Rhizosphere soil nitrification ability controls nitrogen-use efficiency in rice growth period
Abstract
Nitrification in the rhizosphere is a crucial process in controlling nitrogen-use efficiency (NUE) in flooded paddy soils. To understand the relationship between the nitrification ability of the rhizosphere and NUE, pot experiments using 15N tracer technique were conducted to investigate the impacts of the rhizosphere soil net nitrification rate on NUE and denitrification losses at different rice growth stages in two paddy soils, which were sampled from Jurong (JR) and Yancheng (YC) in Jiangsu Province in China. The results showed that the nitrification rate in JR rhizosphere soil was lower than in YC rhizosphere soil at all rice growth stages. The abundance of ammonia-oxidizing bacteria (AOB), ammonia-oxidizing archaea (AOA), and pH in YC rhizosphere soils were always higher than in JR rhizosphere soils. Rice yield, biomass, NUE, leaf glutamine synthetase (GS) activity, and nitrate reductase (NR) activity were higher in JR soils with low nitrification rates than in YC soils with high nitrification rates (p < 0.05). In contrast, denitrification loss from JR soil (12.69%–23.41%) was lower than that from YC soil (26.83%–40.98%; p < 0.05) for each rice growth stage. The biomass and NUE decreased significantly as the net nitrification rate, the abundance of AOA and AOB of both the JR and YC rhizosphere soils increased (p < 0.05), and the denitrification loss was enhanced as the rhizosphere nitrification rate increased in the JR and YC soils (p < 0.05) during the rice growth period. The rhizosphere-dominant AOB community Nitrosospira is the key factor affecting the nitrification rate and then decreasing rice NUE. In general, the rhizosphere nitrification rate in paddy soils is a primary factor controlling the rice NUE and denitrification loss.
1 INTRODUCTION
Nitrogen (N) is a significant element in rice growth and N-based fertilisers are used extensively in paddy fields to promote rice yields (Zhou et al., 2020). To increase rice yields and meet food demands in China, large amounts of N fertiliser are applied to paddy fields (Liu et al., 2019; Yang et al., 2019). High N fertiliser inputs increase rice yields but decrease N use efficiency (NUE) by 20%–40% and cause environmentally harmful impacts, including water and air pollution, N leaching, and N2O emissions (Coskun et al., 2017a; Wang et al., 2017; Zhang et al., 2011). Hence, it is necessary to enhance the rice NUE and reduce N loss to increase financial profit and decrease the negative environmental effects of N fertilisers in paddy fields.
Many N fertilization and water management strategies have been used to improve NUE and reduce N losses in paddy fields (Wang et al., 2020). However, the dynamics of N transformation within the soil, particularly nitrification, has been neglected (Liu et al., 2019; Wang et al., 2017). Nitrification is an important N transformation process that determines the forms of inorganic soil N. In soils with high nitrification rates, such as alkaline soils and well-aerated soil, microorganisms rapidly convert immobile ammonium (-N) to highly mobile nitrate (-N). In contrast, in soils with low nitrification rates, such as acidic and waterlogged soils in subtropics, -N is the dominant inorganic form of N. The chemical form of N determines its fate in soil, as -N is more difficult to lose via leaching and runoff than -N (Liu et al., 2019).
Nitrification is known to be one of the critical elements controlling the NUE and N loss of the applied N fertiliser (Gao et al., 2020). The nitrification process directly or indirectly leads to leaching (Liu et al., 2020), nitrous oxide (N2O) emission (Li & Lang, 2014) and affects the emission of other reactive N pollutants such as nitrous oxide (NO) (Kirk & Kronzucker, 2005) and NH3 (Nicolaisen et al., 2004). The and produced by nitrification can promote denitrification in anaerobic soil through a coupled nitrification and denitrification process (Cai, 2002; Yang et al., 2017). Furthermore, many factors that affect nitrification ability, including the quantity, form, and application location of N fertiliser, root exudates, and water management, can also influence the NUE (Coskun et al., 2017b; Sahrawat, 2008). Increasing evidences have indicated the application of nitrification inhibitors is an effective strategy for decreasing N loss in paddy soils and thereby increasing the NUE.
In addition, plant N preferences can affect plant NUE. A good match between plant N preferences and soil mineral N forms (regulated by the characteristics of soil N transformations) can increase NUE and crop yield (Zhang et al., 1997). It is well known that rice prefers to take up -N (Zhang et al., 2022). Zhang et al. (2016) demonstrated that in soils with low nitrification rates, -N-preferring crops would take up more -N. Many reports indicated that rice had higher NUE under mixed -N and -N supply than -N only supply in hydroponic experiments (Kronzucker et al., 1997). Therefore, -N produced by nitrification is also very important for N nutrition of rice plants. However, soil conditions are much more complex than hydroponics, and NUE in rice and soil nitrification rates throughout the growth period of rice are difficult to measure and maintain in field trials. Thus, soil and plant observations are needed to better understand the relationship between NUE and soil nitrification rate in rice.
Oxygen concentration is an essential factor that influences the nitrification rate (Yang et al., 2016; Zhang et al., 2011). It has been well documented that an oxidizing environment exists near rice roots (Yao et al., 2020), so nitrification mainly takes place in the rice root rhizosphere or oxidized topsoil even though these areas are low in oxygen, because nitrifiers might adapt to low oxygen environment. Rice rhizosphere oxygen concentration is mainly controlled by root conditions, such as root length, percentage porosity of root, and diffusion path consumed (Armstrong & Beckett, 1987). The biomass, length, and percentage porosity of root will be different at different rice growth stages, root system, and percentage porosity of root increased with the development of rice plants, which may transport more O2 from the root to rhizosphere soil (Li et al., 2007; Li et al., 2008). Thus, rice root condition at different rice growth stages is expected to have a variety of effects on the nitrification rate in the rhizosphere soil. However, most studies have focused on a certain stage, such as the seeding stage but do not actually measure the rice full growth period. Soil pH is also a key factor affecting the nitrification rate as it is an acid-sensitive process and the abundance and community of ammonia-oxidizing bacteria (AOB) and archaea (AOA) in soil with different pH may shift (Yu et al., 2020). To date, many studies have explored the connection between the nitrification rate and NUE in rice fields, but few have considered the nitrification taking place in the rice root rhizosphere. Accordingly, research into the impact of nitrification on NUE-containing rice growth stages is rare. The rice rhizosphere is the main site of nitrification in flooded paddy fields, and rhizosphere nitrification plays a crucial role in regulating the coupling of soil N forms and N uptake by rice plants. The dynamic change of nitrification rate in rice rhizosphere and its coupling mechanism with N uptake by rice remain to be clarified during rice growth.
