Antioxidant, anti-inflammatory, and anticancer function of Engleromyces goetzei Henn aqueous extract on human intestinal Caco-2 cells treated with t-BHP
Abstract
High body mass index (high BMI, obesity) is a serious public health problem, and “obesity-induced oxidative stress, inflammation, and cancer” have become modern epidemic diseases. We carried out this study to explore a functional beverage that may protect against obesity-induced diseases. The Engleromyces goetzei Henn herbal tea is such a candidate. For this study, we carried out LC–MS analysis of E. goetzei Henn aqueous extract (EgH-AE); then used the Caco-2 cell line for the model cells and treated the cells with t-BHP to form an oxidative stress system. An MTT assay was used for testing the biocompatibility and cytoprotective effects; reactive oxygen species and malondialdehyde determination was used for evaluating the antioxidative stress effect; TNF-α and IL-1β were used for observing the anti-inflammatory effect, and 8-OHdG for monitoring anticancer activity. The results of this study demonstrate that the EgH-AE has very good biocompatibility with the Caco-2 cell line and has good cytoprotective, antioxidant, anti-inflammatory, and anticancer properties. It is clear that EgH-AE, a kind of ancient herbal tea, may be used to develop a functional beverage that can be given to people with a high BMI to protect against obesity-induced diseases.
1 INTRODUCTION
With the change in living environment, people are now more and more easily able to obtain food, especially high-calorie food. At the same time, due to the increasing pace of lifestyle, fast food has become the food of choice for most people, and these foods are usually rich in calories, sugar, and fat, coupled with factors such as the constant reduction of exercise time, obesity is becoming more common in the population (Brewer & Balen, 2010).
Currently, obesity is one of the most common public health problems facing the world today and has been listed as one of the top 10 health risk factors by the World Health Organization. According to global deaths for all ages, a high body mass index (obesity) is fifth among all risk factors and was responsible for the death of about 5,019,360 people in 2019 (The Institute for Health Metrics and Evaluation, 2020). The prevalence of obesity is also very high. Taking China as an example, 16.4% of adults are obese, a population of 150 million, while the overweight adult population (including obese patients) is close to 500 million, or 50.7% of the adult population (Xiao & Yang, 2022). At present, “obesity-induced oxidative stress” (Lin et al., 2019; Shen et al., 2020; Taherkhani et al., 2021), “obesity-induced inflammation” (Battineni et al., 2021; Kim & Lee, 2021; Kwaifa et al., 2020; Land Lail et al., 2021; Sudhakaran & Doseff, 2020), and “obesity-induced cancer” (Annett et al., 2020; Kern et al., 2018; Ostrand-Rosenberg, 2021; Rajesh & Sarkar, 2021; Yau et al., 2018) have become very worrying modern diseases and pathological changes and pose a major challenge for global public health.
In terms of pathogenesis, oxidative stress is usually an upstream event of inflammation and cancer (Reuter et al., 2010). Reactive oxygen species (ROS) can induce the synthesis and release of inflammatory factors such as TNF-α and IL-1β, by activating the NFκB signaling pathway. In addition, high levels of ROS in cells attack guanine bases in DNA easily and form 8-hydroxydeoxyguanosine (8-OHdG), and 8-OHdG can induce the occurrence of various cancers. Therefore, in the face of the above diseases, in addition to clinical treatment, appropriate use of antioxidant functional foods could also form a very important strategy for the prevention and treatment of obesity-induced diseases.
At this stage, antioxidant functional foods are mainly derived from plants, such as grapes, blueberries, kiwifruit, green tea, etc.; these foods are rich in antioxidants, such as blueberries rich in anthocyanins, kiwi fruit rich in vitamin C, green tea rich in tea polyphenols, and these substances play a significant role in maintaining the health of the body and slowing down the oxidation of the body (Ramarathnam et al., 1995). At the same time, edible and medicinal fungi are a kind of functional food that is rich in nutrients and has vigorous antioxidant activity; at present, edible and medicinal dual-use fungi have become another important source of antioxidant functional food (Mwangi et al., 2022).
Engleromyces goetzei Henn. (EgH) (Fungal, Ascomycetes, Hypocreaceae, Hypocrea), the shape is irregularly spherical, 2–20 cm in diameter, nearly smooth in appearance with fine black spots, the whole is pink or light flesh, anaphase becomes milky white, grayish to brown, the inside is light red to gray–white, solid, hard after drying, interwoven by short and irregular branched mycelium, containing a large number of spherical oil droplets, mature bamboo fungus surface is often uneven, or with irregular protrusions, the fungus meat has a slightly bitter taste after chewing (Yunnan Institute of Botany, 1975). In China, EgH parasitic on high mountain bamboo poles and matures in the rainy season from July to August, this fungus is mainly distributed between 25–30° N and 98–103° E, that is, Northwest Yunnan, Southwest Sichuan, and Southeast Tibet. The terrain of this region is steep, and many mountains are arranged in parallel in a north–south direction. It is one of the regions with the highest biodiversity in Asia and even in the world. EgH is also a native functional food and medicine with a long tradition of use in Yunnan, Sichuan, Tibet, and other places. It is often used by local residents to make tea with boiling water and is used to treat infection, inflammation, and cancer (Jikai et al., 2002; Wang, Zhang, Li, et al., 2015). An earlier experimental study found that EgH has a broad-spectrum antibacterial effect (Yunnan Institute of Botany, 1975). Zhang et al. (2019) found experimentally that EgH has an antiproliferation effect. Wang's study (Wang, Zhang, Wang, et al., 2015) found that EgH inhibits cholesterol ester transfer protein. These are the only studies found in the literature on the biological activity of EgH based on cellular and in vivo experiments.
