Analysis of the microbial community structure and flavor components succession during salt-reducing pickling process of zhacai (preserved mustard tuber)
Abstract
The salt-reducing pickling method has been applied to the industrial production of zhacai. In order to reveal the succession of the microbial community structure and flavor components during the pickling process, this study used PacBio Sequel to sequence the full length of 16S rRNA (bacteria, 1400 bp) and ITS (fungi, 1200 bp) genes, and detected flavor components simultaneously, including organic acids, volatile flavor components (VFC), monosaccharides, and amino acids. Eleven phyla and 148 genera were identified in the bacterial community, and 2 phyla and 60 genera in the fungal community. During the four stages of pickling, the dominant bacterial genera were Leuconostoc, Lactobacillus, Leuconostoc, and Lactobacillus, while the dominant fungal genera were Aspergillus, Kazachstania, Debaryomyces, and Debaryomyces, respectively. There were 32 main flavor components (5 organic acids, 19 VFCs, 3 monosaccharides, and 5 amino acids). Correlation heat mapping and bidirectional orthogonal partial least squares (O2PLS) analysis showed that the flora having close relation to flavor components included 14 genera of bacteria (Leuconostoc, Clostridium, Devosia, Lactococcus, Pectobacterium, Sphingobacterium, Serratia, Stenotrophomonas, Halanaerobium, Tetragenococcus, Chromohalobacter, Klebsiella, Acidovorax, and Acinetobacter) and 3 genera of fungi (Filobasidium, Malassezia, and Aspergillus). This study provides detailed data regarding the microbial community and flavor components during the salt-reducing pickling process of zhacai, which can be used as a reference for the development and improvement of salt-reducing pickling methods.
1 INTRODUCTION
Zhacai is a type of pickle processed using the tuber of the mustard plant Brassica juncea as the raw material. Zhacai is produced in three major areas in China, Fuling in Chongqing, Yuyao in Zhejiang, and Xiapu in Fujian. Among them, Fuling is well known as the “hometown of zhacai,” because it is the origin of Brassica juncea varieties as it is located at the intersection of Yangtze River and Wujiang River, and has a unique climate, soil, and other geographical environments which are especially suitable for growing Brassica juncea (Fan et al., 2016). In 1898, Qiu Shou'an, a pickle merchant, created the pickling method for this mustard tuber and named it “zhacai (pressed vegetable)” because the process includes pressing treatment utilizing wood to expel the brine from the tuber (He & Hou, 2012). As early as 1910–1930, Fuling realized industrialized and large-scale production of zhacai (Yuan, 2008). Presently, the raw mustard tuber output from Brassica juncea in Fuling accounts for 70% of production within the entire country, and it is the largest zhacai production and export zone.
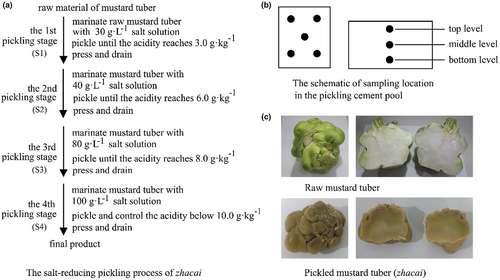
A multistage pickling method is adopted during industrial production of zhacai. The traditional method is summarized as “three times pickled and pressed,” as can be seen from the name, the product is pickled three times by immersing the raw tuber in 40, 80, and 140–160 g·L−1 (w/v) salt solution in a cement pool for about 7, 20–30 days, and 4–6 months, respectively. At the end of each pickling stage, the tuber is taken out and pressed to remove the brine, and then transferred to the next pickling stage (the tuber reaches its final pickling product at the end of the third stage) (He et al., 2013). We have reported on this traditional pickling method in a previous article (Yang et al., 2021). The other method is a salt-reducing pickling method which has been developed in recent years. This technique includes four pickling stages, the salt concentration within the brine is about 30, 40, 80, and 100 g·L−1, for one to four stages, respectively. The acidity of the tubers at each stage is an important factor in process control. When the acidity in the first three stages reaches 3, 6, and 8 g·kg−1, respectively, the pickling is terminated, and the tuber is then pressed and transferred to the next stage. In the fourth stage, the acidity of the final product is controlled and must be less than 10 g·kg−1 (as shown in Figure 1a). This new method reduces salt concentrations in the brine, shortens the total pickling time (less than 4 months), and lowers the workload of subsequent desalination treatment; in addition, the flavor and other organoleptic qualities of the zhacai are equivalent to that achieved using the traditional method.
The salt-reducing pickling method has been applied to the industrial production of zhacai. However, the succession of the microbial community structure and the formation of flavor components under these processing conditions have not been clarified. The characteristic flavors of zhacai include salty, umami, sour, and sweet tastes and unique and rich aroma, which is deeply loved by consumers. Zhacai is used as an appetizer and seasoning for Sichuan cuisine. The formation of zhacai's flavor is complex, it includes not only the flavor components of raw mustard tuber but also the flavor components formed by the chemical conversion of endogenous enzymes and microorganisms during the pickling process. The metabolic activities of microorganisms play a key role in the formation of flavor quality. Carbohydrates in the tubers can be degraded into low-molecular sugars (especially monosaccharides) for use by other microorganisms, or converted into organic acids. Monosaccharides and organic acids produce sweet and sour tastes, respectively. The free amino acids produced by degradation of proteins produce umami, sweet, or bitter taste. Microorganisms can also metabolize and produce volatile flavor components (VFCs), such as esters, aldehydes, and alcohols, that are the main components of zhacai's aroma (Li, 2003). Thus, microorganisms probably contribute to the aroma and umami, sour, and sweet flavors of zhacai. Zhacai depends mainly on natural microbial fermentation and its long pickling time, and multiple steps can also easily be affected by temperature, environmental, and other factors, making its flavor unstable. Studying and clarifying the evolution process of the microorganisms and flavor components, and exploring the correlations between them, will help to improve flavor stability through process control.
The third-generation sequencing platform PacBio SMRT (single-molecule real-time) has been used to study microbial diversity. It is capable of full-length sequencing 16S rRNA genes (1400 bp) in bacteria and the ITS gene (1300 bp) in fungi with 99% accuracy (Fichot & Norman, 2013; Roberts et al., 2013; Tedersoo et al., 2020), and improves the accuracy of microbial community structure analysis. This study investigated the entire process of the salt-reducing pickling method, with tuber and brine samples collected and analyzed at various stages and time points. The full-length 16S rRNA and ITS genes were sequenced using the PacBio Sequel platform, the flavor components (organic acids, monosaccharides, VFCs, and amino acids) were detected simultaneously, and correlation heat mapping and the bidirectional orthogonal partial least squares (O2PLS) were used to analyze the correlations between the microbial community and flavor components and infer the core functional flora (Huang et al., 2018; Zhao et al., 2020), and PICRUSt2 (Phylogenetic Investigation of Communities by Reconstruction of Unobserved States) was used to predict the function of bacterial and fungal communities based on 16S rRNA and ITS gene sequences, respectively (Douglas et al., 2020). Using these techniques and analyses, it was expected that a reference could be provided to develop and improve the salt-reducing pickling method.