Denitrification is the primary mechanism of N loss in rice fields, accounting for over 40% of the N in the applied fertiliser (Liu et al., 2022; Reddy & Patrick, 1986; Yang et al., 2019). The -N concentration is the most critical factor controlling denitrification in flooded paddy soils (Zhou et al., 2012). Ammonium (-N) fertiliser is the main N fertiliser used in paddy fields. The -N produced by the nitrification of -N fertiliser can cause denitrification when it diffuses into anaerobic bulk soils (Cai, 2002; Yang et al., 2017). Therefore, N loss by denitrification in flooded paddy fields could be controlled by the nitrification rate.
In the present study, two paddy soils with dramatically different soil nitrification rates (an acidic soil with a low nitrification rate and an alkaline soil with a high nitrification rate) were chosen. The rice yield, NUE, N loss, and rice rhizosphere nitrification ability at different rice growth stages were measured. The study aimed to: (1) explore the correlation between rice yield, NUE, denitrification N loss, and rhizosphere nitrification rate at different rice growth stages in soils with different nitrification abilities; (2) estimate the impact of the rice rhizosphere nitrification rate on rice growth, NUE, and denitrification loss in soils. Because denitrification is a substrate-dependent procedure, NUE is significantly impacted by the rhizosphere nitrification rate; therefore, it was supposed that the denitrification loss and NUE of rice would be significantly impacted by the rhizosphere nitrification rate in paddy soils.
2 MATERIALS AND METHODS
2.1 Soil samples
Two rice soils, an acidic soil from Jurong (JR; 31°56 N, 119°10′E), which is mature through Xiashu loess, and is classified as clay soil in international soil classification, and an alkaline soil from Yancheng (YC; 31°16′N, 105°27′E), formed from lake sediments, and is classified as loam soil in international soil classification in Jiangsu, were collected. The Oryza sativa L. cv. Nanjing 9108 was used in this study as the rice cultivar, and the rice seeds were obtained from Jiangsu Academy of Agricultural Science (Nanjing, China). Two soil samples were gathered from the plough layer (0–20 cm) before rice transplantation, air-dried, sieved in a 2 mm sieve, and mixed well prior to the pot experiment and lab study. The properties and texture of the two rice soils are shown in Table 1.
| Site | Soil | pH | -N (mg kg−1) | -N (mg kg−1) | Available P (mg kg−1) | Available K (mg kg−1) | TN (g kg−1) | SOC (g kg−1) | C/N | Clay (%) <2 μm | CEC cmol kg−1 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 28°15′N, 116°55′E | JR | 5.94 | 12.63 | 24.48 | 49.60 | 51.9 | 0.96 | 8.3 | 8.6 | 35.6 | 13.15 |
| 31°16′N, 105°27′E | YC | 8.38 | 42.25 | 6.04 | 37.91 | 93.6 | 1.20 | 9.2 | 7.7 | 21.8 | 10.56 |
- Note: Soils JR and YC were collected from Jurong and Yancheng, respectively, in Jiangsu province, China. TN is total nitrogen content. SOC is soil organic carbon content. C/N is the carbon and nitrogen ratio. -N and -N are the mineral N contents after 2 weeks of preflooding. Soil pH, CEC, soil organic carbon (SOC), soil texture, soil available P, soil available K, and TN were determined according to the soil agrochemical analysis process (Novamsky et al., 1974).
2.2 Pot experiment
This pot experiment was implemented in a greenhouse at Yangzhou University, Yangzhou city, Jiangsu province, during the rice-growing season in 2020. Throughout the pot experiment, the inside temperature of the greenhouse was kept at 30 ± 3°C during the day and at 20 ± 3°C during the night; humidity and natural illumination conditions were kept during the whole experiment.
There were two pot experiments. The first pot experiment was a 15N labeling experiment to determine the rice plant biomass, NUE, and denitrification N loss at four major rice growth stages: tillering (5 July), jointing (17 July), heading (24 August), and grain filling (20 September). There was a total of 30 PVC pots (fifteen pots of each paddy soil, with triplicates of each rice growth stage and three pots used for harvest). 7.0 kg of air-dried rice soil (oven-dry basis) mixed with KH2PO4 at the rate of 100 mg kg−1 was placed in each PVC pot (30 cm depth, 15 cm inside diameter). The soil was then flooded with deionised water, immersed to a depth of about 2.0 cm for moving -N in the primary basis for 2 weeks by denitrification. On June 15, three homogeneous rice plants with three true leaves were placed in the middle of each pot.
For each stage, three pots of each soil were randomly chosen for the 15N-labeled trial. 15N-labeled (NH4)2SO4 (10.32% atom% surplus) as the N source was added at 50 mg N kg−1 soil. Pots that were to be 15N labeled during other processes were treated with unlabeled N fertiliser to ensure that rice growth occurred. The labeled or unlabeled N fertiliser was added by the injector to the soil at a depth of 3–5 cm to avoid NH3 volatilisation. All the pots were kept continuously submerged and placed in a completely random design throughout the experiment. Other conditions, including water, weed control, and insecticide, were the same as for the field experiments. The potash fertiliser and phosphate fertiliser were in sufficient supply. The rice was harvested on 13 October 2020. The whole rice plant (roots and shoots) and soils were destructively sampled at five days from the start of 15N accretion during each process to determine the concentration and isotopic constitution of -N, -N, organic N, and plant N, and to estimate the uptake efficiency and recovery of the added 15N. N losses were estimated from the 15N balance.