We, therefore, considered exploring whether the traditional remedy using EgH tea can be used to prevent and treat obesity-induced oxidative stress, inflammation, and cancer. In this study, we investigated the antioxidant, anti-inflammatory, and anticancer effects of E. goetzei Henn aqueous extract (EgH-AE) on Caco-2 cells treated with t-BHP. The results support the possibility that EgH-AE may be developed as a functional beverage to protect the health of obese patients.
2 MATERIALS AND METHODS
2.1 Chemicals and reagents
Bi-distilled deionized water system (Laboratory Water Purification System; Shanghai Hitech Instrument Co., Ltd), rotary evaporator (RE-2000A Rotary Evaporator; Shanghai Yarong Biochemical Instrument Factory), ultrasonic extractor (SB25-12DTD Ultrasonic Cleaner; Ningbo Xinzhi Biotechnology Co., Ltd.), and freeze dryer (SCIENTZ-10N Freeze Dryer; Ningbo Xinzhi Biotechnology Co., Ltd.) were used in this study, High-speed centrifuge (Hunan Xiangyi Experiment Equipment Co., Ltd.), Vortex mixer (Haimen Kylin-bell Lab Instruments Co., Ltd.), Microporous membrane filters (0.22 μm; Tianjin Jinteng Experiment Equipment Co., Ltd.), Thermo Vanquish (Thermo Fisher Scientific), Thermo Q Exactive Focus (Thermo Fisher Scientific).
The chemicals used were all of high purity or analytical reagent grade. 2-Chloro-l-phenylalanine, Folin–Ciocalteu reagent, phenol, petroleum ether, ethyl acetate, n-butanol, 95% ethanol, Na2CO3, NaNO2, Al(NO3)3, NaOH, and H2SO4 were obtained from Sinopharm Chemical Reagent Co. Ltd. 2,2-diphenyl-1-pycrilhydracyl acid (DPPH) was purchased from Tixiai Chemical Industry Development Co., Ltd. LC–MS grade acetonitrile (ACN) was purchased from Fisher Scientific. Formic acid was obtained from TCI. Ammonium formate was obtained from Sigma-Aldrich. Ultrapure water was generated using a Milli-Q system. 2,2′-Azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) and tert-butyl hydroperoxide (t-BHP) were obtained from Shanghai McLean Biochemical Technology Co., Ltd. Ethyl acetate and glacial acetic acid were purchased from Guangdong Chemical Reagent Engineering Technology R&D Center, China. DMEM high glucose medium came from Gibco, USA. Ascorbic acid (Vit. C), anhydrous sodium acetate, and anhydrous ethanol were purchased from Beijing Solarbio Science & Technology Co., Ltd. 3-(4,5-Dime thylthiazol-2-yl)-2.5-diphenyltetrazolium bromide (MTT) kit and 8-hydroxy-2 deoxyguanosine (8-OHdG) kit were purchased from Shanghai Enzyme Link Biotechnology Co., Ltd. Malondialdehyde (MDA) kit was from Nanjing Jiancheng Bioengineering Institute. Interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) kits were purchased from Wuhan Boster Bioengineering Co., Ltd. ROS kit was from Jiangsu Jingmei Biological Technology Co., Ltd.
2.2 Preparation of Engleromyces goetzei Henn extracts
Natural E. goetzei Henn (EgH) were harvested from Yunling-Lasha Mountain Nature Reserve, Yunnan Province, China during the summer of 2021. Figure 1 shows a sample image of EgH and the preparation protocol of the EgH extracts.
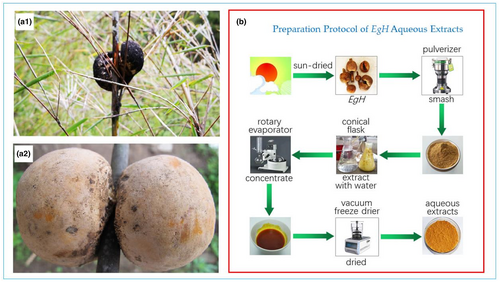
The sun-dried EgH sample was smashed and passed through a 100-mesh sieve. Place 50 g of EgH powder into a 500 mL conical flask, and add 500 mL of petroleum ether according to a material-to-liquid ratio of 1:10. At 20°C, 60% power, 40 Hz ultrasonic extraction for 1 h. After standing in the dark for 24 h, the supernatant was decanted using a pipette, suction filtered, and the filtrate concentrated by rotary evaporation. The remains were freeze-dried to obtain the petroleum ether extract of EgH. The filter residue was dried at 40°C, and the above operation was repeated using ethyl acetate, n-butanol, 95% ethanol, and distilled water to successively extract filter residue, the ethyl acetate extract, n-butanol extract, 95% ethanol extract, and aqueous extract of EgH were obtained, respectively, and stored at 4°C for future use.
2.3 DPPH assay
2.4 ABTS assay
2.5 Measurement of total phenolic, flavonoid, and polysaccharide content
The total phenolic content was quantified using Folin–Ciocalteu reagent and gallic acid standard (Ainsworth & Gillespie, 2007). Briefly, 1 g/mL of EgH-AE was mixed in a test tube containing 5 mL 10% Folin–Ciocalteu reagent. The mixture was then allowed to react for 3–8 min, after which 4 mL of 7.5% Na2CO3 was added. The mixture was then placed in a dark room for 1 h, and the absorbance was measured at 765 nm. The total phenolic content is expressed as gallic acid equivalents (μg GAE/mg).