2 MATERIALS AND METHODS
2.1 Sample collection
This study investigated the salt-reducing pickling process for zhacai, as described above. The raw mustard tubers were marinated in 30, 40, 80, and 100 g·L−1 (w/v) salt solutions, respectively, during the four stages of the pickling process. The acidity of the tubers was controlled at 3.0, 6.0, 8.0, and 10.0 g·kg−1 for the four stages, respectively. At the end of the first three stages, the tuber was taken out of the brine, pressed until most water flows out, and then transferred to the next stage. The pickling times of the four stages were 7, 20, 37, and 36 days, respectively (Figure 1a). The color, flavor, and quality of zhacai were qualified after being reviewed by enterprise personnel. Samples of brine and tuber were collected on days 4 and 7 of the first stage, days 4, 10, 16, and 20 of the second stage, days 2, 10, 20, and 37 of the third stage, and days 5, 10, 15, 21, 28, and 36 of the fourth stage (as shown in Table 1). To ensure uniformity of sampling, the rectangular cement pickling pool was divided into three layers (i.e., top, middle, and bottom). The four corners and center of each layer were selected as sampling points (5 points per layer, 15 points in total, Figure 1b). Approximately 1 kg tuber and 500 mL of brine were collected at each point. Tuber or brine samples collected at the same time points were pooled and homogenized. The tuber samples were divided into three portions for parallel biochemical analyses. The brine samples were used to analyze microbial community structure.
| Pickling stage | Sample | Pickling days | Titratable acidity3 (g·kg−1) | NaCl concentration (g·kg−1) | Nitrite concentration (mg·kg−1) | Organic acid concentration (g·kg−1) | Monosaccharide concentration (μg·kg−1) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxalic acid | Malic acid | Lactic acid | Acetic acid | Succinic acid | Glucose | Fructose | Galactose | Total | ||||||
| Raw material | S0 | 0 | 0.77 ± 0.06q | 5.06 ± 0.17q | 0.48 ± 0.012g | 0.55 ± 0.04c | 0.34 ± 0.05e | 0.69 ± 0.05j | 0.39 ± 0.04i | 2.58 ± 0.11k | 9.33 ± 0.36a | 9.26 ± 0.41a | 0.02 ± 0.01l | 18.97 ± 0.76a |
| First | S11 | 4 | 1.81 ± 0.07p | 13.85 ± 0.10p | 0.88 ± 0.011d | 0.73 ± 0.09c | 0.53 ± 0.06d | 3.65 ± 0.15i | 0.71 ± 0.06h | 2.84 ± 0.07j | 8.12 ± 0.23b | 8.45 ± 0.54b | 0.05 ± 0.01l | 16.88 ± 0.79b |
| S12 | 7 | 3.22 ± 0.05o | 20.75 ± 0.11o | 0.79 ± 0.046e | 0.83 ± 0.06bc | 0.62 ± 0.09d | 5.41 ± 0.10h | 1.07 ± 0.08g | 3.14 ± 0.11i | 8.62 ± 0.46b | 7.57 ± 0.56c | 0.06 ± 0.01l | 16.50 ± 1.05b | |
| Second | S21 | 4 | 3.43 ± 0.13n | 22.41 ± 0.09n | 0.95 ± 0.046c | 0.84 ± 0.08b | 0.68 ± 0.04d | 5.82 ± 0.09g | 1.18 ± 0.10g | 3.49 ± 0.04h | 7.13 ± 0.17c | 7.34 ± 0.64c | 0.14 ± 0.02l | 14.83 ± 0.63c |
| S22 | 10 | 4.48 ± 0.10m | 26.64 ± 0.08m | 1.10 ± 0.048b | 0.86 ± 0.07ab | 0.82 ± 0.08cd | 8.25 ± 0.16f | 1.88 ± 0.08f | 4.24 ± 0.20g | 6.92 ± 0.64c | 7.16 ± 0.42cd | 0.38 ± 0.05k | 14.75 ± 1.15c | |
| S23 | 16 | 5.33 ± 0.08l | 28.16 ± 0.09l | 0.87 ± 0.048d | 0.81 ± 0.07bc | 0.89 ± 0.04c | 10.22 ± 0.12e | 2.19 ± 0.12e | 4.84 ± 0.13f | 5.89 ± 0.56d | 7.34 ± 0.47c | 0.62 ± 0.03j | 14.43 ± 1.12c | |
| S24 | 20 | 6.19 ± 0.05j | 31.17 ± 0.10k | 0.96 ± 0.048c | 0.76 ± 0.05bc | 0.93 ± 0.03c | 11.62 ± 0.14d | 2.02 ± 0.07ef | 5.26 ± 0.08e | 5.17 ± 0.23e | 7.40 ± 0.64c | 0.81 ± 0.04i | 14.25 ± 1.01cd | |
| Third | S31 | 2 | 6.07 ± 0.08k | 35.81 ± 0.14j | 0.75 ± 0.038ef | 0.74 ± 0.05c | 0.87 ± 0.04c | 11.84 ± 0.09d | 1.97 ± 0.04f | 5.01 ± 0.03f | 5.59 ± 0.32de | 6.89 ± 0.67cd | 1.07 ± 0.12h | 14.41 ± 1.23c |
| S32 | 10 | 6.79 ± 0.06i | 49.63 ± 0.18i | 0.73 ± 0.017f | 0.78 ± 0.02bc | 0.94 ± 0.03c | 12.77 ± 0.26c | 2.37 ± 0.11d | 5.73 ± 0.09d | 5.36 ± 0.52de | 6.39 ± 0.57d | 1.29 ± 0.15g | 13.92 ± 0.67cd | |
| S33 | 20 | 7.29 ± 0.04h | 57.32 ± 0.24h | 0.85 ± 0.035d | 0.88 ± 0.04ab | 1.12 ± 0.07b | 13.09 ± 0.49c | 2.91 ± 0.14c | 6.23 ± 0.08b | 4.78 ± 0.60e | 5.79 ± 0.74de | 1.45 ± 0.12f | 12.88 ± 1.50d | |
| S34 | 37 | 8.07 ± 0.05g | 66.71 ± 0.16g | 0.71 ± 0.035f | 0.93 ± 0.02a | 1.41 ± 0.16a | 13.42 ± 0.08b | 2.75 ± 0.09c | 6.54 ± 0.14a | 4.25 ± 0.45f | 5.88 ± 0.86de | 1.76 ± 0.13e | 12.89 ± 1.52d | |
| Fourth | S41 | 5 | 8.10 ± 0.06f | 70.49 ± 0.13f | 0.88 ± 0.012d | 0.84 ± 0.05bc | 1.33 ± 0.07a | 13.70 ± 0.15b | 2.94 ± 0.10c | 6.17 ± 0.07b | 4.03 ± 0.19f | 5.42 ± 0.64e | 1.94 ± 0.16d | 12.47 ± 1.07de |
| S42 | 10 | 8.28 ± 0.03e | 76.67 ± 0.20e | 1.26 ± 0.057a | 0.82 ± 0.02bc | 1.14 ± 0.04b | 14.04 ± 0.16a | 3.22 ± 0.07b | 5.93 ± 0.12c | 4.38 ± 0.53f | 5.28 ± 0.42e | 2.19 ± 0.14c | 12.95 ± 1.17d | |
| S43 | 15 | 8.47 ± 0.07d | 81.19 ± 0.12d | 0.90 ± 0.012cd | 0.76 ± 0.04c | 1.28 ± 0.12ab | 14.20 ± 0.08a | 3.49 ± 0.20a | 5.72 ± 0.13d | 3.23 ± 0.19g | 5.67 ± 0.33de | 2.24 ± 0.09bc | 12.39 ± 0.50de | |
| S44 | 21 | 8.69 ± 0.08c | 83.72 ± 0.14c | 1.10 ± 0.058b | 0.73 ± 0.02c | 1.18 ± 0.11b | 14.31±0.09 a | 3.26 ± 0.12b | 5.84 ± 0.10cd | 3.12 ± 0.13g | 4.92 ± 0.66ef | 2.49 ± 0.15a | 11.90 ± 1.03de | |
| S45 | 28 | 8.94 ± 0.03b | 85.47 ± 0.10b | 1.10 ± 0.050b | 0.72 ± 0.05c | 1.09 ± 0.03bc | 14.23±0.10 a | 3.30 ± 0.06b | 5.63 ± 0.12d | 3.08 ± 0.18g | 4.10 ± 0.43f | 2.46 ± 0.19ab | 11.09 ± 0.84e | |
| S46 | 36 | 9.31 ± 0.06a | 87.15 ± 0.06a | 0.94 ± 0.031cd | 0.70 ± 0.02c | 0.97 ± 0.09c | 14.05 ± 0.07a | 3.17 ± 0.09b | 5.57 ± 0.13d | 3.36 ± 0.20g | 4.35 ± 0.38f | 2.33 ± 0.19b | 11.53 ± 0.67e | |
- Note: The superscript letters which represent significant markers of ANOVA test at the level of p < 0.05.
