Exploring the Multifaceted Potential of Elaeagnus angustifolia L.: A Comprehensive Review of Its Nutritional, Pharmacological, and Environmental Significance
ABSTRACT
Elaeagnus angustifolia L. is commonly known as oleaster or Russian olive. It is an economically important plant due to its wide applications and ability to thrive under harsh environmental conditions, including various edaphic and topographical circumstances. Different parts of the E. angustifolia serve multiple purposes, including food, nutraceuticals, fuel, timber, windbreaks, and erosion control. Furthermore, the abilities of E. angustifolia as a nitrogen fixer, bio-monitoring, and bio-absorbent characteristics make it an essential component of soil fertility improvement programs. This review elucidates the biochemical characteristics of E. angustifolia, encompassing the nutritional and bioactive compounds present in its various parts, including sugars, proteins, minerals, and complex carbohydrates. Additionally, recent findings about the therapeutic potential of E. angustifolia, including its analgesic, anti-inflammatory, antioxidant, cardioprotective, antimicrobial, and anticancer properties, were summarized. Likewise, wound healing effects are described as possible starting points for the development of innovative cosmeceuticals. In short, this review advances current knowledge and provides deep insights into the versatile applications of E. angustifolia in focusing on human health concerns and targeted development of nutraceuticals, pharmaceuticals, and environmental sustainability.
Abbreviations
-
- Bcl2
-
- B cell leukemia/lymphoma 2, N-Histidine-tagged
-
- DPPH
-
- 2,2′-diphenyl-1-picrylhydrazyl radical
-
- EAE
-
- E. angustifolia water extract
-
- E. angustifolia
-
- Elaeagnus angustifolia
-
- FRAP
-
- fluorescence recovery after photobleaching
-
- HDL
-
- high-density lipoprotein
-
- HER2
-
- human epidermal growth factor receptor 2
-
- HRE2
-
- human epidermal receptor 2
-
- IL-6
-
- interleukin-6
-
- JNK1/2
-
- c-Jun N-terminal Kinase 1 and 2
-
- KRAS
-
- Kirsten Rat Sarcoma viral oncogene homolog
-
- LDL
-
- low-density lipoprotein
-
- LPFI
-
- Lequesne Pain and Function Index
-
- PGA
-
- patient global assessment
-
- SKBR3
-
- Sloan-Kettering Institute's Breast Cancer 3
-
- SOD
-
- superoxide dismutase
-
- STAT3
-
- signal transducer and activator of transcription 3
-
- TC
-
- total cholesterol
-
- TNF-α
-
- tumor necrosis factor-α
-
- VAS
-
- Visual Analog Scale
1 Introduction
Medicinal plants and herbs have long been used due to the abundance of valuable bioactive compounds (Mohammadhosseini et al. 2019). Among these plants, E. angustifolia (commonly known as Russian Olive) is remarkably known as a beneficial species due to its diverse pharmacological properties. This deciduous, long-lived, mesophytic, oligotrophic, photophilic, and riparian plant grows near water corridors. It is native to southern Europe and the Middle Eastern and Central Asian regions. This plant has fast growth, and it is well characterized by olive-like edible nutritious fruit (Fonia et al. 2009). Due to its resemblance to an olive tree, it is also called an oleaster and Russian olive. The leaves, bark, flowers, fruits, and seeds of the E. angustifolia plant are being used to prepare decoctions, which are traditionally used to treat different ailments of humans and livestock. Several studies have demonstrated the anti-inflammatory, antioxidant, cardioprotective, antimicrobial, and anticancer properties of this plant. Also, several phyto-complexes and juices obtained by E. angustifolia have been reported as anticancer, hypocholesterolemic, and cardiovascular protective agents. E. angustifolia's flavonoids and phenolic components, which scavenge free radicals, inhibit oxidative enzymes, and chelate metal ions, shield cells from oxidative stress and lower the risk of degenerative diseases (Farzaei et al. 2015, 2019; Niknam et al. 2016; Tehranizadeh et al. 2016; Hamidpour et al. 2017, 2019).
The diverse array of phytochemicals, such as polysaccharides, amino acids, saponins, carotenoids, vitamins, flavonoids, coumarins, phenol carboxylic acids, tannins, and volatile oils in leaves, fruits, and flowers beckons further exploration as a promising reservoir of potential nutritional and nutraceutical compounds (Bucur et al. 2008). This review article comprehensively discusses the taxonomy, distribution, botanical description (e.g., root, leaf, stem, flower, fruit, and seed), phytochemical constituents (for instance carbohydrate, polysaccharides, protein, fatty acids, lipids, phenolic compounds etc.), effectiveness of its extracts for improving sexual health, its multipurpose uses, and recent ethnomedicinal and pharmacological benefits of E. angustifolia.
2 Taxonomy and Distribution
E. angustifolia, with a chromosomal count of 2n = 2x = 12, is a member of the Elaeagnaceae family. This family comprises three genera: Elaeagnus, Sheperdia, and Hippophae, with a total of 77 species worldwide (Asadiar et al. 2013), primarily found in the Northern Hemisphere's temperate zones, some in eastern Australia and tropical southeast Asia (Bartish and Swenson 2004). E. angustifolia is a deciduous and monoecious shrub that grows to a height of 10 m, and its wild form has a denser canopy (Ersoy et al. 2013). The native regions of E. angustifolia include the temperate regions of the Mediterranean, southwestern Asia, and Irano-Turano regions. E. angustifolia is supposed to have originated in southern Russia, while in Pakistan and Turkey, it was introduced from Kazakhstan (Gaskin et al. 2019).