To analyze total flavonoid content in EgH-AE (Zhishen et al., 1999), 1 g/mL of each sample was mixed with 300 μL of 5% (w/v) NaNO2 and 300 μL of 10% Al(NO3)3, The mixture was then allowed to react for 6 min, after which 1 mL of 1 mol/L NaOH and 3.4 mL of 30% ethyl alcohol was added. The mixture was then placed in a dark room for 15 min, and the absorbance was measured at 510 nm. The total flavonoid content is expressed as rutin equivalents (μg RE/mg).
To analyze the total polysaccharide content of EgH-AE (Dubois et al., 1951), 1 g/mL of each sample was mixed with 1 mL of 5% phenol and 5 mL of 1.84 g/mL H2SO4. The mixture was then placed in a dark room for 30 min, and the absorbance was measured at 490 nm. The total polysaccharide content is expressed as glucose equivalents (μg GE/mg).
2.6 LC–MS analysis
LC–MS was performed in electrospray ionization (ESI) mode with a liquid chromatography instrument (U3000; Thermo Fisher) combined with a mass spectrometer (QE Plus; Thermo Fisher). Accurately add 300 μL of water prepared with 2-chloro-l-phenylalanine (4 ppm) solution for redissolution EgH-AE. The EgH-AE was centrifuged at 17,435 g at 4°C for 10 min and filtered through a 0.22 μm membrane. An ACQUITY UPLC* HSS T3 chromatographic column (1.8 μm, 2.1 × 150 mm; Waters) was used. The column was maintained at 40°C. The flow rate and injection volume were set at 0.25 mL/min and 2 μL, respectively. For LC-ESI (+)-MS analysis, the mobile phases consisted of (B2) 0.1% formic acid in acetonitrile (v/v) and (A2) 0.1% formic acid in water (v/v). Separation was conducted under the following gradient: 0–1 min, 2% B2; 1–9 min, 2%–50% B2; 9–12 min, 50%–98% B2; 12–13.5 min, 98% B2; 13.5–14 min, 98%–2% B2; 14–20 min, 2% B2. For LC-ESI (−)-MS analysis, the analytes were carried out with (B3) acetonitrile and (A3) ammonium formate (5 mM). Separation was conducted under the following gradient: 0–1 min, 2% B3; 1–9 min, 2%–50% B3; 9–12 min, 50%–98% B3; 12–13.5 min, 98% B3; 13.5–14 min, 98%–2% B3; 14–17 min, 2% B3. Mass spectrometric detection of metabolites was performed on Q Exactive Focus (Thermo Fisher Scientific) with ESI ion source. Simultaneous MS1 and MS/MS (Full MS-ddMS2 mode, data-dependent MS/MS) acquisition were used. The parameters were as follows: sheath gas pressure, 30 arb; aux gas flow, 10 arb; spray voltage, 3.50 and −2.50 kV for ESI(+) and ESI(−), respectively; capillary temperature, 325°C; MS1 range, m/z 100–1000; MS1 resolving power, 70,000 FWHM; the number of data-dependent scans per cycle, 3; MS/MS resolving power, 17,500 FWHM; normalized collision energy, 30 eV; dynamic exclusion time, automatic. The identification of the chemical composition was first confirmed by the exact molecular weight (molecular weight error ≤30 ppm), and then the Human Metabolome Database (HMDB) (http://www.hmdb.ca, accessed 5 August 2022), METLIN (http://metlin.scripps.edu, accessed 10 August 2022), MassBank (http://www.massbank.jp/, accessed 5 August 2022), LipidMaps (http://www.lipidmaps.org, accessed 5 August 2022), mz Clound (https://www.mzcloud.org, accessed 5 August 2022), and BioNovoGene self-built standard product database confirmed that the chemical composition was obtained.
2.7 Cell culture
Caco-2 cells (human colon adenocarcinoma cells) were purchased from Kunming Cell Bank of Type Culture Collection, Chinese Academy of Sciences. The cells were inoculated into a 75 cm2 cell culture flask containing 15 mL culture medium, placed in a 37°C, 5% CO2, and high humidity incubator, and the culture medium was changed every 24 h. The culture medium comprised 84% DMEM high glucose medium, 15% fetal bovine serum, 1% penicillin (1000 U/mL), and 1% streptomycin (1000 μ g/mL). The cells were subcultured after 80%–90% of the bottom of the bottle.
2.8 MTT assay
Also called a cell viability assay, this was carried out by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method (Mosmann, 1983). Briefly, the Caco-2 cells were seeded into a 96-well cell culture plate with 1 × 105 cells/well, and treated with different concentrations of EgH-AE (0.05, 0.1, 0.2, 0.4, 0.8, 1.6, 3.2, 6.4, and 12.8 mg/mL) for 24 h at 37°C under 5% CO2. The cell viability (%) was determined using a commercial kit (Cat. No. 16H12B56; Shanghai Enzyme Link Biotechnology Co., Ltd.). All samples were prepared according to the instructions for the use of the MTT kit. Absorbance was measured at 490 nm using a microplate reader (DNM-9602G; Beijing Pulang New Technology Co., Ltd.).