The second pot experiment was to determine the nitrification rate of the rice rhizosphere at the four major rice growth stages. A total of 24 PVC pots (twelve pots of each paddy soil) were utilized. 7 kg of air-dried rice soil (oven-dry basis) mixed with KH2PO4 at the rate of 100 mg kg−1 was weighted and divided into two parts. 1.0 kg was placed in a nylon bag (15 cm depth, 10 cm diameter) as rhizosphere soil and placed in the center of the PVC pot (30 cm depth, 15 cm internal diameter). 6.0 kg was placed around the nylon bag. Three rice plants at 35-d old were placed in the middle of the nylon bag. Unlabeled N fertiliser was used with the same dosage and application method as in pot experiment 1. For each stage, three pots of each soil were randomly chosen to obtain the rice rhizosphere soil.
2.3 Sampling and measurements
Rice plants (including aboveground plants and roots) harvested at each rice stage in the first experiment were cleaned with water, heated at 105°C for 30 min and then dried to a constant weight at 70°C to measurement biomass. The rice plants were separated into shoots and roots, crushed in a mortar, and passed through a 0.1 mm screen for analysis of N concentration and 15N atom%. The NUE of rice plants was expressed as the uptake of whole plant dry biomass of 15N applied by the rice plants as shown in Equation (1). After the rice harvest, about 25 g of representative soil samples were obtained by thoroughly mixing the soil and floodwater. The soil sample was then placed in a 250 ml flask, KCl (2.5 M, 80 ml) was added, and the mixture was shaken for 1 h at 200 rpm and 25°C. The concentration of -N and -N in the filtrate was identified with a continuous-flow analyzer (Skalar, Breda, Netherlands). The isotopic composition and 15N enrichment of -N and -N were determined using an isotope ratio mass spectrometer (Sercon Ltd., Crewe, UK), as described by Zhang et al. (2011) and Yang et al. (2017). Briefly, 25 ml of the filtrate were steam-distilled with MgO to separate -N on a semi-micro Kjeldahl, thereafter the sample in the flask was distilled again after added of Devarda's alloy to reduce -N to -N. The liberated NH3 was trapped in boric acid solution. The trapped N was acidified and converted to (NH4)2SO4 using 0.02 mol L−1 H2SO4 solution. The titrated solution was dried at 65°C in an oven and analyzed for 15N excess. The KCl-extracted soil was suspended with distilled water to remove residual mineral N for 6 h, and then, the soil suspension was filtered through a quantitative filter paper, rinsed twice with distilled water, and oven dried at 55°C to measure the soil insoluble organic N and the relative 15N enrichment by mass spectrometry (Yang et al., 2017). Thus, the fate of fertilizer N in plant and soil systems (uptake by rice, remain in soil, and lost) could be quantified.
The rhizosphere soils in the second pot experiment were collected as follows, at each rice growth stage, three pots from each soil were destructively sampled. During each sampling, the nylon bag of root was taken out of the pot and carefully removed to prevent damage to the rice roots. By shaking the root, soil adhering to the roots was bushed off into the sterilized plastic bag as rhizosphere soils after removing all root pieces (He et al., 2013; Yu et al., 2020). Bulk soils were sampled 1.0 cm away from the root nylon bags. Soil samples used for DNA extraction, amplification, and high-throughput sequencing were frozen at −80°C. Soil samples used for chemical analyses were air-dried while those for net nitrification rate were air-dried to achieve 40%–50% moisture content and passed through a 2 mm sieve and thoroughly blended before refrigerating at 4°C for the incubation experiment.
The net nitrification rate of the rice soils was determined according to the method of Yang et al. (2016). Briefly, for each soil, six 250 ml Erlenmeyer flasks were prepared with 20 g soil (oven-dry basis) and preincubated for 1 day at 25°C after adjusting the soil water content to 40% water holding capacity (WHC). (NH4)2SO4 solution was added to each flask at a concentration of 50 mg -N Kg −1 soil. Then, soil adjusted to 60% WHC and incubated for 7 days at 25°C. The soils were extracted at 1 and 7 days to determine the -N and -N concentrations. The net nitrification rates of soils were calculated by the increased concentration of -N divided by the incubation time.
The leaf nitrate reductase (NR) activity and Glutamine synthetase (GS) activity were determined using the specific assay kits (Comin Biotechnology Co. Ltd., Suzhou, China). Leaf samples (1.0 g) were ground in a chilled mortar containing 6 ml of extraction buffer. The crude supernatant was analyzed for NR and GS activity according to the methods as follows: NR activity was determined by a modification method reported by Zhang et al. (2022), and GS activity was determined according to the method, which reported by Han et al. (2017).
The abundance of AOA and AOB in the soils was counted by the real-time quantitative polymerase chain reaction (PCR) based on DNA and cDNA reverse transcribed from RNA. Soil total DNA was extracted from 0.5 g fresh soil samples using Fast DNA®SPIN Kit for Soil (MO BIO Laboratories, Inc., Carlsbad, CA, USA) according to instructions and stored at −80°C. Three replicates were performed for each soil sample. The population size of AOA and AOB was determined by using real-time PCR as described by Yu et al. (2020). The details of PCR primers for AOB and AOA were listed in Table S1.
The V4-V5 region of the 16sRNA gene in each sample at four rice growth stages was analyzed by HiSeq sequencing with the universal primers 515F and 907R. The producers for microbial DNA amplification were conducted by the Meiji Biotech incorporated company (Shanghai, China). The qPCR products were then purified using MinElute PCR purification Kit (Qiangen, Germany), and sequenced with a HiSeq PE250 sequencing platform (Illumina, USA).