- 1 All data are expressed as: .
- 2 Significance markers: IBM SPSS Statistics 23 software is used for one-way ANOVA test, and then is subjected to post hoc comparisons with the Waller–Duncan method and a significance level of 0.05.
- 3 Expressed as g·kg−1 after multiplying by the factor (0.09) appropriate to lactic acid.
2.2 Detection of flavor components
2.2.1 Biochemical indices and organic acids
Titratable acidity (TA), salt, nitrite, and organic acid concentrations of tuber samples were determined by previously established methods (Yang et al., 2021).
2.2.2 Monosaccharides
2.2.3 Volatile flavor components (VFCs)
2.2.4 Amino acids
The concentrations of 22 amino acids in zhacai were determined by ultrahigh-performance liquid chromatography (ACQUITY, Waters, USA) coupled with a triple quadrupole mass spectrometer (Applied Biosystems, USA) using a C18 column (ACQUITY BEH 2.1 × 100 mm, 1.7 μm; Waters, USA). Method details including standard reagents and standard curves are given in Appendix S2. Zhacai sample (50 mg) was weighed into a 2-mL Eppendorf tube, 400 μL 10% formic acid in methanol-ddH2O (1:1, v/v) was added, vortexed for 30 s, and centrifuged at 12000 rpm for 5 min at 4°C. Ten microliters of supernatant was then diluted with 990 μL 10% formic acid in methanol-ddH2O (1:1, v/v) and vortexed for 30 s. The diluted sample (100 μL) was mixed with 100 μL isotopic internal standard (100 ppb), vortexed for 30 s, and filtered through a 0.22 μm membrane into a microvial.
Chromatography conditions were as follows: injection volume 5 μL, column temperature 40°C, mobile phase A 10% methanol in water (with 0.1% formic acid), mobile phase B 50% methanol in water (with 0.1% formic acid), flow rate 0.3 mL·min−1 at 0–8.5 min, and increasing from 0.3 to 0.4 mL·min−1 over 8.5–12.5 min. The gradient elution program was as follows: 0–6.5 min mobile phase B increased from 10% to 30%; 6.5–7 min phase B 30% to 100%; 7–8 min phase B held at 100%; 8–8.5 min phase B 100%–10%; and 8.5–12.5 min phase B held at 10%. Mass spectrometry conditions were as follows: electrospray ionization (ESI) source, positive ionization mode, ion source temperature 500°C, ion source voltage 5500 V, collision gas 6 psi, air curtain gas 30 psi, and atomization and auxiliary gases 50 psi. The taste activity value (TAV) of each compound was obtained by dividing the concentration by its corresponding taste threshold.
2.3 Microbial community structure analysis
Microbial precipitation particles were collected from 20 mL of zhacai brine by centrifuging at 7000 g for 15 min. The particles were washed with 1x TE buffer and microbial macrogenomic DNA was extracted using a DNeasy mericon food kit (Yang et al., 2021). DNA was sequenced at Shanghai Personal Biotechnology Co. Ltd. (China) using a PacBio Sequel platform. The 16S rRNA gene was sequenced using the primer pair 27F (5'–AGAGTTTGATCMTGGCTCAG-3′) and 1492R (5'–ACCTTGTTACGACTT–3') with a sequencing length of 1400 bp. The primers for fungi were ITS1F (5'–CTTGGTCATTTAGAGGAAGTAA–3') and LR3 (5'–CCGTGTTTCAAGACGGG–3') with a sequencing length of 1300 bp.
Cutadapt v.2.3 was used to cut the primer fragment (set at −0 to 10) and discard the unmatched primer sequence (Martin, 2011). The “fastq_mergepairs” module of Vsearch (v.2.13.4_linux_x86_64) was used to merge sequences (Rognes et al., 2016). The “fastq_filter” module was used for quality control of the merged sequences and “derep_fulllength” for removing repetitive sequences. The deduplicated sequences were clustered at 98% similarity level using the “cluster_size” module, then chimeras were removed using the “uchime_denovo” module. A Perl pipeline (https://github.com/torognes/vsearch/wiki/VSEARCH-pipeline) was used to filter chimeras in the sequences following quality control to obtain high-quality sequences. The high-quality sequences were clustered at 97% similarity level using the “cluster_size” module, yielding the amplicon sequence variant (ASV) and operational taxonomic unit (OTU) table. Singleton OTUs (i.e., those with an abundance of one in all samples) and their representative sequence ASVs were removed from the OTU table. The high-quality sequences were processed and analyzed further using QIIME2 (Quantitative Insights Into Microbial Ecology, 2020.8) (Bolyen et al., 2019). The “classify-sklearn” algorithm with default parameters and the pretrained Naive Bayes classifier were used to perform taxonomic annotation of each ASV sequence (https://github.com/QIIME2/q2-feature-classifier) (Bokulich et al., 2018). The ASVs of 16S rRNA genes were annotated using the NCBI database (Stoddard et al., 2015), and ITS genes were annotated using Unite (unite 8.0/its_fungi, release 8.0, http://unite.ut.ee/index.php) (Abarenkov et al., 2010). Thus, taxonomic information on each ASV was obtained and relative abundance tables at each taxonomic level were generated. The α-diversity indices (Shannon, Simpson, Chao1, and Goods coverage) were calculated by QIIME2. Rarefaction curves, box plots of α-diversity indices (Chao1, Shannon, Simpson), microbial profiles at phylum and genus level, and principal component analysis (PCA) plots were all constructed using the R package (v.4.1.1). Linear discriminant analysis effect size (LEfSe) was carried out using python's LEfSe package (http://huttenhower.sph.harvard.edu/galaxy/root?tool_id=PICRUSt_normalize) (Segata et al., 2011). PICRUSt2 (https://github.com/picrust/picrust2) was used to predict the functions of bacterial and fungal communities while figures were generated on the R package. Some data analysis was also performed on the website (https://www.genescloud.cn/) of Shanghai Personal Biotechnology Co., Ltd.