E. angustifolia is naturally present along the edge of roadsides, fields, mountain slopes, and riverbanks (Azmat et al. 2020). It can be grown under various soil and climatic conditions. Its seedling has the potential to tolerate soil salinity, alkalinity, and acidity (up to pH 6) (Kiseleva and Chindyaeva 2011; Azmat et al. 2020). Its seedlings are also shade tolerant; in contrast, the plant continues to grow in high light intensities. This plant also tolerates different temperature ranges from 45°C to 46°C (Kiseleva and Chindyaeva 2011; Enescu 2018). In recent years, E. angustifolia has been promoted in different parts of the world, especially in the USA, Mexico, Canada, and Europe to control erosion, rehabilitate problematic lands, and for ornamental purposes. However, the outstanding regeneration capability of this plant makes it an invasive species in some regions (Katz and Shafroth 2003; Gaskin et al. 2019).
3 Botanical Description
The Elaeagnaceae family belongs to the order Rosales of class Magnoliopsida and includes Genus Elaeagnus. This family comprises small trees, shrubs, and flowering plants, which have hairs or thorns on their leaves and have xerophytic and halophytic properties; all genera have nitrogen-fixing capacity (Nazir et al. 2020). Each genus has a varying numbers of chromosomes: 2n = 22, 24, 26, 28, and varies in the reproductive system. Elaeagnus is monoecious, while Hippophae and Shepherdia are dioecious (Bartish et al. 2002).
3.1 Roots, Stem, and Leaves
E. angustifolia is categorized explicitly by a well-defined taproot system with highly developed lateral roots. Depending upon the soil type and water availability, the lateral root may reach a depth of 12 m (Zhou et al. 2007). Stubbendieck et al. (2003) have noted that the type of soil, its pH, and even its aeration can greatly influence the level of symbiotic nodulation that takes place on their roots. E. angustifolia is predominantly multistemmed and usually possesses 5–6 primary stems that arise from above the crown root surface. The remaining height above ground varies from 4 to 11 m depending on soil and environmental conditions, with approximately 30-cm trunk diameter at breast height. Branches are randomly arranged and covered with reddish-brown or silvery hues with coarse thorns (Zhou et al. 2007).
Leaves are petiolate with oblong-elliptic to lanceolate and occasionally round-ovate lamina shapes, which are alternatively attached to the plants by twigs. The leaf apex could be acute-acuminate or round-obtuse while the leaf margin ranges from revolute to entire, having a round-obtuse or attenuate-cuneate leaf base. The presence of silvery scales (speckles) on both the adaxial and abaxial leaf surfaces is one of the characteristic features of E. angustifolia leaves. There are variations in leaf shapes both among and between the canopies of individual plants. Leaf length and width range from 1 to 4 cm and 2 to 10 cm, respectively (Klich 2000).
3.2 Flower, Fruit, and Seed
Flowering occurs from June to August when it bears silvery-yellow, attractive, and fragrant campaniform flowers of ~3–12 mm length appearing in the form of small auxiliary clusters. The flowers are entomophilous allogamous and complete with a unilocular superior ovary, apically bent style, sticky stigma and four extensive powdery stamens (Pretty Paint-Small et al. 2013). Fruits ripen in August or early September forming fleshy, dry-mealy, and yellow-red classical drupe with berry-like oval shape. Fruit length, width and weight range from 10–20 mm, 6–13 mm and 0.5–1.5 g, respectively (Kiseleva and Chindyaeva 2011).
Fruit of E. angustifolia contain a single seed, covered with a hard seed coat. The length and width of the seed could be 17 and 4.5 mm, respectively with 0.3–0.35 shape index and 0.2–0.4 g in weight (Katz and Shafroth 2003). Normally, the seeds require two to three months of cold stratification period before germination due to the hard seed coat and the presence of a coumarin-like inhibiting substance. Viability of seeds is up to 3 years under standard storage conditions. E. angustifolia can reproduce either sexually through seed or asexually through root suckers, layering and cuttings (Bonner 2008; Brock 2003).
4 Phytochemical Composition
Phytochemical investigations revealed the presence of various bioactive compounds with a range of biological activities; some of these are summarized in Table 1 and are listed below.
| Elaeagnus spp. is in the family riparian trees growing near the rivers or water corridors | Percentage in dried ripe fruits of E. angustifolia | References | |
|---|---|---|---|
| Reducing sugars | 50.67%–55.75% | Abizov et al. (2008), Tehranizadeh et al. (2016) | |
| Total sugar | 60% ± 5% | ||
| Pectic polysaccharides | 3.58% ± 0.3% | ||
| Total flavonoids and polycarboxilic acids | 1.35% ± 0.15% | ||
| Total saponins | 1.96% ± 0.52% | ||
| Ascorbic acid | 5.6 mg% | ||
| B-carotene | 17.5 mg% | ||
| Tannin | 5.03% ± 0.05% | ||
| Composition of the essential oils isolated from the flowers and leaves of E. angustifolia | Flower % | Leaves % | Torbati et al. (2016) |
| Hydrocarbons | 4.69 | 3.02 | |
| Oxygenated compounds | 91.9 | 95.95 | |
| Most abundant mineral found in E. angustifolia fruit | mg/kg | Hamidpour et al. (2019) | |
| Potassium | 8504 | ||
| Sodium | 1731 | ||
| Phosphorus | 635 | ||
| Most abundant phenolic compounds found in E. angustifolia | |||
| 4- hydroxybenzoic acid | 45.8 mg/100 g dry wt. | ||
| Caffeic acid | 32 mg/100 g dry wt. | ||
| Fructose | 27.1% dry wt. | ||
| Glucose | 22.3% dry wt. | ||
| Major fatty acids in E. angustifolia | Percentage (%) | Sahan et al. (2015) | |
| Palmitic acid | 34.31 | ||
| Oleic acid | 26.23% | ||
| Lignoceric | 17.47 | ||
4.1 Carbohydrates
The characteristic taste of ripened E. angustifolia fruit is due to the presence of various carbohydrates. Fructose (27.1%) and glucose (22.3%) are among the major sweetening compounds in E. angustifolia fruits, while sucrose is only a minor sugar (Ayaz and Bertoft 2001). Additionally, minor reducing sugars including xylose, mannose, and rhamnose have also been quantified from the extracts of fruit (Abizov et al. 2008).