The concentration of Caco-2 cells was adjusted to 5 × 105 cells/mL, inoculated 2.0 mL cell suspension in each well of the 6-well plate, and placed in the incubator for culture, changing the solvent every 12 h. After 2 days, discard the cell culture medium, add 1 mL PBS to each well to clean it, and repeat twice. The Caco-2 cells were then preprotected for 12 h with different concentrations (0.0, 1.0, 5.0, and 10 mg/mL) EgH-AE and 0.5 mg/mL Vit C. After that, the cells were treated with 2 μmoL/mL t-BHP. The EgH-AE, t-BHP, and Vit C were diluted with DMEM medium. Each group was equipped with six experimental holes. After 5 h, the cell viability (%) was determined using a commercial kit (Cat. No. 16H12B56; Shanghai Enzyme Link Biotechnology Co., Ltd.). All samples were prepared according to the instructions for the use of the MTT kit.
2.9 Detection of ROS, MDA, 8-OHdG, TNF-α, and IL-1β
The concentration of Caco-2 cells was adjusted to 5 × 105 cells/mL, inoculate 2.0 mL cell suspension in each well of 6 well plate, place it in the incubator for culture changing the solvent every 12 h. After 2 days, discard the cell culture medium, add 1 mL PBS to each well to clean it, and repeat twice. The Caco-2 cells were then preprotected for 12 h with different concentrations (0.0, 1.0, 5.0, and 10 mg/mL) EgH-AE and 0.5 mg/mL Vit C. After that, the cells were treated with 2 mmol t-BHP. The EgH-AE, t-BHP, and Vit C were diluted with DMEM medium. Each group was equipped with six experimental holes. After 5 h, sample twice from each hole taking 100 μL each time into 1.5 mL centrifuge tubes. These were then sealed and stored at −20°C for later determination of ROS, MDA, TNF-α, and IL-1β, 8-OHdG. All samples were prepared according to the instructions for the kits, and absorbance was measured using a microplate reader (DNM-9602G; Beijing Pulang New Technology Co., Ltd).
Reactive oxygen species was measured at 450 nm using the ROS kit (Cat. No. 202204; Shanghai Enzyme Link Biotechnology Co., Ltd). MDA was measured at 532 nm using the MDA kit (Cat. No. 20220221; Nanjing Jiancheng Bioengineering Institute). 8-OHdG, TNF-α, and IL-1β were measured at 450 nm using the 8-OHdG kit (Cat. No 02/2022; Shanghai Enzyme Link Biotechnology Co., Ltd.), and TNF-α and IL-1β kits (Cat. No. 2391811208, and Cat. No. 1141816208; Wuhan Boster Bioengineering Co., Ltd.).
2.10 Statistical analyses
All data in this study were analyzed by one-way ANOVA, followed by an LSD test, the LSD test at 5% precision level was used to ascertain the significance and nonsignificance of different treatment and control groups. Data were processed with GraphPad Prism 7 software and results were expressed as the mean and standard error of the mean (mean ± SD).
3 RESULTS AND DISCUSSION
3.1 Free radical scavenging activity of EgH extracts
In this study, we used DPPH and ABTS radical scavenging assays to evaluate the antioxidant activity of the EgH extracts with different solvents. The results are presented in Figure 2 and Table 1. As shown in Figure 2, the EgH extracts made with five solvents (petroleum ether, ethyl acetate, n-butanol, 95% ethanol, and distilled water), scavenge DPPH and ABTS in a concentration-dependent manner. Of the five extracts, the EgH-AE made using distilled water demonstrates the best antioxidant activity. From Table 1, it is also clear that all of the five extracts made with the five different solvents have relatively strong antioxidant capacity, compared with the other extracts listed in Table 1, with the best extract being EgH-AE.

| Study in | Samples for extracting | IC50 (μg/mL) | Data source | |
|---|---|---|---|---|
| DPPH assay | ABTS assay | |||
| China, 2022 | EgH—aqueous extract | 1.30 | 0.55 | This study |
| EgH—95% ethanol extract | 5.85 | 0.38 | ||
| EgH—ethyl acetate extract | 2.95 | 0.71 | ||
| EgH—n-butanol extract | 8.84 | 1.15 | ||
| EgH—petroleum ether extract | 25.49 | 4.29 | ||
| Japan, 2019 | Vin tea polyphenols from Hunan | 4.51 | — | Xie et al. (2019) |
| Vin tea polyphenols from Guizhou | 4.06 | — | ||
| Vin tea polyphenols from Guangxi | 4.31 | — | ||
| Dihydromyricetin | 3.24 | — | ||
| Italy, 2020 | Tomato pomace from industry 1 | 57.9 | — | Abbasi-Parizad et al. (2020) |
| Tomato pomace from industry 2 | 92.7 | — | ||
| Italy, 2020 | Fucus vesiculosus—assay B200214 | 614 | — | Corsetto et al. (2020) |
| Fucus vesiculosus—assay B290814 | 608 | — | ||
| Portugal, 2020 | Chestnut shells—sample no. 1 | 56.5 | 66.4 | Lameirão et al. (2020) |
| Chestnut shells—sample no. 2 | 59.9 | 69.6 | ||
| Chestnut shells—sample no. 3 | 56.7 | 64.4 | ||
| Chestnut shells—sample no. 4 | 44.1 | 65.3 | ||
| Chestnut shells—sample no. 5 | 63.7 | 50.5 | ||
| China, 2021 | Kiwi leaves—ultrasound extract | 13.67 | 10.88 | Lv et al. (2021) |