2.4 Correlation between microbial community structure and flavor components
The bacterial and fungal genera with the highest relative abundance greater than 1% and the average relative abundances greater than 0.1% and major flavor components (organic acid, VFCs, monosaccharides, and amino acids) were selected for correlation analysis. The Hmisc package in R was used to calculate Pearson's correlation coefficients and p values among bacteria, fungi, and flavor components and to plot heat maps. O2PLS models were constructed in SIMCA 14.1 to analyze correlations among bacteria, fungi, and flavor components. Genera with variable importance in projection (VIP) predictive values greater than 1.0 and significant positive correlations with more than three flavor components were considered to be the core functional flora.
2.5 NCBI sequence number
Sequences of the bacterial 16S rRNA gene and fungal ITS gene were submitted to the Sequence Read Archive (SRA) database of the National Center for Biotechnology Information (NCBI) (https://www.ncbi.nlm.nih.gov/sra), with the registration numbers PRJNA725670 and PRJNA725681, respectively.
3 RESULTS
3.1 Changes in flavor components during pickling
3.1.1 Chemical indices, organic acids, and monosaccharides
The data of titratable acidity, and the NaCl, nitrite, and organic acid concentrations within the tuber samples during pickling process are shown in Table 1. The titratable acidity was selected as the process control factor, which was controlled at 3.0, 6.0, 8.0, and less than 10.0 g·kg−1 for pickling stages 1 to 4, respectively. The mustard tuber was immersed in 30, 40, 80, and 100 g·L−1 (w/v) salt solutions during the four stages, respectively. The salt penetrates into each tuber and the tuber is also dehydrated by salt, therefore the salt concentrations within the tubers gradually increase at each stage, in the ranges 13.85–20.75, 22.41–31.17, 35.81–66.71, and 70.49–87.15 g·kg−1, respectively. The nitrite concentrations were below 1.30 mg·kg−1 during the whole process, which is lower than the 4 mg·kg−1 standard applied to “green food” (Ministry of Agriculture and Rural Affairs of the People's Republic of China, 2012). The major organic acids were lactic, succinic, and acetic acids, their concentrations all showed upward trends during the pickling process.
In total, 11 monosaccharides were detected within the zhacai samples, and complete data are given in Table S1. Table 1 shows the concentrations of the three major monosaccharides and total monosaccharides. The glucose and fructose concentrations gradually decreased from 9.33 and 9.26 μg·kg−1, respectively, in the raw tubers to 3.36 and 4.35 μg·kg−1 by the end of the process. The concentrations of galactose gradually increased from 0.02 to 2.33 μg·kg−1. The total monosaccharide concentration decreased from 18.97 μg·kg−1 in raw tuber to 11.53 μg·kg−1. The polysaccharides in the raw tuber can be degraded into monosaccharides, mostly glucose and fructose, and the two sugars are metabolized and utilized by microorganisms. As reflected by the downward trends of the concentration of glucose, fructose, and total monosaccharide, the consumption and utilization of monosaccharides exceed their production.
3.1.2 VFCs
In total, 38 VFCs, belonging to seven categories (six categories plus “Others”), were identified in the zhacai samples. The complete VFC data are shown in Table S2. Figure 2 shows the concentration changes throughout the process for these seven categories and the VFCs with an OAV greater than 1 (19 in total).
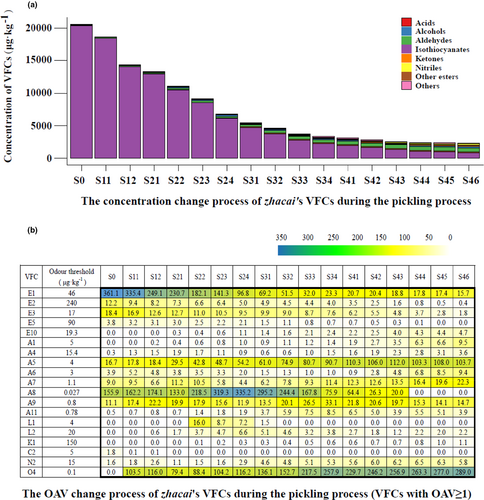
The mustard tuber belongs to Cruciferae family and contains characteristic volatile compound isothiocyanates. Eight isothiocyanates were detected from the raw tuber samples in this study, designated as E1-E8, namely allyl, 2-phenylethyl, 3-butenyl, 3-(methylthio) propyl, butyl, isobutyl, 3-methylbutyl, and pentyl isothiocyanate. Among them, the concentrations of E1, E2, E3, and E5 were 16612.3, 2919.3, 313.6, and 340.0 μg·kg−1, respectively, with an OAV greater than 1, indicating that they were the predominant aroma components of raw tubers, showing a strong pungent aroma. The aldehydes ranked second in the amount of VFCs in the raw mustard tubers. Aldehydes with OAV values greater than 1 included A8 ((E,E)-2,4-decadienal), A5 (phenylacetaldehyde), A9 ((E,Z)- 2,6-nonadienal), A7 (nonanal), and A6 ((E)-2-octenal), and their aromas are fruity (oily cucumber/melon/citrus), green sweet floral hyacinth, green fatty dry cucumber, waxy aldehydic rose, and fresh cucumber, respectively (all description of aroma were retrieved from http://www.thegoodscentscompany.com/search2.html, the same below). Other components with OAV values greater than 1 were C2 (linolenic acid) and N2 (benzenepropanenitrile), and their aromas are faint fatty and powerful nasturtium, respectively.
During the pickling process, the concentrations of isothiocyanates decreased significantly, and the strong pungent aroma became lighter. The other esters (E9–E13), aldehydes, alcohols, ketones, acids, nitriles, and other components increased from 0, 115.0, 0, 6.9, 29.3, 49.9, and 29.4 μg·kg−1, to 135.5, 614.2, 167.6, 197.3, 73.2, 165.9, and 77.4 μg·kg−1, respectively. This indicated that significant amounts of these compounds were produced during the pickling process, which changed the composition of aroma components of the tuber samples and made the aroma softer and richer.