4.1.1 Polysaccharides
Polysaccharides from fruits, vegetables, mushrooms, and medicinal plants are endowed with many biological activities, including anti-inflammatory (Wei et al. 2023) antioxidant (Tang et al. 2023) prebiotic (Antunes et al. 2023) antidiabetic (Zhao et al. 2011) and neuroprotective properties (Zhang et al. 2023). Several polysaccharides, including EAP-1a (Elaeagnus angustifolia Polysaccharide-1a) and EAP-1b (Elaeagnus angustifolia Polysaccharide-1b), were derived from the fruits of E. angustifolia. These polysaccharides are mostly composed of mannose, rhamnose, glucose, galactose, xylose, and arabinose. These compounds promote phagocytosis and nitric oxide generation in RAW 264.7 macrophages (Du et al. 2016). It was found that these polysaccharides have antioxidant properties, mainly comprised of xylose, glucose, rhamnose, mannose, and galactose (Chen et al. 2014). Moreover, most polysaccharides present in the fruit of this plant are water-soluble and possess good emulsifying activity (Sharifian-Nejad and Shekarchizadeh 2019).
4.2 Proteins, Fatty Acids, and Lipids
The fruit of the Russian olive is considered a rich protein source containing both essential and nonessential amino acids (Abizov et al. 2008; Niknam et al. 2016). Their fruit, flower, and seeds are also rich in various fatty acids and derivatives. The flowers of Russian olive primarily contain free fatty acid followed by thioglycolic acid and esters, while the exocarp of the fruit is rich in palmitoleic acid. More than 20 fatty acids of different classes have been quantified from the extracts obtained from E. angustifolia fruit pulp (Hamidpour et al. 2017).
To date, several fatty acids (both saturated and unsaturated; glycolipids and phospholipids) have also been found in E. angustifolia seeds, with linoleic and palmitic acids identified in seed oil (Ayaz and Bertoft 2001; Hamidpour et al. 2017; Abdalla 2019). Among lipophilic substances, the phytosterols β-sitosterol and its acetate form were identified in fruits and barks, while stigmasterol was found in the bark (Si et al. 2011). The leaves were also found to contain several lipids, such as fatty acids, triterpenes, sterols, free alcohols, and esters (Bekker and Glushenkova 1997). In the bark, isocaryophyllene was identified, while limonene, nerolidol, squalene, and ursolic acid were extracted from the essential oils of the flowers, and α and β amyrin were isolated from the leaves (Bekker and Glushenkova 1997; Si et al. 2011).
4.3 Phenolic Compounds and Flavonoids
Phenolic compounds and their derivatives are widely distributed in different parts of the E. angustifolia plant. The concentration of phenolic compounds in the extracts obtained from different plant parts varies with the age and health of the plant and/or time of sampling. Leaves and fruits of E. angustifolia represent good sources of phenolic compounds, such as p-hydroxybenzoic, caffeic and protocatechuic acid. Fruits also contain 4-hydroxybenzoic acid, 4-hydroxycinnamic acid, benzoic acid, ferulic acid and vanillic acid (Ayaz and Bertoft 2001). Leaves were shown to contain a higher total phenolic content than fruits (Carradori et al. 2020).
Flavonoids are a group of polyphenolic compounds with low molecular weight and high antioxidant activity found in most of vegetables and fruits (Okmen and Turkcan 2014). These secondary metabolites exert many biological effects including antioxidant, anti-inflammatory, hypocholesterolemic, antidiabetic and neuroprotective properties (Zeka et al. 2017; Ayaz et al. 2019; Farzaei et al. 2019) and may also have a role in cancer prevention (Arroo et al. 2014). E. angustifolia has high amounts of flavonoids including flavanol, flavanone, proanthocyanidin, isoflavone and anthocyanin in leaves, fruits and flowers (Hamidpour et al. 2019). The leaves contain flavonoids such as (+)-gallocatechin, 4 (+)-catechin, (−)-epigallocatechin, (−)-epicatechin, kaempferol, quercetin, luteolin, isorhamnetin-3-d-gluco-d-galactoferuloyl, isorhamnetin-3-rhamnoglucorhamnoside and isorhamnetin-3-d-gluco-d-galactoside (Hamidpour et al. 2017, 2019). In addition to the already known flavonoids, various novel flavonoids such as 4 (+)-catechin, (−)-epicatechin, (+)-gallocatechin, (−)-epigallocatechin, quercetin, luteolin, isorhamnetin and isorhamnetin-3-O-β-d-galactopyranoside were isolated from the leaves of E. angustifolia (Yuca et al. 2021). These molecules were also detected in fruits of E. angustifolia. Particularly, different flavonoids such as rutin, eleagnoside, isorhamnetin (3, 5, 7, 4-tetrahydroxy-3-methoxy flavone) and isorhamnetin-3-O-β-galactopyranoside were found (Abizov et al. 2008). Seven novel acylated isorhamnetin glycosides (elaeagnosides) A-G (1–7) have also been isolated from the methanol extracts of E. angustifolia petals. Leaves were found to have higher total flavonoid contents than the fruits (Carradori et al. 2020).