2.4 Calculation and statistical analyses
3 RESULTS
3.1 Soil performances
The characteristics of the test soils are presented in Table 1. The soil pH, available K was higher in the YC soil compared with the JR soil, but the soil -N, available P, CEC, TN, and SOC in the YC soil was lower than in the JR soil. The clay content was 35.6% in the JR soil and 21.8% in the YC soil. The nitrate ratio, defined as -N/(-N + -N) was 33.6% in the JR soil, significantly lower than in the YC soil (87.5%).
3.2 Net nitrification rate and abundance of AOA, AOB amoA gene copies
The net nitrification rate of bulk and rhizosphere soil at different rice growth stages, identified at 60% water-keeping ability, is shown in Table 2. The net nitrification rate in the JR and YC rhizosphere soil samples was associated with the rice growth stages, but there was no significant change in the bulk soil. The net nitrification rate in the JR rhizosphere soil decreased from 3.57 to 2.54 mg N kg−1 d−1 as the rice growth progressed, and from 6.69 to 5.84 mg N kg−1 d−1 in the YC rhizosphere soils. The net nitrification rate varied considerably between JR and YC, being dramatically lower in the JR compared with the YC in both bulk and rhizosphere soil at each of the growth stages (p < 0.05). Generally, the net nitrification rate was highest in the rhizosphere soil of YC and lowest in the bulk soil of JR. This difference can be interpreted as a result of the higher amounts of AOB and AOA in the rhizosphere soils than in the bulk soils (Table 3) and soil pH (Table S2). The AOB, AOA, and soil pH were always significantly higher in YC soils than in JR soils, especially in the rhizosphere.
| Soil | Tillering | Jointing | Heading | Filling |
|---|---|---|---|---|
| JR | ||||
| B | 1.14 ± 0.02 b | 2.23 ± 0.16 b | 2.47 ± 0.27 b | 2.04 ± 0.04 b |
| R | 3.57 ± 0.09 a | 3.46 ± 0.37 a | 3.23 ± 0.16 a | 2.54 ± 0.16 a |
| YC | ||||
| B | 5.26 ± 0.10 b | 5.29 ± 0.29 b | 4.89 ± 0.20 b | 5.09 ± 0.12 b |
| R | 6.69 ± 0.08 a | 6.50 ± 0.18 a | 6.29 ± 0.10 a | 5.84 ± 0.26 a |
- Note: The acidic soil JR and alkaline soil YC were collected from Jurong and Yancheng, respectively, in Jiangsu Province, China. Different letters (a, b) indicate significant differences (p < 0.05) for the average values between two studied soils in the same bulk or rhizosphere soil.
| Soil | Tillering | Jointing | Heading | Filling |
|---|---|---|---|---|
| AOB | ||||
| JR | ||||
| B | 1.62+E6(0.21+E6) b | 1.95+E6(0.33+E6) b | 2.37+E6(0.36+E6) b | 1.77+E6(0.12+E6) b |
| R | 3.50+E6(0.19+E6) a | 3.21+E6(0.09+E6) a | 2.49+E6(0.03+E6) a | 2.20+E6(0.19+E6) a |
| YC | ||||
| B | 5.20+E6(0.24+E6) b | 5.66+E6(0.13+E6) b | 4.51+E6(0.16+E6) b | 4.67+E6(0.37+E6) b |
| R | 9.89+E6(0.16+E6) a | 7.40+E6(0.40+E6) a | 7.28+E6(0.20+E6) a | 5.56+E6(0.39+E6) a |
| AOA | ||||
| JR | ||||
| B | 5.83+E6(0.01+E6) b | 9.18+E6(0.01+E6) b | 7.70+E6(0.19+E6) b | 5.84+E6(0.32+E6) b |
| R | 1.35+E7(0.02+E6) a | 1.20+E7(0.02+E7) a | 1.06+E7(0.09+E6) a | 9.57+E6(0.17+E6) a |
| YC | ||||
| B | 8.75+E6(0.64+E6) b | 1.12+E7(0.02+E7) b | 9.33+E6(0.28+E6) b | 8.52+E6(0.30+E6) b |
| R | 1.51+E7(0.06+E7) a | 1.42+E7(0.10+E7) a | 1.41+E7(0.17+E7) a | 1.17+E7(0.17+E7) a |
- Note: The acidic soil JR and alkaline soil YC were collected from Jurong and Yancheng, respectively, in Jiangsu Province, China. Different letters (a, b) indicate significant differences (p < 0.05) for the average values between two studied soils in the same bulk or rhizosphere soil.
Correlation analysis showed that the net nitrification rates of rhizosphere soil of JR and YC were significantly associated with the abundance of AOB, AOA, AOB/AOA, soil pH, and ratio (p < 0.05) (Table S3). AOB, AOB/AOA, and soil pH had significant effects on the net nitrification rate in JR bulk soil. Net nitrification rates in YC were not consistent with those in rhizosphere soils, but the amount of AOB significantly affected the net nitrification rate in YC bulk soil (p < 0.05).
