Escherichia coli isolates from vegetable farms in Addis Ababa, Ethiopia: Antimicrobial susceptibility profile and associated resistance genetic markers
Abstract
The use of animal manure to fertilize soil is an emerging concern contributing to the transfer of antimicrobial-resistant pathogens to vegetables. Hence, assessing antimicrobial susceptibility profile of Escherichia coli in vegetable farms is essential to design appropriate interventions against antimicrobial resistance (AMR) in the food chain. This study assessed antimicrobial resistance profile and associated genetic markers among E. coli isolated from vegetable farms fertilized with animal manure in Addis Ababa, Ethiopia. A total of 1044 samples were collected using convenience sampling: soil (n = 271), manure (n = 375), and vegetables (n = 398) from 81 vegetable farms in Addis Ababa, Ethiopia. Antimicrobial susceptibility test was conducted for 100 E. coli isolates and antimicrobial resistance genes (ARGs) were tested by polymerase chain reaction (PCR). Of the 1044 collected samples, 25.3% were positive for E. coli, with significantly higher prevalence in the manure sample and samples collected from Akaki Kality sub-city (p < .05). The highest resistance rate was recorded for tetracycline (72%), followed by streptomycin (63%), and sulfamethoxazole +trimethoprim (56%). Multidrug resistance was detected in 61% of the E. coli isolates. The aac(3)-IV (76.9%), bla TEM (65.4%), aadA (60.3%), tet(A) (58.3%), and sulI (51.7%) were the commonly detected resistance genes. The current study showed a high burden of antimicrobial resistance among E. coli isolated from manure-amended vegetable farms, with potential of playing a significant role in the dissemination of antimicrobial resistance in the food chain. Efforts should be made to reduce the burden of resistant organisms and ARGs through prudent use of antimicrobials in livestock and application of appropriate composting techniques before using manure as fertilizer.
1 INTRODUCTION
Antimicrobial resistance (AMR) is designated by the United Nations as a public health crisis (IAGC, 2019) that complicates the treatment of infections and is known to increase morbidity and mortality (Smith & Coast, 2013). Antimicrobial resistance results in about 700,000 deaths worldwide. If no action is taken to reduce the burden of antimicrobial resistance, by 2050, the estimated number of deaths will reach up to 10 million, resulting in a cumulative economic loss of more than $100 trillion (O'Neill, 2016).
The emergence of antimicrobial-resistant gram-negative bacteria to the last-line antimicrobials, such as carbapenems and colistin, indicates the severity of the problem (Liu et al., 2016). Since the late 1990s, the development of new antimicrobials has slowed dramatically, which has increased the necessity of preserving the efficacy of the current antimicrobials (Buchy et al., 2020). Even if the role of antimicrobial use in the development of antimicrobial resistance is well studied, the distribution and transmission of AMR is complex and poorly understood (Woolhouse et al., 2015). Host, bacterial, and environmental factors, exposure to antimicrobials in the animal production system, and poor waste disposal methods could contribute to the transmission of AMR (Holmes et al., 2016).
Escherichia coli, a member of the bacterial family Enterobacteriaceae, is one of the most widespread bacterial species globally. It is a commensal organism in the intestinal tract of animals and humans. However, various strains of E. coli are responsible for a broad spectrum of diseases in humans and animals (Ramos et al., 2020). Antimicrobial-resistant E. coli is the most frequent cause of hospital- and community-acquired infections (Pitout, 2012). Due to its capacity to carry different plasmids, E. coli is a major contributor to the horizontal transmission of drug resistance markers to other microorganisms (Salyers et al., 2004).
Multidrug-resistant E. coli strains are responsible for transmitting antimicrobial resistance to the environment through several transmission pathways (Galindo-Méndez, 2020). Thus, the environment plays a critical role by serving as a reservoir for the transmission of antimicrobial resistance genes (ARGs) (González Zorn & Escudero, 2012). E. coli serves as an indicator organism to monitor antimicrobial resistance, as it has a wide range of hosts (Bakshi et al., 2023; Fang et al., 2019).