4.4 Alkaloids
Different alkaloids were detected in roots, bark, and aerial parts. Elaeagnin or calligonin, which is structurally a tetrahydroharman, is among the most notable alkaloids obtained from E. angustifolia (Tehranizadeh et al. 2016). Also, 2-methyl-l,2,3,4-tetrahydro-β-carboline, 1,2-dihydroharmaline, dihydroharmane, 3,3-dimethyl-1,3-dihydro-indol-2-one, harmane, harmol, N-methyl-1,2,3,4-tetrahydro-β-carboline, N-methyltetrahydroharmol, tetrahydroharmine, tetrahydroharmol, and 2,3,4,9-tetrahydro-1-methyl-1H-pyrido[3,4-b] indole were identified from the bark of E. angustifolia (Tolkachev et al. 2008; Si et al. 2011).
4.5 Saponins and Tannins
Saponins and tannins have different biological activities including anthelmintic, hemostatic, antimicrobial, chemopreventive, angiogenesis, analgesic, cardioprotective, antitumor, and anti-inflammatory activity (Motevalian et al. 2017). Variable concentrations of these compounds were observed in different organs of the E. angustifolia plant. Prominently, the fruit possesses significant amounts of saponins, whereas the maximum contents of tannins were found in bark, followed by the leaves and annual branches (Hamidpour et al. 2017).
4.6 Vitamins, Minerals, and Other Nutrients
E. angustifolia is recognized as a rich reservoir of different nutrients, including vitamins, minerals, sterols, steroids, alkanes, polyphenols (deca-, undeca-, and dodecaisoprenols), trans-ethylcinnamate, cycloartenol, tocopherols, triterpene, aldehydes, citrostadienol, 24-methylenecycloartanol, C21-C31 hydrocarbons (mainly C29), C16-C26 fatty alcohols, and α and β-amyrins (Kiseleva and Chindyaeva 2011). Vitamins profile includes important vitamins, including vitamins A (provitamin A, β-carotene), vitamin B, ascorbic acid (vitamin C), and vitamin K. Different minerals have also been found in E. angustifolia, with significant amounts of potassium, calcium, sodium, and phosphorous, and their abundance varies temporally and spatially (Abizov et al. 2008; Niknam et al. 2016).
5 Uses
5.1 Source of Food, Feed and Shelter for Wildlife, Domestic Animals and Humans
E. angustifolia is an excellent resource for both wildlife and humans alike as it provides feed, food, shelter (nest), windbreak, and controls soil erosion. Domestic animals, humans, birds, and honeybees feed on the branches, twigs, leaves, flowers, and fruits of E. angustifolia (Katz and Shafroth 2003).
5.2 Bio-Fertilizer and Bio-Monitor Activity
With the increasing population, the demand for food substantially enhanced, which demands the need to rehabilitate and recultivate contaminated and less fertile soils for sustainable agriculture. E. angustifolia is among those available stress-tolerant plant species that can be efficiently used to cope with toxic heavy metals (Pb, Cd, Zn, Ni, and Hg) through bio-adsorption. Furthermore, fruit powder of E. angustifolia acts as a good bio-absorbent and symbiotically fix atmospheric nitrogen simultaneously (Follstad Shah et al. 2009). The leaves of E. angustifolia can also act as bio-fertilizers because of the rich amount of nitrogen-lignin contents (> 3%) and the ease of biodegradation (de Castro et al. 1990). Moreover, the presence of some allelopathic chemicals in the litter of E. angustifolia inhibits microbial nitrification; hence, it is very helpful in soil nitrogen availability. By fixing atmospheric nitrogen through symbiosis with Frankia bacteria, E. angustifolia improves soil fertility and greatly improves degraded croplands, even in nutrient-poor and saline environments (Khamzina et al. 2009; Pretty Paint-Small et al. 2013). The hardy nature of E. angustifolia and its adaptability across a wide range of soils in different geographical and ecological conditions, make it a potent bio-fertilizer and bio-monitor agent. Bio-monitoring involves the ecological assessment of soil health and environmental interactions by using biological indicators such as soil microbe, vegetative dynamics, and insect pollinators and bio-absorbent is the ability of plants to absorb heavy metals (Nistratov et al. 2020; Sardar et al. 2013).
5.3 Biological Insecticide
Increased use of synthetic insecticides which are mostly nonbiodegradable, poses a serious threat to the environment and human health. In this context, E. angustifolia offers a natural alternative. Extracts and fractions obtained from various parts of E. angustifolia, such as roots, leaves, flowers and fruits have shown promising insecticidal activity to control moth (Ephestia cautella) and different food grain storage pests including red flour beetle (Tribolium castaneum) (Khan et al. 2016; Niknam et al. 2016). Hence, E. angustifolia could also be used as a potential biological insecticide to control different insects of field and horticultural crops.
5.4 Medicinal Uses
Medicinal plants are a rich source of valuable natural compounds having various pharmaceutical properties. These include antioxidants, wound healing, analgesic and anti-inflammatory applications in osteoarthritis and respiratory disorders, even demonstrating antimicrobial, antimutagenic, and anticancer properties, possessing cardiovascular and metabolic effects and helping in sustaining sexual health (Mohammadhosseini et al. 2019, 2021; Tehranizadeh et al. 2016). E. angustifolia and its constituents have long been used in the prevention or suppression of several diseases as summarized below (Figure 1).