3.3 Nitrifying community composition
Results indicated that Crenarchaeota dominated the AOA phylotypes in both JR and YC soil (Table 4). The proportions of Crenarchaeota were significantly higher in JR rhizosphere soil and bulk soil than in YC at all rice growth stages (p < 0.05). As for AOB, the dominant phylotype was Nitrosospira in YC bulk and rhizosphere soil and JR bulk soil, and it was relatively stable at different rice growth stages (Table 5).
| Soils | Phylotypes | Tillering | Jointing | Heading | Filling |
|---|---|---|---|---|---|
| JR | |||||
| B | Crenarchaeota | 79.89 ± 0.86 a | 69.94 ± 0.65 a | 78.47 ± 1.98 a | 79.31 ± 0.18 a |
| Nitrososphaera | 0.87 ± 0.13 b | 5.57 ± 0.22 a | 1.09 ± 0.29 b | 0.89 ± 0.02 b | |
| Others | 19.25 ± 0.73 b | 24.49 ± 0.43 b | 20.43 ± 1.70 b | 19.79 ± 0.17 b | |
| R | Crenarchaeota | 80.08 ± 0.54 a | 79.21 ± 0.64 a | 79.67 ± 0.34 a | 77.52 ± 0.27 a |
| Nitrososphaera | 0.92 ± 0.07 b | 1.77 ± 0.17 b | 0.74 ± 0.12 b | 1.53 ± 0.44 b | |
| Others | 18.99 ± 0.47 b | 19.02 ± 0.60 b | 19.58 ± 0.40 b | 20.94 ± 0.50 b | |
| YC | |||||
| B | Crenarchaeota | 52.40 ± 1.41 b | 60.47 ± 3.40 b | 54.16 ± 0.86 b | 52.34 ± 1.25 b |
| Nitrososphaera | 2.34 ± 0.25 a | 4.81 ± 0.20 b | 3.49 ± 0.08 a | 4.01 ± 0.43 a | |
| Others | 45.26 ± 1.31 a | 34.72 ± 3.60 a | 42.35 ± 0.78 a | 43.66 ± 0.94 a | |
| R | Crenarchaeota | 52.95 ± 0.57 b | 57.37 ± 1.07 b | 56.54 ± 1.13 b | 52.37 ± 0.48 b |
| Nitrososphaera | 2.99 ± 0.47 a | 4.15 ± 0.02 a | 2.94 ± 0.03 a | 3.64 ± 0.22 a | |
| Others | 44.06 ± 0.75 a | 38.48 ± 1.07 a | 40.52 ± 1.15 a | 44.00 ± 0.62 a | |
- Note: Standard deviations are given in parentheses. Different letters (a, b) indicate significant differences (p < 0.05) for the average values between two studied soils in the same bulk or rhizosphere soil.
| Soils | Phylotypes | Tillering | Jointing | Heading | Filling |
|---|---|---|---|---|---|
| JR | |||||
| B | Nitrosospira | 51.82 ± 15.47 b | 50.66 ± 16.32 b | 40.04 ± 18.10 b | 37.34 ± 2.19 b |
| Nitrosomonadaceae | 4.52 ± 1.89 a | 16.81 ± 8.67 a | 36.15 ± 17.73 a | 23.01 ± 2.45 a | |
| Nitrosomonadales | 11.63 ± 8.95 a | 4.45 ± 3.51 b | 0.70 ± 0.70 b | 3.35 ± 0.29 b | |
| Betaproteobacteria | 0.96 ± 0.71 a | 0.73 ± 0.73 a | 1.51 ± 0.81 a | 0.49 ± 0.24 a | |
| Others | 31.07 ± 6.57 a | 27.35 ± 6.84 a | 21.59 ± 0.58 b | 35.82 ± 1.88 a | |
| R | Nitrosospira | 17.29 ± 3.49 b | 33.60 ± 4.43 b | 30.11 ± 4.58 b | 13.14 ± 1.00 b |
| Nitrosomonadaceae | 32.02 ± 3.79 a | 15.41 ± 8.11 a | 19.05 ± 3.42 a | 45.36 ± 3.55 a | |
| Nitrosomonadales | 3.14 ± 1.61 b | 22.97 ± 5.16 a | 5.56 ± 2.61 a | 1.87 ± 1.75 b | |
| Betaproteobacteria | 1.12 ± 0.32 a | 0.23 ± 0.14 a | 0.00 ± 0.00 a | 1.65 ± 0.68 a | |
| Others | 46.43 ± 5.82 a | 27.79 ± 2.27 a | 45.28 ± 3.71 a | 37.98 ± 2.00 a | |
| YC | |||||
| B | Nitrosospira | 66.58 ± 5.25 a | 60.39 ± 0.61 a | 55.59 ± 0.80 a | 64.56 ± 1.88 a |
| Nitrosomonadaceae | 0.03 ± 0.01 b | 0.02 ± 0.01 b | 0.03 ± 0.02 b | 0.02 ± 0.01 b | |
| Nitrosomonadales | 10.37 ± 1.28 a | 12.03 ± 0.42 a | 13.51 ± 0.43 a | 12.15 ± 1.75 a | |
| Betaproteobacteria | 0.00 ± 0.00 b | 0.01 ± 0.01 a | 0.01 ± 0.01 b | 0.00 ± 0.00 b | |
| Others | 23.02 ± 4.00 a | 27.56 ± 0.64 a | 30.86 ± 0.37 a | 23.26 ± 1.00 b | |
| R | Nitrosospira | 63.41 ± 0.20 a | 68.97 ± 1.62 a | 65.48 ± 0.22 a | 61.34 ± 1.85 a |
| Nitrosomonadaceae | 0.03 ± 0.01 b | 0.01 ± 0.01 b | 0.10 ± 0.08 b | 0.03 ± 0.01 b | |
| Nitrosomonadales | 10.92 ± 0.22 a | 8.02 ± 1.07 b | 8.47 ± 0.32 a | 11.10 ± 0.97 a | |
| Betaproteobacteria | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 a | 0.01 ± 0.01 b | |
| Others | 25.64 ± 0.09 b | 23.00 ± 0.89 b | 25.95 ± 0.41 b | 27.52 ± 0.92 b | |
- Note: Standard deviations are given in parentheses. Different letters (a, b) indicate significant differences (p < 0.05) for the average values between two studied soils in the same bulk or rhizosphere soil.