| Kiwi leaves—maceration extract | 17.59 | 13.67 | ||
| Costa Rica, 2019 | Keitt skin | 11.93 | — | Navarro et al. (2019) |
| Keitt flesh | 17.78 | — | ||
| T. Atkins skin | 9.97 | — | ||
| T. Atkins flesh | 22.51 | — | ||
| Italy, 2022 | Lyophilized olive mill wastewater | 95 | 19 | Spizzirri et al. (2022) |
| China, 2018 | Peptide-A: GAERP | 3730 | 100 | Tao et al. (2018) |
| Peptide-B: GEREANVM | 1870 | 50 | ||
| Peptide-C: AEVG | 2300 | 70 | ||
| China, 2019 | Peptide-A: VPR | 4610 | 4010 | Pan et al. (2019) |
| Peptide-B: IEPH | 770 | 1300 | ||
| Peptide-C: LEEEE | 80 | 160 | ||
| Peptide-D: EEEQ | 150 | 180 | ||
| China, 2020 | Collagen peptide-1: DGPEGR | 4240 | — | Wang et al. (2020) |
| Collagen peptide-2: GPEGPMGLE | 590 | — | ||
| Collagen peptide-3: EGPFGPEG | 370 | — | ||
| Collagen peptide-4: YGPDGPTG | 1760 | — | ||
| Italy, 2019 | Grape leaves—water crude extract | 150 | — | Ferhi et al. (2019) |
| Grape leaves—ethanolic crude extract | 90 | — | ||
| China, 2020 | Butylated hydroxytoluene (BHT) | 30 | 10 | Deng et al. (2020) |
| China, 2020 | Rape bee pollen—crude extract | 76.87 | — | Zhang et al. (2020) |
| Rape bee pollen—flavonoids | 34.19 | — | ||
| Rape bee pollen—phentolamines | 6.41 | — | ||
| China, 2020 | Plantago asiatica—dichloromethane extract | 351 | —— | Dong et al. (2020) |
| Plantago asiatica—ethyl acetate extract | 160 | — | ||
| Plantago asiatica—methanol extract | 153 | — | ||
| Portugal, 2018 | Saco sweet cherry—total extract | 21.88 | — | Gonçalves et al. (2018) |
| Saco sweet cherry—colored fraction | 31.39 | — | ||
| Saco sweet cherry—noncolored fraction | 210.86 | — | ||
In the latter experiments of this study, we used only EgH-AE as our test sample, because: (1) in the earlier experiments we found that the antioxidant effect of EgH-AE had the best effect of the five extracts; (2) this study aimed to simulate the general preparation process of the beverage to be drunk, since this fungus has been used for a long time as a herbal tea in Western Yunnan, China; (3) to obtain the most accurate results of antioxidant, anti-inflammatory, and antigenotoxic activity for the extract, it is best that the samples are not contaminated by solvents, and are nontoxic.
3.2 Chemical composition of EgH-AE
The total phenolic, flavonoid, and polysaccharide content in EgH-AE is shown in Table 2. It is clear that the main chemical components in EgH-AE are polysaccharides. This means that the polysaccharides in EgH-AE possibility perform the main antioxidant activity. This finding is consistent with other studies (Ren et al., 2015; Wang et al., 2018). In other words, the main antioxidant components of EgH-AE are polysaccharides. In fact, fungal polysaccharides are widely distributed in the cell walls of fungi. Medicinal fungal polysaccharides are widely known as “biological response modifiers”.
| Chemical composition | Measurement (mean ± SD) |
|---|---|
| Total phenolic content (TPC) (μg GAE/mg) | 6.155 ± 0.239 |
| Total flavonoid content (TFC) (μg RE/mg) | 5.758 ± 0.857 |
| Total polysaccharide content (μg GE/mg) | 47.310 ± 2.029 |
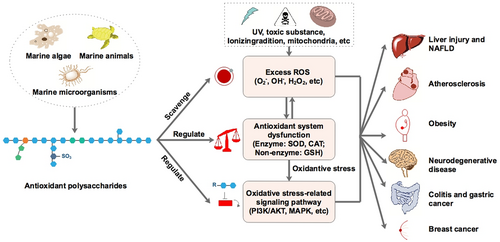
Zhong et al. (2019), illustrated the antioxidant mechanisms and pharmacological effects of polysaccharides (Figure 3).
3.3 Identification of the chemical constituents of EgH-AE
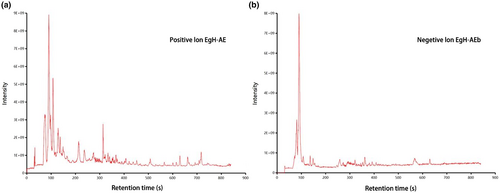
This is the first time to analyze the composition of EgH-AE. A nontargeted UPLC–MS/MS-based method was used to analyze EgH-AE, and the obtained data then underwent bioinformatics analysis. The positive and negative ion flow diagrams of EgH-AE are shown in Figure 4. A total of 196 species of chemical constituents of EgH-AE were identified, including 111 species in positive ion mode and 85 species in negative ion mode. According to the peak response intensity of the total ion chromatogram, the 10 substances with the highest content in the EgH-AE can be preliminarily determined (Table 3). Among them, Pipecolic acid, 1,2,3-Trihydroxybenzene, and Taurine have antioxidant activity (Aruoma et al., 1988; Sroka & Cisowski, 2003; Wang et al., 2021). Pipecolic acid, Azelaic acid, 1,2,3-Trihydroxybenzene, and Taurine have anti-inflammatory activity (Letavic et al., 2002; Marcinkiewicz & Kontny, 2014; Mastrofrancesco et al., 2010; Sharma et al., 2014). 5-Aminolevulinic acid, Azelaic acid, and Taurine have cancer activity (Abo-Zeid et al., 2018; Baliou et al., 2020; Manosroi et al., 2007). Azelaic acid has antibacterial activity (Nazzaro-Porro, 1987). These results indicated that the EgH-AE had various biological activities. At the same time, it can also be inferred that the EgH-AE has a variety of potential effects as a functional beverage.