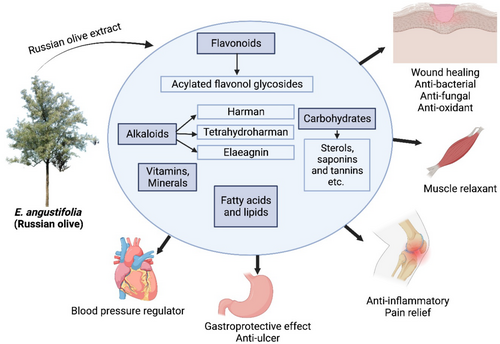
5.4.1 Analgesic and Anti-Inflammatory Properties
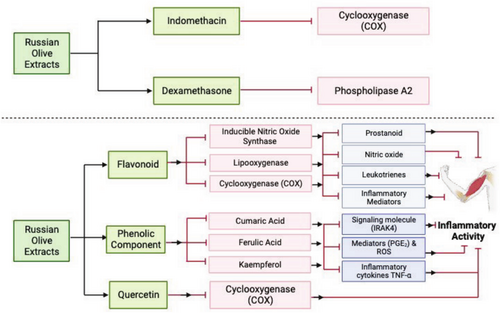
Polyphenols present in E. angustifolia such as cumaric acid, ferulic acid, and kaempferol have been reported to be involved in suppressing the release and production of inflammatory mediators (COX-1, COX-2, PGE2 and nitric oxide), signaling molecules (IRAK4, Src, and Syk), inflammatory cytokines (TNF-α and interleukin-6) and ROS production (Farahbakhsh et al. 2011). In addition, Motevalian et al. (2017) observed that E. angustifolia fruit extract possesses acute and chronic anti-inflammatory activities in rat paw edema through diverse mechanisms (Figure 2). Similarly, Ahmadiani et al. (2000) also reported the anti-nociceptive and anti-inflammatory activities of E. angustifolia fruit extract.
5.4.2 Osteoarthritis
The pain-relieving and muscle-relaxant properties of E. angustifolia extracts provide relief to patients with osteoarthritis. However, it is important to note that long-term muscle relaxation is considered harmful because strengthening of quadriceps muscles is required, along with muscle relaxation for osteoarthritis treatment (Hosseinzadeh et al. 2003; Panahi et al. 2016). Studies have shown that fruit extracts of E. angustifolia have efficacy in alleviating osteoarthritic symptoms. A water extract derived from fruit was shown to reduce serum matrix metalloproteinase 3 and 13 and pathological signs in mice with monosodium iodoacetate-induced osteoarthritis (Heydari Nasrabadi et al. 2022).
More convincingly, Nikniaz et al. (2014) demonstrated that the administration of 15 g/day of E. angustifolia medulla and whole fruit powders to women affected by knee osteoarthritis resulted in a decrease of matrix metalloproteinase-1, TNF-α and an increase of the anti-inflammatory cytokine IL-10. These data are consistent with the work of Karimifar et al. (2017) who observed the administration of an E. angustifolia fruit water extract, at a daily dose of 200 mg, for 4 weeks, to knee osteoarthritis affected patients of 40–80 years old, resulted in an improvement of visual analog scale (VAS), Lequesne pain and function index (LPFI) and patient global assessment (PGA). Also, osteoarthritic patients 50–80 years old treated with a daily dose of 300 and 400 mg of an E. angustifolia fruit water extract for 7 weeks experienced improvements measured as Western Ontario and McMaster Universities Osteoarthritis Index, VAS, LPFI, and PGA index. This study identified the alkaloids (Harmine and Harmaline) and polyphenols in fruit extracts that reduce oxidative stress (Panahi et al. 2016; Nikniaz et al. 2014).
5.4.3 Wound Healing
E. angustifolia extracts contain different chemicals in the highest concentrations, such as vitamins A, B, K, and E, which are important for wound healing (Natanzi et al. 2012; Esmaeili and Niknam 2013; Hamidpour et al. 2019). Natanzi et al. (2012) demonstrate that the water fruit extracts of E. angustifolia improve the hydroxyproline content, wound healing, and histological score of rats with skin wounds. Also, the flower's soft extract derived from E. angustifolia (cream-based) showed a wound healing effect on rabbits with scarred skin. Extracts have been reported to significantly impact all the phases of the wound healing process including hemostasis, inflammatory response, proliferation, and tissue repair (Bucur et al. 2008).
5.4.4 Antimicrobial Activity
E. angustifolia extracts derived from its leaves, flowers and fruits have shown significant antimicrobial activity both against bacteria and fungi. Methanolic phyto-complex from flowers, leaves and fruits showed antimicrobial activities against several bacteria with some differences. Particularly, flower extract was the most active against gram-positive bacteria (Bacillus cereus, Staphylococcus aureus, Staphylococcus epidermidis and Enterococcus faecalis), followed by leaves and fruit extracts (Incilay 2014). This difference likely reflects the higher amount of volatile compounds (terpenes, alcohols, aldehydes, ketones and esters) in flowers than in leaves and fruits. These volatile compounds are endowed with antimicrobial effects.
Methanolic extract of the whole plant and its fractions of increasing polarity were tested against several pathogenic bacteria and fungi. The methanolic extract of the whole plant and the water fraction had no effects on Pseudomonas aeruginosa. The water fraction had the highest potency against Escherichia coli, while the ethylacetate fraction was the most active against S. aureus and P. aeruginosa. No antibacterial effects were observed with the chloroform fraction. The crude extract was active against Aspergillus fumagatus but inactive against Aspergillus flavis and Aspergillus niger. The water fraction inhibited only A. flavis; the ethylacetate fraction was active against A. flavis and A. niger, without any effects on A. fumagatus. The chloroform fraction had no activity, while the n-hexane fraction was the most active against all the tested strains (Khan et al. 2016). The antimicrobial activities of a methanolic extract obtained from E. angustifolia L. leaves were demonstrated for Bacillus subtilis, Staphylococcus aureus, Listeria monocytogenes, Enterococcus faecalis, Yersinia enterocolitica, Salmonella typhimurium, with the highest effect on Yersinia enterocolitica (Okmen and Turkcan 2014). Therefore, it can be effectively used for preventing and treating several bacterial and fungal diseases including mastitis in animals and dental hygiene in humans (Incilay 2014).