More pronounced differences were observed in the compositional diversities (Shannon diversity, sobs, and Simpson) of AOA as compared to those in AOB (Table S4 and S5). Principal coordinates analysis (PCoA) was performed to examine beta (β-) diversities across all samples based on Bray-Curtis distance matrices (Figure 1 and Table S6). The first two components in PCoA explained 69% and 89% of the variation in the soils. The separation of the different growth stages was not apparent, while the separation between JR and YC soil in the PcoA plots was clear. This illustrates that the composition of the nitrifying community was significantly affected by soil pH.
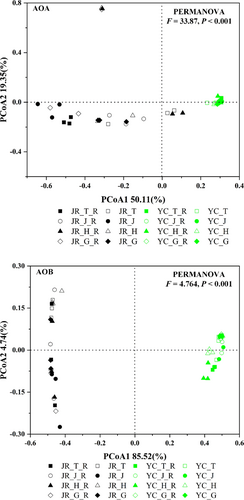
3.4 Rice biomass, NUE, NR, and GS activity, yield, and composition
The biomass and NUE differed in different soils and during different rice growth stages (Table 6). The biomass of the JR soil samples ranged from 14.61 to 114.85 g per pot and was considerably higher than that of the YC soil samples at all four rice growth stages (p < 0.05). Similarly, the NUE values for the JR soil samples were markedly higher than for the YC soil samples at the four rice growth stages (p < 0.05).
| Characters | Soil | Tillering | Jointing | Heading | Filling |
|---|---|---|---|---|---|
| Biomass (g) | JR | 14.61 ± 0.25 a | 60.24 ± 2.61 a | 97.39 ± 1.66 a | 114.85 ± 2.54 a |
| YC | 13.69 ± 0.26 b | 53.72 ± 2.60 b | 81.14 ± 0.57 b | 94.45 ± 0.67 b | |
| NUE (%) | JR | 37.14 ± 1.17 a | 63.64 ± 3.94 a | 74.77 ± 0.95 a | 80.95 ± 3.66 a |
| YC | 28.32 ± 2.02 b | 49.33 ± 2.64 b | 57.89 ± 3.77 b | 57.54 ± 1.33 b | |
| NR (μmol g−1h−1fw) | JR | 0.25 ± 0.02 a | 0.29 ± 0.01 a | 0.53 ± 0.01 a | 0.66 ± 0.03 a |
| YC | 0.18 ± 0.01 b | 0.26 ± 0.04 b | 0.43 ± 0.01 b | 0.38 ± 0.02 b | |
| GS (nmol g−1min−1fw) | JR | 16.61 ± 0.34 a | 17.12 ± 0.63 a | 17.84 ± 0.43 a | 21.85 ± 0.29 a |
| YC | 14.90 ± 0.28 b | 15.62 ± 0.28 b | 16.47 ± 0.43 b | 16.57 ± 0.32 b |
- Note: The acidic soil JR and alkaline soil YC were collected from Jurong and Yancheng, respectively, in Jiangsu Province, China. Different letters (a, b) indicate significant differences (p < 0.05) for the average values between two studied soils in the same bulk or rhizosphere soil.
In leaves, the NR activity of JR increased from tillering stage to grain-filling stage, while that of YC increased from tillering to booting stage but decreased from booting to grain-filling stage (Table 6). The NR and GS activities of both JR and YC increased from tillering stage to grain-filling stage, and the NR and GS values of JR were always significantly higher than those of YC (Table 6). The correlation analysis showed that biomass and NUE increased significantly in both JR and YC soils by increasing NR and GS activities with R2 values ranging from 0.915 to 0.999 (p < 0.05) (Table S7), except for the relationship between NR activity and NUE in JR soils. The rice biomass in the JR and YC soils increased significantly as the rhizosphere soil native -N content increased (p < 0.05) (Table 7). In contrast, the biomass decreased significantly as the rhizosphere soil native -N content increased (p < 0.05). Correlation analysis showed that the relationship between the rice biomass and the net nitrification rate, AOA, AOB abundance, and AOA/AOB in rhizosphere soils in both JR and YC were consistent with the binomial relationships with R2 values of 0.982 (p < 0.05) and 0.999 (p < 0.05), respectively. The relationships between NUE and net nitrification rate, soil pH, -N, -N, and abundances of AOA and AOB were also significantly correlated in JR and YC rhizosphere soils (p < 0.05). However, only the relationship between biomass and soil pH and -N content was significantly correlated in JR and YC bulk soils (p < 0.05). The relationship between NUE in JR and YC was significantly related to bulk soil pH, -N, and -N content.
| Parameter | Soil | NNR | Soil pH | -N | -N | AOB | AOA | AOB/AOA | |
|---|---|---|---|---|---|---|---|---|---|
| Biomass | JR | B | 0.713 | 0.955* | 0.829 | 0.948* | 0.355 | 0.162 | 0.842 |
| R | 0.982* | 0.851 | 0.980* | 0.979* | 0.978* | 0.999* | 0.948* | ||
| YC | B | 0.648 | 0.973* | 0.783 | 0.996* | 0.706 | 0.012 | 0.651 | |
| R | 0.999* | 0.982* | 0.967* | 0.996* | 0.917* | 0.938* | 0.920* | ||
| NUE | JR | B | 0.844 | 0.987* | 0.915* | 0.974* | 0.495 | 0.158 | 0.859 |
| R | 0.923* | 0.925* | 0.925* | 0.975* | 0.940* | 0.997* | 0.905* | ||
| YC | B | 0.492 | 0.995* | 0.942* | 0.999* | 0.812 | 0.034 | 0.811 | |
| R | 0.988* | 0.998* | 0.977* | 0.998* | 0.947* | 0.969* | 0.945* | ||
- Note: The acidic soil JR and alkaline soil YC were collected from Jurong and Yancheng, respectively, in Jiangsu Province, China. * indicate significant at p < 0.05.