There are a few studies conducted to identify E. coli strains in different food items and different farm environments in Ethiopia to understand its epidemiology, prevalence, and resistance profile (Dejene et al., 2022; Gemeda et al., 2023). However, the prevalence and antimicrobial resistance profile of E. coli from vegetable farms that apply dairy and poultry manure to their farms as fertilizer have not been well studied. Understanding this is essential to develop effective strategies to reduce the spread of AMR. Thus, this study aims to assess the spread of AMR in E. coli isolates from vegetables, manure, and soil from vegetable farms in Addis Ababa, Ethiopia.
2 MATERIALS AND METHODS
2.1 Study design, study area, and sample collection
A cross-sectional study was conducted in Addis Ababa, the capital city of Ethiopia, from February 2022 to March 2023. Vegetable farms that use animal manure as fertilizer were selected from four sub-cities of Addis Ababa (Akaki-Kality, Nifas-Silk Lafto, Arada, and Gulelle). Based on the availability of different numbers of farms in each sub-city, 81 vegetable farms were visited: 41 from the Akaki Kality sub-city, 23 from Nifas-Silk Lafto, 14 from the Gulelle sub-city, and 3 from the Arada sub-city. Of the vegetable farms, 75.3% used composted manure, while the remaining 24.6% reported that they used animal manure without any treatment. Most of the vegetable farms, 95% (77 of 81), used dairy manure to fertilize their farms, while only 5% (4 of 81) used poultry litter.
From these farms, 1044 samples were collected: Akaki-Kality (n = 482), Nifas-Silk Lafto (n = 252), Arada (n = 72), and Gulelle (n = 238). Based on the sample type, 375 manure, 271 soil, and 398 vegetable samples were collected.
2.2 Escherichia coli isolation
Escherichia coli was cultured, as per the method described in ISO 16654:2001 (Mritunjay & Kumar, 2017). Briefly, 10 grams of the sample was suspended in 90 mL of buffered peptone water (BPW) and mixed by shaking to form a slurry. The slurry was incubated at 37°C for 24 hours. Enrichment and isolation of presumptive E. coli colonies and the biochemical test were performed, as described previously (Quinn et al., 2011). For identification of E. coli O157:H7, first, the E. coli isolates were inoculated on a sorbitol MacConkey agar plate, and all non-sorbitol fermenter E. coli isolates were further tested using the PROLEX E. coli O157 latex test reagent kit as per the manufacturer's instruction.
2.3 Antimicrobial susceptibility testing
To represent the 264 E. coli isolates, one hundred E. coli isolates were selected proportionally from all the sample types (vegetables n = 30, dairy cattle manure n = 46, and soil n = 24) for the antimicrobial susceptibility testing. Antimicrobial susceptibility testing of the E. coli isolates was performed, according to the Clinical and Laboratory Standards Institute (CLSI) guidelines using the Kirby–Bauer disk diffusion method on Muller–Hinton agar plates (Oxoid, CM0337, Basingstoke, England) (CLSI, 2022). Antimicrobials used in the current study were: ampicillin (10 μg), nalidixic acid (30 μg), sulfamethoxazole+ trimethoprim (1.25/23.75 μg), sulfisoxazole (1000 μg), chloramphenicol (30 μg), ceftriaxone (30 μg), amoxicillin+clavulanic acid (20/10 μg), streptomycin (10 μg), kanamycin (30 μg), ciprofloxacin (5 μg), tetracycline (30 μg), gentamicin (10 μg), amikacin (30 μg), azithromycin (15 μg), and meropenem (10 μg). Antimicrobial disks used in this study were all from Sensi-Disc, Becton Dickinson & Company, Loveton, USA. E. coli isolates were considered multidrug-resistant (MDR) when they were resistant to at least one agent in three or more antimicrobial classes (Rafailidis & Kofteridis, 2022). E. coli ATCC 25922 was used for quality control when conducting antimicrobial susceptibility tests.