5.4.5 Respiratory Disorders
Folk medicine has long employed decoction and infusion made from the bark, leaf, flower, and fruit to treat respiratory conditions. Beyond documented antimicrobial activities of E. angustifolia, this plant also exhibited notable anti-inflammatory and antioxidant effects, further justifying its potential as a nutraceutical against respiratory diseases. Specifically, extracts of E. angustifolia can be effectively used in the treatment of excessive mucus production, airway hyper-responsiveness, shortness of breath, sore throat, flu, cold, fever, cough, and wheezing (Ge et al. 2009). This traditional use of E. angustifolia is further substantiated by a recent study by Mamashli et al. (2022). He observed that E. angustifolia fruit water extract exerts protective effects toward carrageenan-induced acute lung injury through several mechanisms involving the decrease of IL-6, TNF-α and oxidative stress.
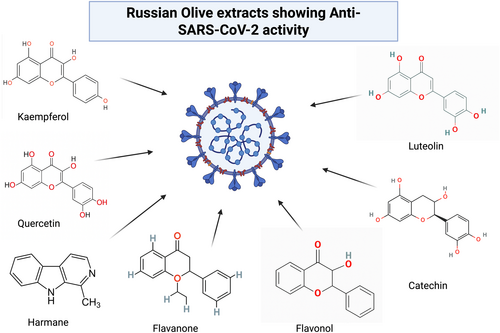
Moreover, E. angustifolia have potential biochemicals such as kaempferol, quercetin, flavanone, flavanol, catechin, luteolin, harmane, etc. that assist in curing emerging diseases, for instance, COVID-19 (as shown in Table 2). Several studies demonstrated the anti-inflammatory, and immune-modulatory properties of these chemicals in coping with COVID-19 (Figure 3) (Anand et al. 2021; Ang et al. 2020; Boukhatem and Setzer 2020; Chakravarti et al. 2021; Chojnacka et al. 2020; Mazraedoost et al. 2021; Nile et al. 2021; Sohail et al. 2021; Srivastava et al. 2020; Wink 2020). However, clinical studies are needed to validate the efficacy of E. angustifolia extracts in coping with COVID-19, standardize the appropriate dosage, and elucidate the mechanism of action.
| Plant | Chemical (extracts) | Active against | Mechanism of action | References |
|---|---|---|---|---|
| Natural herbs, fruit and vegetables | Flavanone, flavanol, triterpenes, quinone and curcumin derivatives | Anti-SARS-CoV-2 activity | Bind with 3 chymotrypsin-like protease (3CLpro), spike protein (TMPRSS2), and angiotensin-converting enzyme 2 (ACE2), ultimately inhibit COVID-19 inoculation and replication in host cell | da Silva Antonio et al. (2020) |
| Natural plants | Natural extracts like flavonoids, terpenoids, diarylheptanoids, and coumarins | Anti-SARS-CoV-2 activity | Effective inhibitors of the SARS-CoV proteases | Chakravarti et al. (2021) |
| Dysphania ambrosioides (Mexican-tea) | Flavonoid glycoside like rutin and nicotiflorin | Anti-SARS-CoV-2 activity | Effectively inhibit the SARS-CoV-2 RNA-dependent RNA polymerase (RdRp) and SARS-CoV-2 main protease (MPRO), thus inhibiting the viral life cycle inside host | Chakravarti et al. (2021) |
| Red onion (Allium cepa) | Flavonoid content as Quercetin | Anti-SARS-CoV-2 activity | Effectively inhibit the viral life cycle and entry in host cell, by binding with SARS-CoV MPRO and SARS-CoV Spike (S) protein | Chakravarti et al. (2021), Srivastava et al. (2020) |
| Natural plants | Flavonol such as Kaempferol | Anti-SARS-CoV-2 activity | Specifically bind to Spike glycoproteins of coronavirus and Inhibit the papain-like proteases (PLPRO), 3CLpro and CoV MPRO | Anand et al. (2021), Nile et al. (2021), Sohail et al. (2021) |
| Broussonetia papyrifera | Polyphenols | Anti-SARS-CoV activity | Inhibition of CoV cysteine proteases | Chojnacka et al. (2020) |
| Curcuma sp. | Polyphenols like curcumin and its derivatives | Anti-SARS-CoV-2 activity | Target the MERS-CoV 3CLpro, hence preventing the viral entry in host cell | Chakravarti et al. (2021), Mazraedoost et al. (2021) |
| Giloy (Tinospora cordifolia) | Sitosterol | Anti-SARS-CoV-2 activity | Exhibiting strong anti-COVID properties by binding with COVID-19 protease | Srivastava et al. (2020) |
| Natural plants | Vitamin C (ascorbic acid) | Anti-SARS-CoV-2 activity | Immunomodulator agent and antioxidant property | Nile et al. (2021) |
| Herbs | Luteolin | Antiviral activity | Specifically bind and interact with S2 domain of SARS-CoV-2 S proteins, and inhibiting the entry of virus (HIV-luc/SARS pseudo-type virus) | Boukhatem and Setzer (2020), Sohail et al. (2021) |
| Euphorbia hirta L. | Tannins extracts | Strong antiretroviral activity | Inhibit viral replication | Sytar et al. (2021) |
| Anagallis arvensis | Saponin | Antiviral activity against HSV-1, poliovirus-2 and SARS-CoV | Inhibit virion to attach, enter, absorb and penetrate into the host cell, also protect the host cell from structural injury | Anand et al. (2021) |
| Green tea | Catechin | Therapeutic agent for COVID-19 | Bind with ACE2 of the host and receptor domains of viral S-protein | Anand et al. (2021) |
| O. sanctum | Luteolin | Anti-SARS–CoV property and most potent against coxsackievirus A16 and enterovirus-71 infections | Bind with human receptor, and inhibiting the entry of SARS–CoV, as well as it disrupts viral RNA replication | Anand et al. (2021), Chakravarti et al. (2021) |
| Zygophyllaceae | β-Carboline alkaloids (harmine, harmaline) | Anti HSV, MCMV, influenza | DNA intercalation, inhibition of topoisomerase, DNA polymerase, reverse transcriptase | Wink (2020) |
| Allium cepa L. | Linolenic acid, palmitic acid, oleanolic acid | Anti-SARS-CoV-2 activity | Target specifically MPRO and 3CLpro | Kim (2021) |
| Medicinal plants | Terpenoids | Antiviral activity | Inhibit the activity of protease in viruses, by hampering specific amino acid | Sohail et al. (2021) |
5.4.6 Sexual Health
The extracts from E. angustifolia flowers have shown effectiveness in recovering sexual function in females by widening blood vessels, decreasing swelling in the genital system, and enhancing the relaxation of smooth vaginal muscles (Akbarzadeh et al. 2014). Furthermore, a clinical trial suggested the efficacy of this plant in improving sexual interest/arousal in women (Zeinalzadeh et al. 2019). Furthermore, studies show mild estrogenic activity in menopausal women (Emaminia et al. 2020; Jalalvand et al. 2021).