The rice grain yield and its components in JR and YC soils were measured, and the results are shown in Table 8. Obviously, higher biomass and NUE resulted in higher rice grain yield. The rice grain yield in JR soil was significantly higher than in YC soil. Additionally, the yield components of spikelet per panicle, panicle number, and seed-setting rate in JR soil were significantly higher than in YC soil (p < 0.05). However, the t-tested results exhibited no significant difference in 1000-grain weight between JR and YC soils (p > 0.05).
| Soil | Grain yield (g pot-1) | Spikelet per panicle | Panicle number (pot-1) | 1000-grain weight (g) | Seed-setting rate (%) |
|---|---|---|---|---|---|
| JR | 45.02 ± 2.00 a | 119.65 ± 0.80 a | 25.00 ± 1.00 a | 24.62 ± 0.61 a | 90.86 ± 0.22 a |
| YC | 37.66 ± 1.00 b | 112.29 ± 1.14 b | 22.00 ± 1.00 b | 23.89 ± 0.88 a | 87.00 ± 0.15 b |
- Note: The acidic soil JR and alkaline soil YC were collected from Jurong and Yancheng, respectively, in Jiangsu Province, China. Different letters (a, b) indicate significant differences (p < 0.05) for the average values between two studied soils in the same bulk or rhizosphere soil.
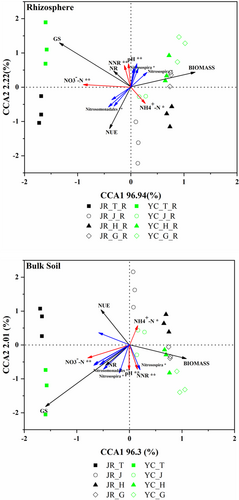
We used canonical correspondence analysis (CCA) to investigate the potential relationships among nitrifying community composition, soil properties, and rice plant indices. The first two CCA factors explained 98% of the community variation in bulk soils and 99% in rhizosphere soils (Figure 2). Nitrifying community scores among different rice growth stages were distinct in both rhizosphere and bulk soils. Soil properties (-N, -N, NNR (net nitrification rate), pH) were positively correlated in rhizosphere and bulk soils. However, rice plant indices (NUE, biomass, NR, GS) were not positively correlated with nitrifying communities in both rhizosphere and bulk soils.
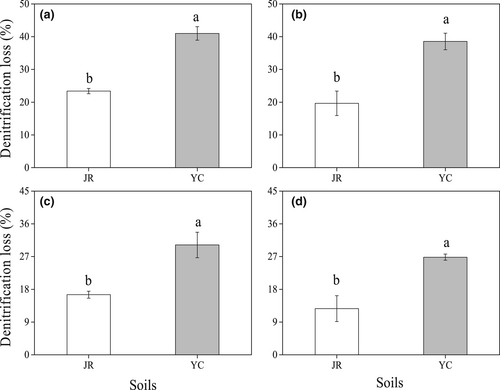
3.5 Denitrification loss
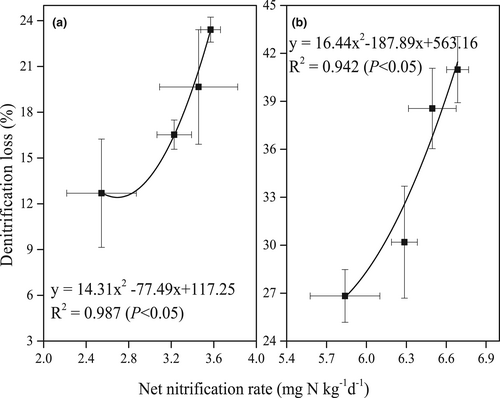
Denitrification loss, corresponding to the amount of unrecovered 15N at different rice growth stages, is displayed in Figure 3. The amount of denitrification N loss in JR soil (12.69%–23.41%) was significantly lower than in YC soil (26.83%–40.98%; p < 0.05). The N loss and net nitrification rates were significantly correlated both in JR (R2 = 0.9226, p < 0.05, Figure 4a) and YC (R2 = 0.9879, p < 0.05, Figure 4b) soils.
4 DISCUSSION
4.1 Nitrification in flooded paddy soil
After two weeks of preflooding, there was still a high -N content in the YC soils (Table 1). This may have been because nitrification can occur under flooding conditions, producing (Gao et al., 2020). Additionally, the denitrification process, which increases at higher concentrations of natural, soil-decomposable C, could not proceed because of the lack of readily dissoluble natural C (Lan et al., 2015; Yang et al., 2019).
The concentration of -N and O2 in paddy fields are the major factors affecting the nitrification nitrifiers. If the -N concentration is sufficient for nitrifiers, the nitrification rate will increase significantly with the increase of O2 concentration (Li et al., 2008). -N still existed in soils under flooded conditions after rice planting (Table S8), this show that nitrification occurred under flooded situations. This result indicated that the rice roots received oxygen via aerenchyma, following which nitrification occurred in the rhizosphere (Wells et al., 2014). Previous studies have shown that the nitrification rate decreases with increasing distance from the root surface (Yao et al., 2020). The nitrification rate under long-term flooded conditions was significantly lower than under growing rice conditions, indicating the importance of the rhizosphere to nitrification relative to oxidized topsoil (Wang et al., 2017). Briones et al. (2002) observed that the rice root surfaces were rich in ammonia-oxidizing bacteria, which also indicated the significance of nitrification in the rice rhizosphere. Our result showed that the abundance of AOA and AOB were significantly higher in rhizosphere soil in both JR and YC than in bulk soil (Table 3), and showed a significant and positive correlation with net nitrification rate (Table S3). Furthermore, leaf NR activity values in JR and YC soils increased significantly with the plant growth (Table S7), suggesting the increase of -N in rhizosphere soil. Our results follow the relationship between NR activity and -N supply under hydroponic conditions (Kaiser & Huber, 2001; Li et al., 2008).