2.4 Detection of antimicrobial resistance genes
Bacterial DNA was extracted using the boiling method, as described previously (Islam et al., 2016). E. coli isolates that were phenotypically resistant to tetracycline, ampicillin, gentamicin, streptomycin, and sulfonamide were screened for eight antibiotic resistance genes: tetracycline-resistant genes (tet(A), tet(B), and tet(C)), aminoglycoside-resistant genes aminoglycoside acetyltransferase (aac (3)-IV), and adenylyl transferase gene (aadA), sulfonamide-resistant genes (sulI and sulII), and β-lactamase gene (bla TEM), using conventional polymerase chain reaction (PCR). These ARGs were selected, based on the prevalence of phenotypic resistance to antimicrobials tested in this study. The PCR conditions and primer sequences used for this assay are shown in Table 1. The PCR product was observed using agarose gel electrophoresis.
| Antimicrobial | Resistance gene | Primer sequence 5′–3′ | Annealing temp (°C) | Length (bp) | References |
|---|---|---|---|---|---|
| Tetracycline | tet(A) |
F: GCTACATCCTGCTTGCCTTC R: CATAGATCGCCGTGAAGAGG |
50 | 210 | Bryan et al. (2004) |
| tet(B) |
F: TTGGTTAGGGGCAAGTTTTG R: GTAATGGGCCAATAACACCG |
60 | 659 | Ng et al. (2001) | |
| tet(C) |
F: CTTGAGAGCCTTCAACCCAG R: ATGGTCGTCATCTACCTGCC |
60 | 418 | Ng et al. (2001) | |
| Ampicillin | bla TEM |
F: TTTCGTGTCGCCCTTATTCC R: ATCGTTGTCAGAAGTAAGTTGG |
60 | 403 | Mohammed et al. (2016) |
| Gentamicin | aac(3)-IV |
F: CTTCAGGATGGCAAGTTGGT R: TCATCTCGTTCTCCGCTCAT |
55 | 286 | Van et al. (2008) |
| Streptomycin |
aadA |
F: TATCCAGCTAAGCGCGAACT R: ATTTGCCGACTACCTTGGTC |
55 | 447 | Van et al. (2008) |
| Sulfonamides | SulI |
F: TTCGGCATTCTGAATCTCAC R: ATGATCTAACCCTCGGTCTC |
55 | 822 | Van et al. (2008) |
| SulII |
F: CGGCATCGTCAACATAACCT R: TGTGCGGATGAAGTCAGCTC |
50 | 721 | Srinivasan et al. (2005) |
- Note: F, forward primer; R, reverse primer.
2.5 Statistical analysis
Descriptive statistical methods were used to summarize the number of samples collected and their prevalence. The chi-square test was used to assess the association of different antimicrobial resistance patterns with the source of E. coli isolates. Statistical Product and Service Solutions (SPSS) version 26 was used to perform the descriptive analysis. Principal component analysis (PCA) and hierarchal clustering were used to investigate the association between the antimicrobial resistance pattern and the source of the isolates. JMP Pro 17 was used to plot the heat map representation with a dendrogram and the PCA. A p-value less than 0.05 was considered an indicator of a statistically significant association.
3 RESULTS
3.1 Prevalence and distribution of E. coli isolates
From the 1044 collected samples, 264 (25.3%) were positive for E. coli. None of the E. coli isolates were E. coli O157:H7. The prevalence of E. coli in the manure sample (32.3%;121 of 375) and in those samples collected from Akaki Kality sub-city (32.2%;155 of 482) was significantly higher compared to the prevalence of E. coli collected from other samples and those from other sub-cities, respectively (p < .05). Moreover, the prevalence of E. coli was 23.6% (64 of 271) and 19.8% (79 of 398) in soil and vegetable samples, respectively. A similar 20% prevalence of E. coli was observed in Gulelle (48 of 238) and Nifas-Silk Lafto (52 of 252) sub-cities, whereas the lowest prevalence was detected in Arada sub-city at 12.5% (9 of 72). The prevalence and distribution of E. coli isolated from samples collected in the four sub-cities and from different sample types are shown in Table 2.