Although the concentration of isothiocyanates present decreased significantly, it was still the category with the highest concentration of VFCs in the final pickled product (S46), and the OAVs of E1 and E3 were greater than 1. The other ester component E10 (octanoic acid ethyl ester) also showed an OAV greater than 1, its aromas have been described as fruity wine, waxy, and sweet apricot. The second category was still the aldehydes, and there were seven aldehydes with an OAV exceeding 1, namely A5, A7, A9, A1 (hexanal), A6, A11 (2-undecenal), and A4 ((E,E)-2,4-heptadienal). The aromas of A1, A11, and A4 have been described as fresh green fatty aldehydic grass, oil and green grass, and fatty green oily aldehydic vegetable, respectively. The concentration of O4 (dimethyl trisulfide) reached 28.90 μg·kg−1, but its odor threshold was as low as 0.1 μg·kg−1 (Van Gemert & Nettenbreijer, 2011), so its OAV value was the highest (289) among all of the VFCs in the final product (S46). Its aroma is described as sulfurous cooked onion savory meaty. Other components with OAV values above 1 included L2 ((E)-2-octen-1-ol), K1 (3,5-octadien-2-one), and N2, with the aromas of L2 and K1 described as green citrus vegetable fatty and fruity fatty mushroom, respectively. They probably make a great contribution to the aroma of the final pickling product (zhacai).
3.1.3 Amino acids
In total, 21 amino acids were detected within the zhacai samples. The complete dataset is shown in Table S3, while Figure 3 shows the major amino acids. The taste threshold value (TAV) refers to the literature (Zhuang et al., 2016). The TAV of all amino acids in the raw tuber was below 1, so their content probably is not high enough to produce a taste.
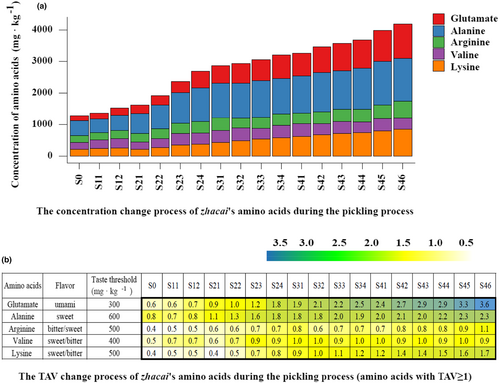
During the pickling process, the concentration of five major flavoring amino acids, glutamate, alanine, arginine, valine, and lysine, increased from 164.8, 477.4, 214.1, 215.8, and 218.6 mg·kg −1 in raw tuber to 1091.9, 1360.1, 539.9, 351.1, and 849.9 mg·kg −1 by the end of the process. In the final pickling sample (S46), the TAV of glutamate, alanine, lysine, and arginine reached 3.6, 2.3, 1.7, and 1.1, respectively. These data indicate that a large amount of flavoring amino acids are produced in the pickling process, which probably contributes to zhacai's overall flavor. Glutamate is an important umami taste amino acid. Alanine elicits a sweet and umami taste and is widely used as a flavor enhancer in soy sauce, fish sauce, and other condiments, to make the taste richer and more umami and intense (Kawai et al., 2002; Lioe et al., 2004; Wakinaka et al., 2019). These two amino acids may contribute toward the strong umami and sweet aftertaste of zhacai. The lysine and arginine present sweet/bitter and bitter/sweet tastes, respectively, which probably also contribute to flavor. The concentration of γ-aminobutyric acid (GABA) in the raw tuber was as high as 1350.7 mg·kg −1, and its concentration showed an increasing trend during pickling, reaching 2767.5 mg·kg −1 in the final pickled sample (S46). γ-Aminobutyric acid is not a flavoring amino acid, but it has important physiological functions.
3.2 Microbial community structure succession during pickling
Tables S4 and S5 show the PacBio Sequel sequencing data and α-diversity indices of the bacteria and fungi, and Tables S6 and S7 show the OTU table of bacteria and fungi, respectively. In the bacterial community, the average number of non-singletons per sample was 4989.47 ± 1087.24 and the 95% confidence interval of the sequence length was 1478.39–1479.09 bp. Eleven phyla and 148 genera were identified in total. In the fungal community, the average number of nonsingletons per sample was 5811.65 ± 2104.19 and the 95% confidence interval of the sequence length was 1228.75–1230.20 bp. Two phyla and 60 genera were identified overall.
Figure S1 shows the rarefaction curves for the bacteria and fungi. As sequencing depth increased, the curves plateaued, indicating that the vast majority of species had been identified. The “Goods coverage” index for bacteria and fungi (Tables S4 and S5) was always greater than 0.97, which also indicated that most species had been identified and that the sequencing depth was sufficient. Figures S2a,b show the diversity indices and PCA plots for the bacterial and fungal communities, respectively. Paired t-tests showed significant differences in the bacterial Chao 1 indexes between stages 3 and 4 (p = .0024, <.01), and in the bacterial Shannon index between stages 1 and 4 (p = .034, <.05). Significant differences were also detected in the fungal Shannon index between stages 1 and 3 (p = .036, <.05) and between stages 1 and 4 (p = .0082, <.01), and in the Simpson index between stages 1 and 3 (p = .029, <.05) and stages 1 and 4 (p = .0027, <.01). This suggests that the significantly reduced bacterial and fungal diversity observed in the fourth stage of processing may have been due to the high salt concentration (100 g·L−1). Figure S2c displays the PCA plots from both communities at a genus level. Stages 1 and 4 samples exhibited distinct separation but the other stages were not clearly clustered.
The succession pattern of bacterial community structure is shown in Figure 4a. In the first stage, the dominant phyla (average relative abundance >1%) included Firmicutes (average relative abundance 53.20%), Proteobacteria (44.38%), and Bacteroidetes (1.67%). The dominant genera were Leuconostoc (40.26%), Psychrobacter (10.50%), Lactobacillus (6.23%), Stenotrophomonas (6.19%), Klebsiella (5.29%), Ralstonia (5.04%), Acinetobacter (2.91%), Lactococcus (1.68%), Pelomonas (1.61%), Pseudoalteromonas (1.26%), Devosia (1.19%), Weissella (1.14%), and Sphingobacterium (1.09%). In the second stage, the abundance of Firmicutes increased (increasing to 64.86%), Proteobacteria decreased (down to 33.38%), and Bacteroidetes maintained its previous level (1.23%). At the genus level, Lactobacillus proliferated rapidly, becoming the dominant genus (abundance 43.47%) and the abundance of Leuconostoc decreased to 15.78%, ranking second. The other major genera included Ralstonia (4.72%), Stenotrophomonas (3.31%), Klebsiella (3.29%), Psychrobacter (3.04%), Erwinia (2.46%), Pediococcus (2.32%), Acinetobacter (1.93%), Pelomonas (1.53%), Citrobacter (1.32%), and Providencia (1.05%). In the third stage, Firmicutes (67.21%) and Proteobacteria (31.05%) were still the dominant phyla, followed by Bacteroidetes (1.26%). The dominant genera changed significantly as salt concentrations increased to 80 g·L−1. Leuconostoc became the dominant genus (24.75%) followed by salt-tolerant Staphylococcus (18.06%), in addition to Lactobacillus (15.55%), Halomonas (9.57%), Psychrobacter (8.29%), Pediococcus (6.05%), Stenotrophomonas (3.49%), and Ralstonia (1.30%). In the fourth stage, the salt concentration increased further (100 g·L−1) and Firmicutes (70.05%) and Proteobacteria (28.99%) remained the dominant phyla. Lactobacillus (50.94%) became the dominant genus again, and the salt-tolerant genera proliferated, which included Halomonas (8.67%), Chromohalobacter (6.63%), Staphylococcus (2.38%), Alkalibacterium (2.26%), Pseudoalteromonas (1.61%), Salinivibrio (1.26%), and Halanaerobium (1.02%). The other genera present included Weissella (6.04%), Aerococcus (3.12%), Psychrobacter (2.63%), Ralstonia (2.16%), Arcobacter (1.69%), and Leuconostoc (1.19%).