| Forecast name | Formula | m/z | Retention time | Ppm | Pos/neg | Sample 1 | Sample 2 | Sample 3 |
|---|---|---|---|---|---|---|---|---|
| Pipecolic acid | C6H11NO2 | 130.09 | 129.9 | 4.26 | pos | 17,290,152,176 | 17,821,126,683 | 17,119,511,902 |
| 5-Aminolevulinic acid | C5H9NO3 | 132.06 | 237.1 | 22.53 | pos | 4,112,449,899 | 67,426,893.66 | 50,857,584.73 |
| Azelaic acid | C9H16O4 | 187.10 | 302.7 | 6.29 | neg | 3,691,034,120 | 3,707,804,857 | 2,461,219,953 |
| Geranyl diphosphate | C10H20O7P2 | 314.09 | 251.4 | 3.71 | pos | 2,993,091,621 | 2,999,381,346 | 2,487,338,137 |
| Guanine | C5H5N5O | 152.06 | 130.9 | 0.66 | pos | 2,044,394,650 | 2,108,126,077 | 2,029,668,974 |
| Muramic acid | C9H17NO7 | 252.11 | 89.1 | 0.40 | pos | 1,736,443,873 | 1,707,489,902 | 1,729,676,138 |
| 2-Dehydro-3-deoxy-l-rhamnonate | C6H10O5 | 145.05 | 248.7 | 25.05 | pos | 1,703,424,066 | 1,518,257,904 | 2,175,279,889 |
| 4-Hydroxycinnamoylagmatine | C14H20N4O2 | 276.14 | 268.5 | 1.23 | pos | 1,133,579,959 | 807,621,617.5 | 1,111,552,004 |
| 1,2,3-Trihydroxybenzene | C6H6O3 | 127.04 | 259.9 | 10.83 | pos | 1,089,956,426 | 1,340,486,189 | 345,604,868.5 |
| Taurine | C2H7NO3S | 124.04 | 490.3 | 22.08 | neg | 1,018,296,245 | 178,820,049.7 | 416,833,992.6 |
3.4 In vitro biocompatibility of EgH-AE
Biocompatibility, which is sometimes called “cytotoxicity”, is the most commonly used term to describe the appropriate biosafety requirements of agents or biomaterials. Their presence in bodies will not cause cell damage or affect the function of cells. Biocompatibility is also described as the ability of agents or biomaterials to have an appropriate host response in a specific application. One of the most common methods for testing biocompatibility is the MTT assay (Aoki & Saito, 2020; Mastrogiovanni et al., 2019; Stan et al., 2019).
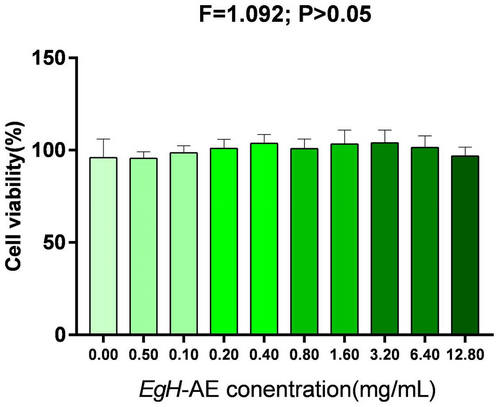
To assess biocompatibility, the Caco-2 cells were treated with different concentrations of EgH-AE for 24 h. After 24 h of exposure, MTT test results (Figure 5) showed that cell viability remained statistically unchanged when compared to the untreated cells (0.00 mg/mL group). The viability of the cells incubated in the presence of the EgH-AE showed a slight change of 0.0%–10% for all of the tested doses, proving that the extract had no harmful effects on the intestinal cells.
3.5 Cytoprotective effect of EgH-AE against t-BHP-induced damage
We evaluated the cytoprotective effect of EgH-AE against oxidative stress induced with t-BHP, by MTT assay at a concentration of 2 μmol/mL for 5 h. The results of cell viability for the different groups are shown in Figure 6.