| Characteristics | Category | No. of samples tested | No. (%) of positive samples | Chi square | p-value |
|---|---|---|---|---|---|
| Sample type | Manure | 375 | 121 (32.2) | 16.29 | .00028 |
| Soil | 271 | 64 (23.6) | |||
| Vegetable | 398 | 79 (19.8) | |||
| Sub-city | Arada | 72 | 9 (12.5) | 24.46 | .00002 |
| Gulelle | 238 | 48 (20.2) | |||
| Akaki-Kality | 482 | 155 (32.2) | |||
| Nifas-Silk Lafto | 252 | 52 (20.6) | |||
| Total | 1044 | 264 (25.28%) |
3.2 Antimicrobial susceptibility profile of E. coli isolates
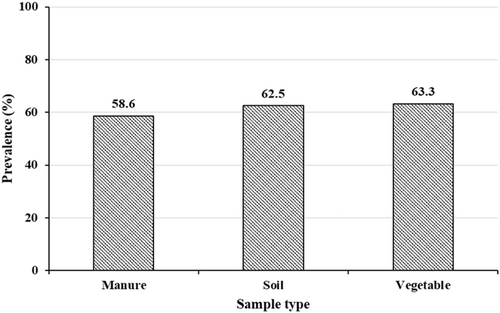
One hundred E. coli isolates were tested for their antimicrobial susceptibility. The highest resistance rate was detected for tetracycline (72%), followed by streptomycin (63%), and sulfamethoxazole+ trimethoprim (56%). Regardless of the source, all the E. coli isolates were susceptible to chloramphenicol and meropenem. Multidrug resistance was observed in 61% of the E. coli isolates, where 58.6%, 62.5%, and 63.3% of the E. coli isolates from manure, soil, and vegetables, respectively, were MDR (Figure 1). Among isolates from vegetables, 50%, 80%, 54.5%, and 83.3% of isolates from lettuce, cabbage, kale, and swiss chard, respectively, were MDR.
The highest rate of resistance to gentamicin was recorded for E. coli isolates from soil, 62.5% (15 of 24), followed by ampicillin, 58.3% (14 of 24), kanamycin, 45.8% (11 of 24), and sulfisoxazole, 58.3% (14 of 24). On the other hand, E. coli isolates from vegetables showed the highest rate of resistance to tetracycline: 76.6% (23 of 30), 30% (9 of 30) to nalidixic acid, and 23.3% (7 of 30) to ciprofloxacin. Resistance to amikacin was recorded in E. coli isolates from manure (8.7%), followed by 3.3% for isolates from vegetables, whereas all isolates from soil were susceptible (Table 3).
| Antimicrobials | No. of samples from different sources (% resistant) | Overall % R n = 100 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Manure (%) n = 46 | Soil (%) n = 24 | Vegetable (%) n = 30 | ||||||||
| R | I | S | R | I | S | R | I | S | R | |
| Tetracycline | 33 (71.7) | 0 | 13 (28.3) | 16 (66.6) | - | 8 (33.3) | 23 (76.6) | - | 7 (23.3) | 72 |
| Chloramphenicol | 0 | 0 | 46 (100) | 0 | 0 | 24 (100) | 0 | 0 | 30 (100) | 0 |
| Ampicillin | 24 (51.2) | 2 (4.3) | 20 (43.4) | 14 (58.3) | 2 (8.3) | 8 (33.3) | 14 (46.6) | 2 (6.6) | 14 (46.6) | 52 |
| Gentamicin | 22 (47.8) | 7 (15.2) | 17 (36.9) | 15 (62.5) | 3 (12.5) | 6 (25) | 15 (50) | 2 (6.6) | 13 (43.3) | 52 |
| Streptomycin | 28 (60.6) | 13 (28.2) | 5 (10.8) | 16 (66.6) | 3 (12.5) | 5 (20.8) | 19 (63.3) | 6 (20) | 5 (16.6) | 63 |
| Kanamycin | 17 (36.9) | 11 (23.9) | 18 (39.1) | 11 (45.8) | 2 (8.3) | 11 (45.8) | 12 (40) | 3 (10) | 15 (50) | 40 |
| Amikacin | 4 (8.7) | 9 (19.5) | 33 (71.7) | 0 | 8 (33.3) | 16 (66.6) | 1 (3.3) | 4 (13.3) | 25 (83.3) | 5 |
| Nalidixic acid | 9 (19.5) | 9 (19.5) | 28 (60.6) | 3 (12.5) | 3 (12.5) | 18 (75) | 9 (30) | 5 (16.6) | 16 (53.3) | 21 |
| Ciprofloxacin | 9 (19.5) | 2 (4.3) | 35 (76.0) | 3 (12.5) | - | 21 (87.5) | 7 (23.3) | 2 (6.6) | 21 (70) | 19 |
| Ceftriaxone | 8 (17.4) | 7 (15.2) | 31 (67.4) | 3 (12.5) | 6 (25) | 15 (62.5) | 4 (13.3) | 8 (26.6) | 18 (60) | 15 |
|
Amoxicillin+ Clavulanic acid |
20 (43.5) | 14 (30.4) | 12 (26.0) | 11 (45.8) | 7 (29.1) | 6 (25) | 12 (40) | 12 (40) | 6 (20) | 43 |
|
Sulfamethoxazole +Trimethoprim |
25 (54.3) | - | 21 (45.6) | 14 (58.3) | - | 10 (41.6) | 17 (56.6) | - | 13 (43.3) | 56 |
| Sulfisoxazole | 21 (45.6) | 25 (54.3) | 14 (58.3) | - | 10 (41.6) | 16 (53.3) | - | 14 (46.6) | 51 | |
| Azithromycin | 3 (6.5) | 43 (93.5) | 1 (4.1) | - | 23 (95.8) | 3 (10) | - | 27 (90) | 7 | |
| Meropenem | 0 | 0 | 46 (100) | 0 | 0 | 24 (100) | 0 | 0 | 30 (100) | 0 |
- Abbreviations: S, susceptible; I, intermediate; R, resistant.