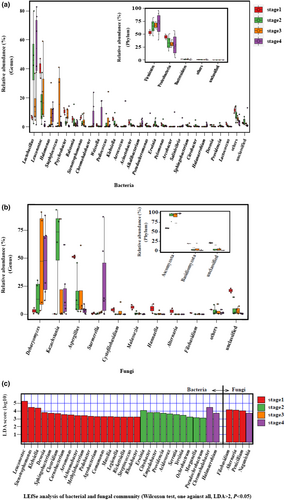
The succession pattern of fungal community structure is shown in Figure 4b. In the first stage, the dominant phyla (average relative abundance >1%) included Ascomycota (average relative abundance 59.75%) and Basidiomycota (19.20%). The dominant genera were Aspergillus (51.24%), Malassezia (6.28%), Hannaella (5.01%), Cystofilobasidium (3.91%), Debaryomyces (3.12%), Alternaria (2.70%), and Filobasidium (1.19%). In the second stage, the abundance of Ascomycota increased sharply (92.66%), whereas Basidiomycota levels decreased (2.75%). At the genus level, Kazachstania proliferated rapidly and became the dominant genus (60.12%), followed by Aspergillus (16.37%) and then Debaryomyces (14.45%). In the third stage, Ascomycota (91.33%) remained the dominant phylum, followed by Basidiomycota (5.38%). At the genus level, Debaryomyces proliferated rapidly and became the dominant genus (47.19%), followed by Kazachstania (21.64%), Aspergillus (19.15%), Cystofilobasidium (2.76%), and Starmerella (1.66%). In the fourth stage, Ascomycota (98.37%) was still the dominant phylum and Debaryomyces remained the dominant genus (47.82%), followed by Kazachstania (17.76%), Aspergillus (3.70%), and Starmerella (28.37%).
Linear discriminant analysis effect size (LEfSe) can be used to identify species with significant differences and a sense of biomarker between groups. Figure 4c shows the LEfSe analysis of the bacterial and fungal communities at the genus level. There were 18, 10, and 2 genera in bacterial community, which were enriched in stages 1, 2, and 4 respectively, and 3 and 1 genera in the fungal community were enriched in stages 1 and 4, respectively. These genera can be taken as biomarker (the most representative genus that can be distinguished from that in other stages) in their respective stages.
3.3 Correlation between microbial community structure and flavor components
During the four pickling stages, there were 38 bacterial genera and 12 fungal genera with the highest relative abundance measured at greater than 1% and their average relative abundances calculated at greater than 0.1%, and 32 main flavor components (5 organic acids, 19 VFCs, 3 monosaccharides, and 5 amino acids). These data were subjected to correlation analysis.
3.3.1 Correlation analysis by heat mapping
Figure 5 shows the Pearson correlation heat maps of the bacteria and fungi against the main flavor components. Mostly significant negative correlations were observed between the five organic acids and the genera of the bacteria and fungi.
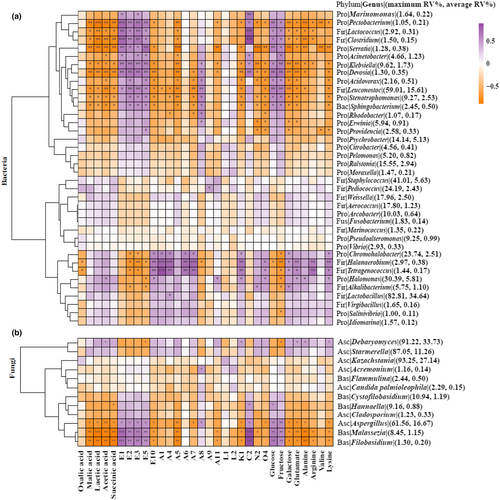
The isothiocyanates (E1, E2, E3, and E5) showed a significantly positive correlation with bacterial genera Marinomonas, Pectobacterium, Lactococcus, Clostridium, Serratia, Acinetobacter, Klebsiella, Devosia, Acidovorax, Leuconostoc, Stenotrophomonas, and Sphingobacterium, and was also positively correlated with fungal genera Aspergillus, Malassezia, and Filobasidium. Ester E10 was significantly positively correlated with Chromohalobacter, Halanaerobium, Tetragenococcus, and Halomonas.
Some aldehydes (A1, A4, A6, and A7) were positively correlated with Chromohalobacter, Halanaerobium, and Tetragenococcus. A8 was significantly positively correlated with the bacterial genera Klebsiella, Devosia, Acidovorax, Leuconostoc, Stenotrophomonas, Sphingobacterium, Rhodobacter, and Erwinia, and with the fungal genera Acremonium and Aspergillus.
Monosaccharides (glucose and fructose) were significantly positively correlated with the bacterial genera Pectobacterium, Lactococcus, Clostridium, Serratia, Acinetobacter, Klebsiella, Devosia, Acidovorax, Leuconostoc, Stenotrophomonas, and Sphingobacterium, and positively correlated with the fungal genera Aspergillus, Malassezia, and Filobasidium. Galactose was significantly positively correlated with the bacterial genera Chromohalobacter, Halanaerobium, Tetragenococcus, and Alkalibacterium.
The five amino acids were significantly positively correlated with the bacterial genera Chromohalobacter, Halanaerobium, Tetragenococcus, Halomonas, and Alkalibacterium, and with the fungi genus Debaryomyces.
3.3.2 Correlation analysis by O2PLS modeling
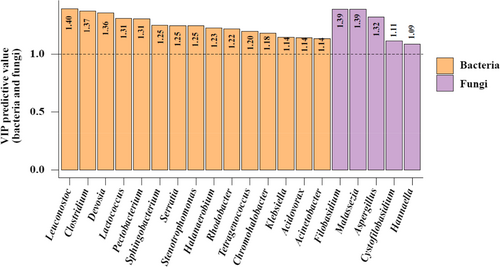
An O2PLS model was constructed using SIMCA 14.1, with X representing the bacterial and fungal data (50 genera in total) and Y representing the flavor component data. The parameters R2X (cum), R2Y (cum), and Q2 (cum) used for evaluating the validity of the O2PLS model were 0.996, 0.966, and 0.538, respectively. All were greater than 0.5, and the first two were closer to 1, which indicated that the fitting effect of the model was good, its prediction was statistically significant (Li et al., 2019; Wang, 2016; Zheng et al., 2018), and the bacteria and fungi data helped explain the flavor components to a certain extent. Figure 6 shows the bacterial and fungal genera with VIP predictive values greater than 1.0, which probably have close correlations with the flavor components.