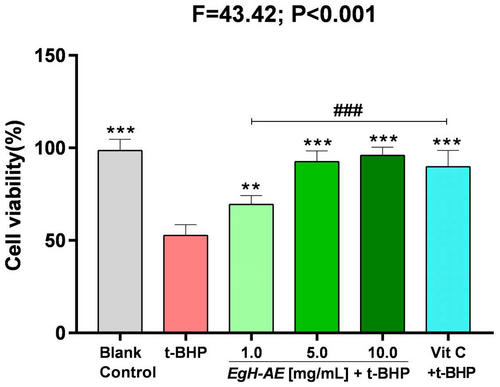
It is clear that: (1) After 5 h, the cell viability of the cells that received the 2 μmol/mL t-BHP treatment, decreased significantly to about 50% that of the blank control group (p < .001). This means that t-BHP treatment can badly damage Caco-2 cells; (2) Compared with the t-BHP only group, the cell viability in the Vit C + t-BHP group increased significantly (p < .001), indicating that the positive reference agent, Vit C, has a good cytoprotective effect; (3) Compared with the t-BHP only group, the cell viability in the EgH-AE + t-BHP groups also increased significantly (p < .01, p < .001, p < .001), indicating that the EgH-AE groups achieved a positive cytoprotective effect, and this effect is very closely dose-related, a result that is similar to other studies found in the literature (Antognoni et al., 2020; Bedoya-Ramírez et al., 2017; Dai et al., 2020).
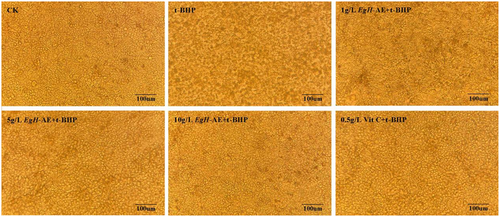
The effect of different treatments on the morphology of Caco-2 cells is shown in Figure 7. The cells of the CK group grow well, the adherent wall is firm, the cells are tightly connected in a polygonal shape, the size is uniform, the edges are distinct, and the paving stone-like single-layer mosaic is arranged. After 5 h of 2 μmoL/mL t-BHP treatment, Caco-2 cells increased the intercellular space, the morphology of the cells became blurred, and the cells began to fall off in large quantities. After the cells were preprotected by different concentrations of EgH-AE, the cell morphology was less and less affected by t-BHP with the increase of EgH-AE treatment concentration, and the cell morphology of the 10 g/L EgH-AE + t-BHP group was similar to that of the CK group. At the same time, after the cells were preprotected by Vit C, the effects caused by t-BHP were primarily avoided, but the protection degree of 10 g/L EgH-AE could not be achieved.
3.6 Antioxidant activity of EgH-AE in Caco-2 cells
Figure 8 illustrates the main biomarkers for indicating cellular oxidative stress and inflammation. In this study we chose: ROS and MDA as the biomarkers for proving antioxidant activity (Bartolomei et al., 2021; Lin et al., 2020; Wang et al., 2020); TNF-α and IL-1β as the biomarkers for proving anti-inflammation activity (Lameirão et al., 2020; Mastrogiovanni et al., 2019; Park et al., 2022); and 8-OHdG as the biomarker for proving anticancer activity (Jee et al., 2020; Reuter et al., 2010; Tiwari & Mishra, 2017; Yadav et al., 2016), although it can be also be used as a biomarker of oxidative stress.
The results of ROS determination are shown in Figure 9a: (1) The levels of ROS in Caco-2 cells treated with 2 μmoL/mL t-BHP, after 5 h, increased significantly in the t-BHP group compared with the blank control group (p < .001). This shows that t-BHP treatment can induce strong oxidative stress in Caco-2 cells; (2) Compared with the t-BHP group, the levels of ROS in the Vit C group decreased significantly (p < .01), indicating that the positive reference agent, Vit C, is a good antioxidant; (3) Compared with the t-BHP group, the cellular ROS levels in each EgH-AE group decreased in a dose-dependent manner (ns, p < .05, p < .001), indicating that EgH-AE is a very good ROS scavenger, and this scavenging effect has a good dose-effect relationship.
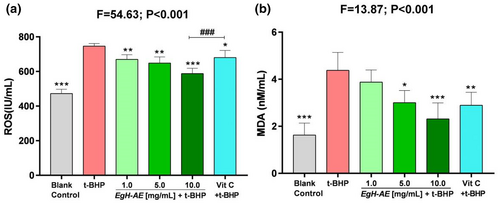
Malondialdehyde levels are shown in Figure 9b: (1) The levels of MDA in Caco-2 cells after 5 h of 2 μmoL/mL t-BHP treatment, increased significantly in the t-BHP group compared with the blank control group (p < .001). The figure shows that t-BHP treatment can induce strong oxidative stress in Caco-2 cells; (2) Compared with t-BHP only group, the content of MDA in the Vit C group decreased significantly (p < .01), illustrating that Vit C is a good antioxidant; (3) Compared with the t-BHP group, the cellular MDA levels in each EgH-AE group decreased in a dose-dependent manner (ns, p < .05, p < .001). This indicates that EgH-AE has a positive effect in preventing oxidative damage of membrane lipids, and this effect has a good dose-effect relationship.
3.7 Anti-inflammatory activity of EgH-AE
The results for TNF-α levels are shown in Figure 10a: (1) The levels of TNF-α, in the Caco-2 cells after 5 h treatment with 2 μmol/mL t-BHP, increased significantly in the t-BHP group compared with the blank control group (p < .001). It can be seen that t-BHP treatment can induce strong inflammation in the Caco-2 cells; (2) Compared with t-BHP only group, levels of TNF-α in the Vit C group decreased significantly (p < .01), indicating that Vit C is a very good anti-inflammatory agent; Compared with the 1.0 and 5.0 EgH-AE groups, Vit C has better anti-inflammation activity (p < .01 and p < .05); (3) Compared with the t-BHP group, levels of cellular TNF-α in each of the EgH-AE groups decreased in a dose-dependent manner (p < .001, p < .001, p < .001), indicating that EgH-AE is a very good anti-inflammatory agent, and this effect also has a dose-effect relationship.