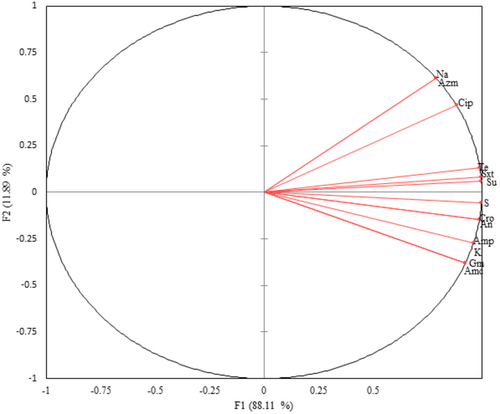
The principal component analysis grouped E. coli isolates that were resistant to sulfisoxazole, sulfamethoxazole+ trimethoprim, tetracycline, and streptomycin together, showing a similar trend of occurrence within these isolates. In addition, resistance to sulfisoxazole, sulfamethoxazole+ trimethoprim, tetracycline, and streptomycin among E. coli isolates was strongly correlated. However, E. coli isolates that were resistant to nalidixic acid, ciprofloxacin, and azithromycin stand separately, as they do not have a similar trend of resistance to other antibiotics (Figure 2).
3.3 Distribution of antimicrobial resistance genetic markers among E. coli isolates
Phenotypically resistant E. coli isolates were tested for their corresponding resistance genes. Resistance genes encoding for aminoglycoside resistance aac(3)-IV were detected in 76.9% (40 of 52) of tested isolates, followed by bla TEM detected in 65.4% (34 of 52), and aadA was detected in 60.3% (38 of 63) of isolates. On the other hand, tet(A), tet(B), and tet(C) were detected in 58.3% (42 of 72), 31.9% (23 of 72), and 4.2% (3 of 72) of tetracycline-resistant isolates, respectively. Moreover, sulI and sulII were detected in 51.7 (29 of 56) and 41% (23 of 56) of sulfisoxazole-resistant isolates, respectively. Based on sample type, the highest prevalence of the resistance genes was observed in isolates from soil: aac(3)-IV (86.6%), aadA (81.2%), bla TEM (71.4%), and sulI (57.1%). The detailed distribution of AMR genes in E. coli isolates from different sample sources is shown in Table 4.