5.4.7 Antioxidant Effect
The experiment of power assays, DPPH (2,2′-diphenyl-1-picrylhydrazyl radical), and FRAP (fluorescence recovery after photobleaching) assays confirm that the methanolic extracts derived from the seeds and peels of E. angustifolia showed the antioxidant property. The seed extracts have a higher amount of flavonoids and phenolic compounds than the peels and flesh. Therefore, seed extracts have the highest antioxidant activity (Faramarz et al. 2015). Yalcin and Sogut (2014) experiment showed that the ethanol/water extracts present in the peel, leaves, mesocarp, and their mixture also exhibit higher antioxidant properties compared to the methanol/water and ethanol/acetone extracts.
Sarvarian et al. (2022) observed that the E. angustifolia extracts added to the orange juice increase the antioxidant capacity. In addition, crackers prepared with E. angustifolia L. fruit flour have higher polyphenol amounts and antioxidant effects than the control foods (Incedayi and Erol 2023). Likewise, its fruit flour is added to ice creams, which improves the chemical, sensory, and physical properties along with boosting antioxidant capacity (Çakmakçı et al. 2015). Thus, data suggest that the E. angustifolia extracts are used for nutraceutical products.
5.4.8 Antimutagenic and Anticancer Activity
E. angustifolia contains methanolic extracts that have antioxidant and anti-inflammatory properties, thus serve as antitumor and antimutagenic agents (Amereh et al. 2017). In 2014, Okmen and Turkcan used the Ames test in the absence of rat microsomal liver enzyme (-S9) and confirmed that the methanolic extracts of E. angustifolia positively inhibit the mutagenic activities (Okmen and Turkcan 2014).
An E. angustifolia water extract (EAE) has demonstrated selective cytotoxic activity against cancer cells by inhibiting MDA-MB-231 and MDA-MB-436 proliferation while minimal affecting nontumorigenic epithelial cell line, MCF 10A. The phyto-complex acts on these cancer cells through the induction of early and late apoptosis as evidenced by an increase of pro-apoptotic markers (Bax and cleaved caspase-8) and a decrease in the antiapoptotic protein Bcl2. Furthermore, it is found that EAE inhibits cancer cell colony formation partly due to the upregulation of p53 and the inhibition of signal transducer and activator of transcription 3 (STAT3) phosphorylation (Fouzat 2021).
In another study, the similar EAE extract inhibited the antiproliferating activity and colony formation of Human Epidermal Receptor 2 (HER2)-positive breast cancer, Sloan-Kettering Institute's Breast Cancer 3 (SKBR3, breast cancer cell line) and ZR75-1 cell (human breast cancer cell line) by modulating mesenchymal–epithelial transition. Specifically, EAE extracts activate the E-cadherin and β-catenin upregulation, vimentin, p-β-catenin, and fascin downregulation. Thereby promoting epithelial cell development. Additionally, EAE modulates the deregulation of JNK1/2 (c-Jun N-terminal Kinase 1 and 2) and promotes E-cadherin and β-catenin expression while maintaining reduced expression of vimentin and fascin (Jabeen et al. 2020). Apart from breast cancer, EAE extract also possesses anticancer effects when observed in oral carcinoma cell lines such as FaDu and SCC25. Indeed, the phyto-complex inhibited proliferation and induced apoptosis in addition to upregulating E-cadherin expression and inhibiting angiogenesis (Saleh et al. 2018).
Additionally, it was stated that E. angustifolia fruit extract prevented colorectal cancer in Drosophila melanogaster with a KRAS (Kirsten Rat Sarcoma viral oncogene homolog) gene mutation (Fouzat 2021; Zakaria et al. 2023). The chemopreventive potential of this plant was investigated also for fruit extracts. Indeed, in vivo data showed the ability of an E. angustifolia fruit extract to inhibit diethyl nitrosamine-induced hepatocarcinogenesis in rats through several mechanisms involving inflammation and oxidative stress to prevent colorectal cancer cells (Amereh et al. 2017).
5.4.9 Cardiovascular and Metabolic Effects
Antioxidant, free radical scavenging, anti-inflammatory, and wound healing properties of E. angustifolia extracts make them a potential treatment for cardiovascular diseases (acute myocardial infarction and myocardial reperfusion injury) (Okmen and Turkcan 2014). A leaf extract of E. angustifolia has shown a protective effect against myocardial ischemia/reperfusion injury due to the presence of different phytochemicals binding to mono-amino oxidase A (MAO-A) active site in humans (Tehranizadeh et al. 2016). The ability of E. angustifolia extracts to inhibit oxidative stress both directly and indirectly through the increase of superoxide dismutase (SOD) activity contributes to safeguarding cardiac tissues and maintaining cardiac functions compromised by ischemia/reperfusion (Wang et al. 2014). The detailed illustrations involving the mechanism of healing during cardiovascular injury by using E. angustifolia extracts are shown in Figure 4.