3.3.3 Integrated correlation analysis
Based on the microbial community structure analysis, correlation heat mapping and O2PLS modeling, 14 genera of bacteria with VIP predictive values greater than 1.0 and significant positive correlations with at least three flavor components were identified: Leuconostoc, Clostridium, Devosia, Lactococcus, Pectobacterium, Sphingobacterium, Serratia, Stenotrophomonas, Halanaerobium, Tetragenococcus, Chromohalobacter, Klebsiella, Acidovorax, and Acinetobacter. Three genera of fungi also met the above criteria, Filobasidium, Malassezia, and Aspergillus. These 17 genera are inferred to be core functional flora in the fermentation process and are probably closely related to the formation of major flavor components.
3.4 Prediction of bacterial and fungal community function by PICRUSt2
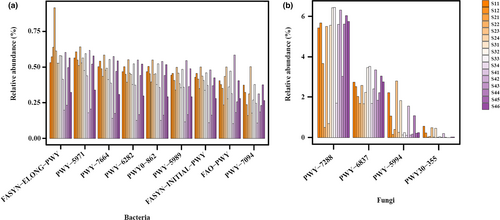
According to the KEGG pathway prediction by PICRUSt2, both bacterial and fungal communities included several pathways relating to fatty acid metabolism (Figure 7).
4 DISCUSSION
This study investigated the whole process of the salt-reducing pickling of zhacai. The microbial community structure during the four stages was analyzed using the PacBio Sequel platform to sequence full-length 16S rRNA and ITS genes, the flavor components were measured (organic acids, VFCs, monosaccharides, and amino acids), the correlations between microbial communities and flavor components were analyzed using correlation heat mapping and O2PLS modeling, the core functional flora was inferred through integrated correlation analysis, and the predicted functions of the microbial communities after using PICRUSt2 analysis were highlighted.
The traditional pickling process for zhacai includes pickling the tubers three times in a high concentration of salt solution (brine). On the third occasion, the salt concentration is the highest at around 140–160 g·L−1 and the immersion time is longer at around 4–6 months. The high salt concentrations inhibit the lactic acid fermentation of the microorganisms and make the acidity increase slowly, ensuring that the microorganisms have a longer time to produce the various flavor components (He et al., 2013). The salt-reducing pickling process investigated in this study includes four pickling stages. Compared with the traditional one, one pickling and one pressing operation are added in this newer technique, and the salt concentrations of the brine in each stage are lower, typically at 80 and 100 g·L−1 in the third and fourth stages, respectively, than utilized in the traditional method. This also means that the acid-producing metabolism stages for the microorganisms are relatively fast, and the pickling time is shortened (about 100 days in total), yet the flavor of the final pickling product (zhacai) is equivalent to that of the traditional method. The pressing treatment plays an important role in the salt-reducing method. It not only helps to expel water from inside tuber and promote the penetration of salt into tuber but also facilitates the outflow of various components inside tuber for microbial metabolism and utilization, accelerating the transformation reactions of chemical components. Thus, although the pickling time is shortened, microorganisms still can complete metabolic transformation of various components and produce large amount of flavor components. Therefore, the addition of pressing operation is necessary for the salt-reducing pickling process.
This study reveals that the most dominant genera of bacteria in the four pickling stages are Leuconostoc, Lactobacillus, Leuconostoc, and Lactobacillus, and the dominant fungal genera were Aspergillus, Kazachstania, Debaryomyces, and Debaryomyces, respectively, whereas in all three pickling stages of traditional method, the most dominant bacterial genus is Lactobacillus, and dominant fungal genus cannot be determined according to a previous report (Yang et al., 2021). The genus Leuconostoc belongs to lactic acid bacteria, its cells are ellipsoidal shaped, Gram positive, nonmotile, and asporogenous (Sahin et al., 2021). They are obligately heterofermentative, and metabolize glucose to produce lactic acid, ethanol, and CO2. They have pentose and pentitol metabolism pathway, which metabolizes L-/D-arabinose, D-ribose, and D-xylose to produce acetate and lactate. They also metabolize citrate and malate to produce diacetyl, acetoin, and 2,3-butanediol, which is an important reaction to produce aroma in dairy products, wine, and other foods (Cogan & Jordan, 1994). They are observed to be dominant in the early and middle stages of kimchi fermentation, and they are selected as starter cultures owing to their good acid-producing activity and contribution to flavor (Jung et al., 2012). The strains of Leuconostoc do not have a strong tolerance to acid and salt, perhaps it is the reason that in the later stage of pickling with high salt concentration, the dominant genus became Lactobacillus. The Debaryomyces are reported to be dominant in fermented dairy and meat products, they can grow under high salt, low water activities (Aw), and other adverse conditions, with remarkable abilities to produce endo- and exopeptidases to degrade proteins into free amino acids, as well as lipases to hydrolyze fat into fatty acids, and thus contribute to flavors (Breuer & Harms, 2006; Flores & Toldra, 2011). The Kazachstania is also normally found in high-salt foods, such as kimchi, Chinese bean peppers, and fermented fish, they are inferred to be related to aroma components, however, its precise roles remain to be investigated (Punyauppa-path et al., 2022).
This study determined that glucose and fructose were the two major monosaccharides in tuber samples during the pickling. Undoubtedly, glucose is the optimum sugar for most microorganisms. It is worth noting that the concentration of fructose in raw tuber and its succession process in pickling stages are close to that of glucose, which suggests that the metabolism of fructose by microorganisms probably also accounts for a large proportion of sugar metabolism. Microorganisms can metabolize fructose in the following ways: (1) fructose is catalyzed by hexokinase to produce fructose-6-phosphate, which is then degraded through glycolysis. (2) Some microorganisms, typically the Leuconostoc cells, have mannitol dehydrogenase, which can directly catalyze fructose to mannitol (Sahin et al., 2021). (3) Some lactic acid bacteria (LAB) have fructophilic characteristics, that is, fructose is their optimum substrate and preferentially metabolized than glucose. Generally speaking, fructophilic LAB are heterofermentative strains because their glucose and fructose metabolism have three pathways leading to the end product of lactic acid, acetic acid, and ethanol, respectively, while homofermentative cells do not have the pathway to ethanol. The fructophilic LAB lack alcohol dehydrogenase (ADH) and/or acetaldehyde dehydrogenase (ALDH) or their activities are relatively lower than that of heterofermentative LAB, which is the important difference between fructophilic and heterofermentative LAB. Therefore, the metabolic pathway of producing ethanol from Acetyl-1-P in fructophilic LAB is blocked and converted to the pathway of producing acetic acid with the catalysis of acetate kinase, simultaneously, the fructose, pyruvate, and O2 act as the electron acceptor to maintain the balance of NAD/NADH. It is reported that more than 69% of fructophilic LAB are related to Leuconostoc spp (Endo et al., 2018).
