Gordon Family Foundation, The Miami Project to Cure Paralysis and The Buoniconti Fund.
CONFLICT OF INTEREST
The authors (G.A., A.L., P.M.W., J.D.G., W.D.D., M.B.B., and D.D.P.) declare that they have a patent filing related to this work.