Additionally, the consumption of whole fruit powder of E. angustifolia appears beneficial for cardiovascular health. A clinical study showed that the daily administration of 15 g of this substance for 10 weeks in postmenopausal women resulted in a decrease in low-density lipoprotein (LDL) cholesterol (Shabani et al. 2021). Furthermore, the administration of 15 g/day of E. angustifolia medulla powder or whole fruit powder for 8 weeks to obese females was reported to reduce total cholesterol, TC/HDL, and LDL/HDL ratios (Nikniaz et al. 2016). Similarly, Belarbi et al. (2011) reported that the administration of E. angustifolia favorably affects total cholesterol, LDL-cholesterol, triglycerides, and HDL-cholesterol in healthy individuals.
These reported beneficial effects of E. angustifolia are mainly attributed due to the presence of bioactive compounds including, polyphenols and alkaloids. In particular, harmine and harmaline alkaloids found in this plant were demonstrated to reduce total cholesterol in rats favoring cholesterol 7 alpha-hydroxylase activity (Vahidi-eyrisofla et al. 2015).
5.4.10 Gastrointestinal Effects
The E. angustifolia contains the carotenoid and fruit methanolic content that has the ulcer-protecting capability (Tehranizadeh et al. 2016). Its extract also has the relaxing property of lumen muscle contraction through the antioxidant pathways, which also act as a barrier to protect the stomach surface from gastric mucosa (Tehranizadeh et al. 2016). In Armenia, the pshatin drug has been derived from the fruit extract of E. angustifolia (specifically polyphenolic compound), which effectively acts against colitis and gastrointestinal tract diseases. Their lead and fruit extracts are effective in treating nausea, flatulence, and vomiting (Hamidpour et al. 2019, 2017).
6 Side Effects
Study-based analysis concludes no detrimental side effects of this plant such as induction of abnormalities or delayed growth, even of excessive usage of extracts, but the pollens of this plant act like allergens and may trigger nasal irritation symptoms (Hamidpour et al. 2017). On the contrary, it is observed that the potent toxicity of E. angustifolia is due to the presence of the high amount of E-ethyl cinnamate in the essential oil of their leaves and flowers, showing notable antifeeding, insecticidal and nematocidal activities. However, the high amount of E-ethyl cinnamate does not cause any noticeable side effects in mammals or humans (Torbati et al. 2016). The aqueous and ethanol extract of E. angustifolia has muscle-relaxant properties, however, long-term use might be harmful because treatment of osteoarthritis requires quadriceps muscle strengthening (Ebrahimi et al. 2014; Panahi et al. 2016). Additionally, studies confirm that the aqueous extract of E. angustifolia fruit in pregnant mice was safe and nonpathogenic (Farzaei et al. 2015).
Notably, the extract of E. angustifolia may reduce the side effects and increase the effectiveness of some chemotherapy medications, for instance, cisplatin (Zakaria et al. 2023). Research has demonstrated that the fruit extract E. angustifolia has cytotoxic and antiproliferation potency on U87 and C6 (human and rat) glioblastoma cancer cells, thus considered a safe pharmaceutical plant to treat glioblastoma cancer (Arab et al. 2022) with no toxic or very minimal effects on normal body cells (Zakaria et al. 2023). However, the anticancer property of E. angustifolia depends upon the type and concentration of extract used (Arab et al. 2022). Therefore, more comprehensive clinical studies are required to find out the potential side effects, toxicity, optimal dosage recommendation to use by patients, and long-term safety of E. angustifolia extracts.
7 Conclusion and Future Perspective
E. angustifolia holds significant potential in improving human health as the different parts are rich sources of different bioactive compounds including flavonoids, phenolic acids, tannins, alkaloids, polysaccharides, water and fat-soluble vitamins, minerals, and lipids. Its potential for incorporation into food matrices such as fruit juices, bakery products, and ice creams offers the possibility of developing functional foods with aided health benefits. However, standardizing extraction processes, determining optimal harvesting times, and establishing quality control measures can be challenging in ensuring product consistency and efficacy. Further, cost-effective extraction methods and efficient processing techniques are essential for the economic viability of valorizing E. angustifolia. Preclinical and clinical studies suggest that this plant could be a resource for the development of nutraceuticals useful in the management of pathological conditions characterized by inflammation and oxidative stress, particularly in cardiovascular and osteoarticular diseases. Nonetheless, there is a need to determine the optimal dosage of E. angustifolia nutraceuticals for therapeutic effects while avoiding potential side effects. Additionally, preliminary data suggest a possible application of E. angustifolia phyto-complexes in the cosmeceutical field. However, rigorous scientific studies are needed to establish the efficacy and safety of E. angustifolia phyto-complexes in cosmeceutical applications. Future research should also focus on exploring the functions of E. angustifolia extracts in combating emerging diseases, such as COVID-19 as it has an active phytochemical profile. Furthermore, well-designed clinical studies are required to evaluate various extract concentrations in managing different diseases and exploring the underlying mechanisms.
Author Contributions
Muhammad Abubakkar Azmat: conceptualization (equal), writing – original draft (equal), writing – review and editing (equal). Aqeel Shahzad: conceptualization (equal), writing – original draft (equal), writing – review and editing (equal). Muhammad Shaban: supervision (equal), validation (equal), visualization (equal), writing – review and editing (equal). Asif Ali Khan: writing – review and editing (equal). Sadaf Shakoor: writing – review and editing (equal).
Acknowledgments
The authors are grateful to Dr. Martin Wiehle from the University of Kassel, Germany, for his valuable suggestions and discussions that greatly contributed to the development of this review. Open Access funding enabled and organized by Projekt DEAL.
Ethics Statement
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.