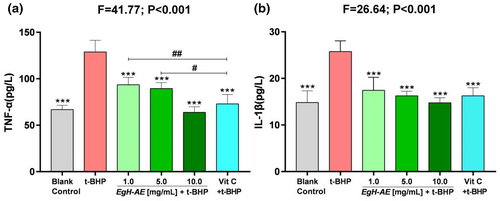
IL-1β levels are shown in Figure 10b: (1) The levels of IL-1β, in the Caco-2 cells after 5 h of treatment with 2 μmoL/mL t-BHP, increased significantly in the t-BHP group compared with the blank control group (p < .001). The figure shows that t-BHP treatment can induce strong oxidative stress in the Caco-2 cells; (2) Compared with the t-BHP group, the levels of IL-1β in the Vit C group decreased significantly (p < .01), indicating that Vit C is a good antioxidant; (3) Compared with the t-BHP group, the levels of cellular IL-1β in each EgH-AE group decreased gradually (p < .001, p < .001, p < .001), indicating that EgH-AE is a very good anti-inflammatory agent, and this effect also has a dose-effect relationship.
3.8 Anticancer activities of EgH-AE
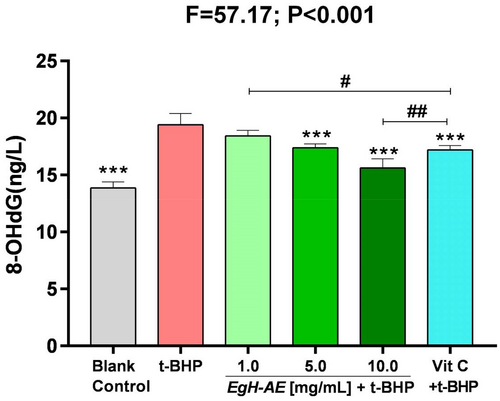
8-OHdG is a special biomarker, because it is one of the endpoints of oxidative stress, but is also an initiator of carcinogenesis (Reuter et al., 2010). The ROS-derived DNA damage includes the generation of 8-hydroxyguanosine, the hydrolysis product of which is 8-hydroxydeoxyguanosine (8-OHdG). 8-OHdG is the most widely used indicator of a radical attack on DNA (Marnett, 2000; Wiseman & Halliwell, 1996). 8-OHdG is strongly implicated in carcinogenesis progression. For example, in breast carcinomas, levels of 8-OHdG have been reported to be 8–17-fold higher in primary breast tumors compared with healthy breast tissue (Musarrat et al., 1996; Valavanidis et al., 2009).
The results of 8-OHdG determination are shown in Figure 11: (1) The levels of 8-OHdG, in Caco-2 cells after 5 h of treatment with 2 μmoL/mL t-BHP increased significantly, in the t-BHP group compared with the blank control group (p < .001). This means that t-BHP can cause extensive DNA oxidative damage, which may then induce DNA base mismatch and sequence point mutation in Caco-2 cells; (2) Compared with the t-BHP group, levels of 8-OHdG in the Vit C group decreased significantly (p < .001), indicating that Vit C is protective against cancer; Compared with the 1.0 EgH-AE group, Vit C is a better anticancer agent (p < .05), but compared with the 10.0 EgH-AE group, Vit C is not as effective an anticancer agent (p < .01); (3) Compared with the t-BHP group, the levels of cellular 8-OHdG in each EgH-AE group decreased in a dose-dependent manner (ns, p < .001, p < .001), indicating that a higher dose of EgH-AE has very good anticancer properties, and this anticancer effect also has a dose-effect relationship.
4 CONCLUSION
In this study, we found that E. goetzei Henn aqueous extract (EgH-AE) is very biocompatible with the Caco-2 cell line, and has good cytoprotective, antioxidant, anti-inflammatory, and anticancer properties. The results of this study support the idea that EgH-AE, the historical herbal tea used in Yunnan province of China, may be used to develop a functional beverage that can be given to people with a high BMI to protect against obesity-induced diseases.
AUTHOR CONTRIBUTIONS
Y.X., X.W., Y.X-Y. and S.R. conceptualized the study; S.R., N.J., and L.L-J. involved in methodology; N.J. provided the software; Y.X-Y. and S.R. involved in validation; N.J. analyzed the data formally; N.J., Z.C-F., C.Y-T., and G.Y-T involved in investigation; X.W. provided the resources; Y.Y-X. and S.R. involved in data curation; N.J. wrote the original draft preparation; Y.X-Y. and S.R. involved in review and editing; N.J. and Y.X involved in visualization process; X.W. involved in supervision, project administration, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
ACKNOWLEDGMENTS
This research was supported by the “Collaborative Innovation Center for Biodiversity and Conservation in the Three Parallel Rivers Region of China, Dali University, Dali, Yunnan, China” and “The Provincial Innovation Team of Biodiversity Conservation and Utility of the Three Parallel Rivers Region, Dali University, Dali, Yunnan, China”.
FUNDING INFORMATION
This research was funded by “Development of diagnostic instrument for oxidative inflammation: determination of blood ROS, MDA and urine 8-OHdG”, grant number “202101BA070001-115”.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
ETHICS STATEMENT
This research did not include biological agents of risk, or clinical trials with humans or animal experiments.
Open Research
DATA AVAILABILITY STATEMENT
Data used for this study are available on request through the corresponding author, although all the relevant data have been provided here.