| Encode resistance to | Source of E. coli isolates | No. of resistant isolates (%) | Tested resistant genes | No. of positive (%) | No. of E. coli isolates having common genes |
|---|---|---|---|---|---|
| Tetracycline | Manure | 33 | tet (A) | 18 (54.5) | 1 (tet (A) and tet (B)) |
| tet (B) | 13 (34.4) | ||||
| tet (C) | 0 | ||||
| Soil | 16 | tet (A) | 8 (50) | 2 (tet (A) and tet (B)) | |
| tet (B) | 7 (43.7) | ||||
| tet (C) | 2 (12.5) | ||||
| Vegetables | 23 | tet (A) | 16 (69.5) | ||
| tet (B) | 3 (13.0) | ||||
| tet (C) | 1 (4.3) | ||||
| Ampicillin | Manure | 24 | bla TEM | 15 (62.5) | |
| Soil | 14 | bla TEM | 10 (71.4) | ||
| Vegetables | 14 | bla TEM | 9 (64.2) | ||
| Gentamicin | Manure | 22 | aac(3)-IV | 18 (81.8) | |
| Soil | 15 | aac(3)-IV | 13 (86.6) | ||
| Vegetables | 15 | aac(3)-IV | 9 (60) | ||
| Streptomycin | Manure | 28 | aadA | 16 (57.1) | |
| Soil | 16 | aadA | 13 (81.2) | ||
| Vegetables | 19 | aadA | 9 (47.3) | ||
| Sulfonamide | Manure | 25 | sulI | 13 (52) | |
| sulII | 10 (40) | ||||
| Soil | 14 | sulI | 8 (57.1) | 1 (sulI and sulII) | |
| sulII | 6 (42.8) | ||||
| Vegetables | 17 | sulI | 8 (47) | 1 (sulI and sulII) | |
| sulII | 7 (41.2) |
3.4 Antimicrobial resistance pattern of E. coli isolates and distribution of antimicrobial resistance genes
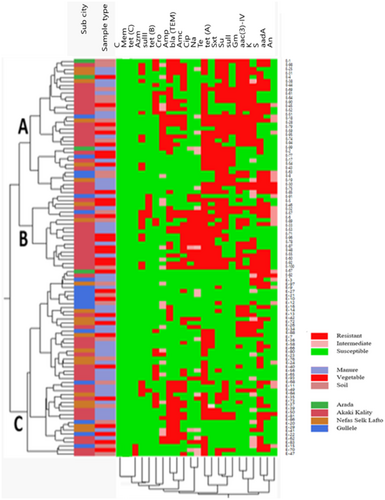
The hierarchical clustering of E. coli isolates based on the phenotypic resistance pattern and distribution of ARGs is shown in Figure 3. E. coli isolates were grouped into three clusters, with Clusters A, B, and C having 34, 19, and 47 E. coli isolates, respectively. In Cluster A, most of the E. coli isolates that were resistant to sulfamethoxazole+ trimethoprim, sulfisoxazole, and tetracycline were grouped together. Moreover, E. coli isolates that were susceptible to azithromycin, ciprofloxacin, and nalidixic acid were grouped together, as also shown in the PCA. In this cluster, only three E. coli isolates were from the Arada sub-city. Whereas in Cluster B, all the isolates were tetracycline-resistant, and most quinolone-resistant isolates were found in this cluster. This cluster is composed of 19 E. coli isolates, interestingly; 15 of the E. coli isolates were from the Akaki Kality sub-city. The overall rate of occurrence of MDR is high among isolates in Clusters A and B and the majority of multidrug-resistant E. coli isolates in these two clusters were from the Akaki Kality sub-city (Figure 3). Cluster C contained a large number of E. coli isolates with less resistance to the tested antimicrobials. It is the only cluster that contained three E. coli isolates positive for tet(C) genes, where one isolate was from the Gulelle sub-city and the other two from the Akaki Kality sub-city.
4 DISCUSSION
Escherichia coli is considered a reliable indicator of food contamination (Disassa et al., 2017). The overall prevalence of E. coli in this study was 25.3%, which is lower than that reported in a study from Belgium, which was 37% (Holvoet et al., 2013) but higher than that mentioned in another study in Ethiopia, where the prevalence of E. coli in soil was 6.8% (Gemeda et al., 2023). The observed difference in the prevalence of E. coli could be the result of ecological and environmental factors. Soil samples in the current study were from vegetable farms fertilized by animal manure, which is believed to have a high load of E. coli. The length of time post application of manure and use of non-composted and composted manure also affects the level of contamination of soil and vegetables by E. coli (Ingham et al., 2004).