This study reveals the main VFCs in salt-reducing pickling zhacai include isothiocyanates, aldehydes, and dimethyl trisulfide. The VFCs in traditional pickling zhacai have been investigated, and Lin and Hua (1986) reported that the characteristic aroma is formed by components such as isothiocyanates, nitriles, and dimethyl trisulfide; esters, heterocyclic compounds, and other oxygen-containing compounds also contribute to aroma, thus forming a unique pungent aroma. Liu (2009) reported that the main volatile components in zhacai pickling are allyl isothiocyanate, benzyl isothiocyanate, dimethyl trisulfide, and phenylpropionitrile. On the whole, our results on salt-reducing zhacai are consistent with those of traditional zhacai. The isothiocyanate is generated from the degradation reaction of glucosinolates, a kind of characteristic components present in mustard tuber (Cruciferae family plant). Glucosinolates and endogenous myrosinase are located in different locations within the tuber cells, and extrusion or pressing can break cells, facilitate contact, and enable hydrolysis of the glucosinolates (Tripathi & Mishra, 2007). The β-D-thioglucose is removed from glucosinolates, leading to the formation of thiohydroximate-O-sulphonate (an organic aglucone), aglucones are unstable and undergo Lossen rearrangement to yield isothiocyanates (ITCs) at neutral pH, while acidic pH and Fe2+ favor the production of nitriles (Tripathi & Mishra, 2007). In addition to endogenous myrosinase, degradation of glucosinates has been reported via many microorganisms; these include Lactobacillus plantarum KW30, Lactobacillus agilis R16, and Pediococcus pentosaceus UM116P (Tian et al., 2018), and the fungi Aspergillus clavatus and Fusarium oxysporum (Smits et al., 1993). This study identified eight ITCs in zhacai, with allyl isothiocyanate (E1) expressed as the dominant component, which was consistent with previous studies (Liu, 2009). Allyl isothiocyanate elicits strong pungent smells, and also has anti-inflammatory, anticancer, antibacterial, and other biological activities (Traka & Mithen, 2009), affording zhacai a variety of physiological and health benefits. Two nitriles are detected, 3-butenenitrile (N1) and benzenepropanenitrile (N2), which are probably also the degrading products of glucosinolates owing to acidic brine conditions, whereas consuming a large amount of nitriles probably exert negative effects on health, such as affecting thyroid function, hepatotoxicity, and nephrotoxicity (Tripathi & Mishra, 2007). It is necessary to develop methods for regulating the glucosinolates' degrading products to be mainly isothiocyanates and avoiding the formation of nitriles for improving the health benefit of zhacai. The second major category of zhacai VFCs was the aldehydes. There are two possible pathways via which aldehydes are produced. Firstly, there is the oxidation of fatty acids. For example, octanoic acid (C1) and linolenic acid (C2) were detected in the raw tubers, and their oxidation reaction probably produce hexanal (A1) and (E)-2-heptenal (A3). Secondly, there is the metabolic activity of microorganisms. PICRUSt2 function prediction (Figure 7) highlighted synthetic pathways for palmitate, oleate, palmitoleate, (5Z)-dodec-5-enoate, and stearate, as well as metabolic pathways for fatty acid elongation, β-oxidation, and salvage in the bacterial community. Palmitate and stearate synthetic pathways and β-oxidation pathways were also apparent in the fungal community. On the other hand, the Ehrlich pathway in yeast is reported to be an important way to produce aldehydes—amino acids are converted to α-keto acids by transamination, then decarboxylated to produce aldehydes, and reduced to alcohols (Hazelwood et al., 2008). Therefore, it can be inferred that bacteria and fungi probably play a role in producing aldehydes (and even subsequently alcohols).
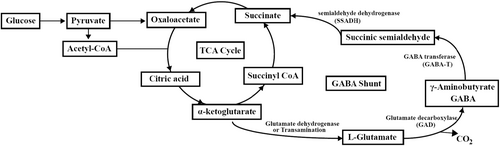
This study observed that the concentration of γ-aminobutyric acid (GABA) in tuber samples increased significantly during the pickling process. Microorganisms can produce GABA through the GABA shunt pathway (Figure 8), the alpha-ketoglutarate generated by the TCA cycle is converted to glutamate, and the glutamate is catalyzed by glutamate decarboxylase (GAD) to produce GABA, which is subjected to further reaction to produce succinic semialdehyde and succinate, and then succinate enters the TCA cycle (Sarasa et al., 2020). LAB, such as Lactobacillus plantarum and Lactobacillus brevis and the yeast Saccharomyces cerevisiae isolated from Chinese paocai are identified to produce GABA (Li et al., 2008; Zhang et al., 2017, 2020). The reaction of producing GABA is related to microbial physiological mechanism to tolerate acid environment. When in an acidic environment, there is more H+ inside microbial cells, the glutamate in the environment is transported into cells and subjected to decarboxylation reaction under the catalysis of GAD enzyme with the consumption of intracellular H+, the product GABA is excluded from the cell through antiporter, and it is an alkaline compound that neutralizes acids in the environment (Sarasa et al., 2020). Therefore, this reaction can help microbial cells balance the acid inside and outside the cells and overcome the acidic environment in fermented food. GABA is a major inhibitory neurotransmitter in the mammal central nervous system and has physiological effects including antihypertensive, antiepileptic, antidepressant, and antidiabetic effects (Zhang et al., 2017), thus affording zhacai a variety of physiological and health benefits.
5 CONCLUSION
The succession patterns of microbial communities during the salt-reducing pickling process of zhacai were elucidated using the PacBio Sequel platform. In the bacterial community, the average number of nonsingletons per sample was 4989.47 ± 1087.24 and the sequencing length was 1400 bp. Eleven phyla and 148 genera were identified. In the fungal community, the average number of nonsingletons per sample was 5811.65 ± 2104.19 and the sequencing length was 1200 bp. Two phyla and 60 genera were identified. During the four stages of pickling, the dominant bacterial genera were Leuconostoc, Lactobacillus, Leuconostoc, and Lactobacillus, while the dominant fungal genera were Aspergillus, Kazachstania, Debaryomyces, and Debaryomyces. The simultaneous development of flavor components in zhacai was also demonstrated. There were 32 main flavor components (5 organic acids, 19 VFCs, 3 monosaccharides, and 5 amino acids). Correlation heat mapping and O2PLS analysis showed that the flora playing key roles in the formation of flavor components included 14 genera of bacteria (Leuconostoc, Clostridium, Devosia, Lactococcus, Pectobacterium, Sphingobacterium, Serratia, Stenotrophomonas, Halanaerobium, Tetragenococcus, Chromohalobacter, Klebsiella, Acidovorax, and Acinetobacter), and 3 genera of fungi (Filobasidium, Malassezia, and Aspergillus). The bacteria and fungi probably produce zhacai flavor components through the metabolic activity of saccharides, glucosinolates, fatty acids, and amino acids.
ACKNOWLEDGEMENTS
This research was funded by the National Natural Science Foundation of China (Grant No. 31801529), the Chongqing Research Program of Basic Research and Frontier Technology (No. cstc2017jcyjAX0249), and the Fundamental Research Funds for the Central Universities, SWU (XDJK2018C070, XDJK2015C135, and SWU113035), and was a scientific research project of the Sichuan Cuisine Development Research Center (CC21Z30).
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
ETHICS STATEMENT
This study does not involve any human or animal testing.
Open Research
DATA AVAILABILITY STATEMENT
All relevant data are within the manuscript and its Supporting Information files. All sequence reads have been deposited at the NCBI Sequence Read Archive. (SRA) under project accession numbers PRJNA725670 and PRJNA725681. Please see https://www.ncbi.nlm.nih.gov/bioproject/PRJNA725670 and https://www.ncbi.nlm.nih.gov/bioproject/PRJNA725681.