The prevalence of E. coli in vegetable samples was 19.8% in this study, lower than that given in a study in south Ethiopia at 31.4% (Alemu et al., 2018) and in Addis Ababa, Ethiopia, 32.1% (Kechero et al., 2019), and higher than in China at 11% (Li et al., 2020). The observed difference in the prevalence of E. coli could be due to the difference in farming practices and the difference in the quality of water used for irrigation, which affects the pathogen level of the produce (Uyttendaele et al., 2015). In addition, the survival of E. coli on manure-amended agricultural farms could be affected by the soil management system of the farm (Franz et al., 2005). In this study, no E. coli O157:H7 was detected, but previous studies reported E. coli O157:H7 in foods of animal origin in Ethiopia (Abebe et al., 2023; Hamid et al., 2018). The common source of contamination of food of animal origin with E. coli O157:H7 is the fresh feces of cattle (Ferens & Hovde, 2010). Manure and soil specimens in the current study might not be contaminated with fresh feces harboring E. coli O.157:H7.
Different studies showed that 30–90% of the antimicrobials administered to animals are excreted as the parent compound in their feces or urine. This could lead to contamination of agricultural soil with antimicrobials and their residues contributing to the selection of resistant organisms in the soil (Gros et al., 2019; Sarmah et al., 2006; Wei et al., 2019; Zhang, He, et al., 2019). In our study, we did not measure the concentration of antibiotics in the collected samples, but different studies showed that antimicrobials were detected in agricultural soil amended with animal manure (Gros et al., 2019; Srichamnong et al., 2021). Thus, soil amendment with animal manure can potentially increase the selection and spread of resistant bacteria (Litt et al., 2021). In this study, E. coli isolates from soil showed a high rate of resistance to ampicillin, gentamicin, kanamycin, and sulfisoxazole, compared to manure and vegetable samples. Thus, soil is an important reservoir of antimicrobial-resistant bacteria and resistance genes (Nesme & Simonet, 2015).
Resistance to some of the tested antimicrobials in this study was lower than those mentioned in other studies in Ethiopia (Messele et al., 2017; Tadesse et al., 2018). For example, the observed resistance for tetracycline in this study was 72%, which was higher than other findings in Ethiopia: 46.4% (Sarba et al., 2019), 8% (Gemeda et al., 2023), and 47.6% (Messele et al., 2017). The difference in resistance of E. coli strains in these studies could be due to the difference in the use of antimicrobials in veterinary practices in different study areas. Tetracycline and oxytetracycline are widely used in dairy and poultry farms in urban and peri-urban areas of Addis Ababa, where animal wastes contaminated by these drugs and antimicrobial-resistant organisms are discharged to the environment, leading to contamination of farms by resistant organisms (Eguale, 2018; Eguale et al., 2016).
Amikacin is not used to treat or prevent infections in livestock in Ethiopia. However, recently, it has been used in the treatment of multidrug-resistant infections in humans at some hospitals in Addis Ababa. In this study, resistance to amikacin was observed among a few E. coli isolates from manure and vegetables from the Akaki Kality sub-city. This might be due to the contamination of Akaki River, with wastewater generated from hospital facilities in Addis Ababa, leading to contamination of manure and vegetables. Akaki River is the main source of water for the farms in the area (Yitayew et al., 2022).
A recent meta-analysis study in Ethiopia showed that E. coli strains from foods, food handlers, animals, and the environment had high MDR profile next to Staphylococcus and Salmonella (Gemeda et al., 2021). In the current study, MDR was observed in 61% of the E. coli isolates, which is higher than 11.8% (Gemeda et al., 2023) and lower than 78.1% (Sarba et al., 2019) in Ethiopia. This variation could be due to the difference in geographical location of the study areas and variation in the number and types of antimicrobials used in animals. Interestingly, similar levels of MDR were reported in a study from South Africa: 61.5% (Fatoba et al., 2022) and 60.5% (Msimango et al., 2023). From the four sub-cities, more MDR E. coli isolates were obtained from the Akaki Kality sub-city. This could be due to the high contamination rate of the Akaki River by waste from health facilities, farms, and other human activities upstream (Tesfaye et al., 2019).
In this study, E. coli isolates from soil have the highest percentage of ARGs (aac (3)-IV, aadA, sulI, and bla TEM) compared to those E. coli isolates obtained from manure and vegetables. The possible explanation for this observation could be that soil from the farms in the current study area is contaminated with human and animal waste from upstream areas with a high human population and livestock. Soil is reported to be the largest environmental reservoir of ARGs, accounting for approximately 30% of known ARGs (Nesme et al., 2014). Moreover, soil-derived ARGs are an important source of resistance genes found in vegetables (Chen et al., 2017).
The prevalence of tet(A) (58.3%) and sulI (51.7%) was lower than those reported in another study in Ethiopia, tet(A) (65.1%) and sulI (54%) (Messele et al., 2017), and another study in Egypt, where tet(A) (64.2%) and sulI (56.7%) were reported (Ramadan et al., 2020). The prevalence of tet(A) and bla TEM in this study was higher than that reported in a study from Tanzania, where the prevalence for both tet (A) and bla TEM was 46% (Sonola et al., 2022). This difference might be attributed to difference in the type of manure amendment, the availability of different soil microbiota and the physicochemical characteristics of the soil, and variations in the distribution of genes encoding for resistance to antimicrobials in different regions (Montealegre Maria et al., 2018; Zhang, Hu, et al., 2019).
In this study, tet(A) was more common than tet(B), showing the dominant role of tet(A) in conferring resistance to tetracycline in E. coli isolates circulating in the study area (Aworh et al., 2021). Additionally, some tetracycline-resistant E. coli isolates in this study were negative for both tet(A) and tet(B). One possible reason for the observed tetracycline resistance in these E. coli isolates could be due to other tet genes like tet(O) and tet(M) (Gao et al., 2012). Similarly, in E. coli isolates resistant to sulfisoxazole and negative to sulI and sulII genes, sulIII could be the gene encoding for the observed phenotypic resistance (Na et al., 2014). These resistance genes are mainly plasmid-mediated and could be sources of resistance transferred from bacteria in manure to grown vegetables (Zhang, Hu, et al., 2019).
5 CONCLUSION
Recycling animal manure into agricultural soil is an emerging concern because it can transfer multidrug-resistant foodborne pathogens to the produce. Our study showed that E. coli isolated from the agricultural environment and the produce were resistant to different antimicrobials and contained different resistant genes. This has a significant role in the dissemination of antimicrobial resistance in the food chain. Therefore, appropriate use of animal manure after proper composting is recommended to minimize the dissemination of foodborne pathogens and antimicrobial resistance in the food chain.
AUTHOR CONTRIBUTIONS
Woinshet Hailu: Conceptualization (lead); data curation (lead); formal analysis (lead); investigation (lead); methodology (lead); software (lead); validation (lead); writing – original draft (lead); writing – review and editing (lead). Haile Alemayehu: Data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); supervision (equal); validation (equal); writing – original draft (equal); writing – review and editing (equal). Lulit Hailu: Data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); resources (equal); validation (equal); writing – review and editing (equal). Girmay Medhin: Conceptualization (equal); data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); software (equal); supervision (equal); validation (equal); writing – original draft (equal); writing – review and editing (equal). Gireesh Rajashekara: Data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); supervision (equal); validation (equal); writing – review and editing (equal). Wondwossen Gebreyes: Data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); supervision (equal); validation (equal); writing – review and editing (equal). Tadesse Eguale: Conceptualization (lead); data curation (lead); formal analysis (lead); funding acquisition (lead); investigation (equal); methodology (equal); resources (lead); supervision (lead); validation (lead); writing – original draft (lead); writing – review and editing (equal).
ACKNOWLEDGMENTS
The authors are grateful to all vegetable farmers in the study area for allowing to visit and collect samples from their farms. We are also grateful to the horticulturalists in the Addis Ababa city administration Urban Agriculture Development Commission and the Sub-cities for providing information on vegetable farms and their support during sample collection.
FUNDING INFORMATION
This study was supported by the Addis Ababa University thematic research project and Sustainable One Health Research Training Capacity (OHEART): Molecular epidemiology of zoonotic foodborne and waterborne pathogens in Eastern Africa, funded by the NIH Fogarty International Center (D43TW008650), through the Global One Health initiative (GOHi).
CONFLICT OF INTEREST STATEMENT
The authors have no conflict of interest to declare.
ETHICS STATEMENT
The Institutional Review Board of Aklilu Lemma Institute of Pathobiology reviewed the protocol for its ethical and methodological standards and approved the conduct of the study (Ref. No. ALIPB IRB/75/2014/22 on April 30, 2022).
Open Research
DATA AVAILABILITY STATEMENT
The authors confirm that all the data are included in the manuscript.