Soil pH was also an essential factor in controlling the nitrification rate (Lehtovirta-Morley et al., 2011; Yang et al., 2016). It is widely accepted that the nitrification rate increases as soil pH increases (Cheng et al., 2004). The rhizosphere soil pH slightly decreased as rice growth progressed, both in JR and YC soil, but the pH in the JR rhizosphere soil was still lower than that in the YC rhizosphere soil (Table S2). The nitrification rate in JR rhizosphere soil was lower than in YC rhizosphere soil at all rice growth stages (Table 2).
4.2 The effect of rhizosphere nitrification rate on N uptake of rice plant
Some hydroponic researches have indicated that rice growth and NUE are increased significantly when both -N and -N sources were provided compared with -N only sources (Li et al., 2008). However, soil conditions are much more complex than solution culture. Rice plants and nitrifiers (AOA and AOB) may compete for the same substrate -N that are formed by mineralization or applied fertilizer N in paddy fields. The concentration and residence time of -N may be shorter in soils with a high nitrification rate compared to those with low nitrification rates. This connection was supported by our results that -N concentration in JR soil with a low nitrification rate was higher than in YC soil with a high nitrification rate (Table S8) at the four rice growth stages. Moreover, the abundance of AOA and AOB in YC soil was significantly higher than that in JR soils. The rice biomass decreased significantly as the native -N concentration in the rhizosphere soil increased (Table 7). This is likely due to the oxidation of -N to -N by nitrification, which reduced the concentration of -N, the preferred N form for rice plants. The negative correlation relationship decreased the effective uptake of -N by rice by enhancing the nitrification rate, meaning that there was competition for -N between the nitrification process and rice plants (Yang et al., 2017).
Our result showed that the rhizosphere nitrification rate both in JR and YC were both significations correlated with the Shannon index of AOB and indicated that AOB control rhizosphere nitrification, which is similar to the results of previous studies (Ai et al., 2013; Zou et al., 2022). Ammonia oxidizers play a key role in controlling the nitrification rate, therefore, their community composition and diversity affect their contribution to nitrification. From Figure 2, we found that the dominant taxa of AOB (Nitrosospira, Nitrosomondale) correlated with both rhizosphere and Bulk soil nitrification rate and NUE, which may indicate that AOB plays an important role in controlling the nitrification rate and rice NUE in JR and YC paddy soils. Our results also showed that Nitrosospira had a positive correlation with rhizosphere nitrification rate and had a negative correlation with rice NUE, while Nitrosomondale had a positive correlation with NUE (Figure 2a), which differs from the bulk soil, which may be because the difference in soil pH, organic carbon contents and inorganic N contents in rhizosphere and bulk soils. As it was reported that the AOB community composition was effluences by soil pH and the contents of nitrogen and carbon (Yang et al., 2020).
These results showed that the NUE in the pot experiment (28%–80.95%; Table 2) was significantly higher than that obtained in the field experiment (20%–40%) (Liu et al., 2019). This was mainly due to the rice growth conditions, such as the N fertiliser being applied in solution and thereby being more easily absorbed by the rice, as well as the N fertiliser being added during the critical period when the rice needed N (Yang et al., 2017). Additionally, less N fertiliser was applied in the pot experiment than in the field (Liu et al., 2019).
Generally, the concentration of -N in paddy soils is influenced by two main factors. The first is the form and dosage of the applied N fertiliser. Rice is an -N-preferring plant (Sun et al., 2015), so -N is the primary N fertiliser utilized in rice cultivation increasing the -N concentration in soil and the NUE in rice. The second is the impact of the soil on N, particularly the nitrification and -N production rates (e.g., mineralisation rate) (Zhang et al., 2016). In soil with a low nitrification capacity and low nitrification/mineralisation ratio, -N is the main inorganic form of N and can be readily absorbed by rice plants. However, in soil with a high nitrification capacity and high nitrification/mineralisation ratio, is the dominant organic N form, which enhanced the risk of N leaching, runoff, and denitrification. Thus, the nitrification process starts the competition for -N from rice plants and nitrifiers, causing a decrease in -N uptake by rice plants; this, in turn, decreases rice plant NUE (Yang et al., 2017).
4.3 The relationship between nitrification rate and denitrification
Two factors controlled denitrification loss. First, the N concentration from substrate -N was the limiting factor influencing denitrification loss under flooded conditions (Li et al., 2016; Yano et al., 2014; Qin et al., 2021). The applied -N-based fertiliser could not be denitrified until it was first nitrified to the -N form of N. This indicated the significance of nitrification as a prerequisite for N loss through denitrification. Nitrification is strictly an aerobic process and, therefore, can only occur in microaerobic zones under flooded conditions. Rice plants respond to long-term waterlogged conditions by forming numerous adventitious roots containing aerenchyma; these facilitate the diffusion of oxygen into the rice roots and rhizosphere soil. Thus, nitrification mainly occurs in the rice rhizosphere under flooded conditions and any factors affecting the soil nitrification rate can also affect denitrification.
The results of the present study showed that denitrification N loss differed with soil pH, which controlled the nitrification rate. Consequently, there was dramatically lower denitrification N loss in the JR soil (12.69%–23.41% of the applied N) than in the YC soil (26.83%–40.98% of the applied N) at equivalent rice-increased growth stages. These results were consistent with previous findings that nitrification inhibitors could dramatically decrease N loss from paddy soils (Sun et al., 2015; Zhou et al., 2020), thereby supporting the theory that rhizosphere nitrification controlled N loss through denitrification. Additionally, soluble organic matter has also been defined as an essential factor that influences denitrification through the provision of readily decomposable natural carbon as denitrification is a heterotrophic process (Lan et al., 2015; Yang et al., 2019). Rice plants release root exudate into rhizosphere soil; this exudate is rich in readily decomposable organic carbon, which can promote the nitrification rate and thereby increase denitrification. This also supports the proposal that rhizosphere nitrification controls N loss through denitrification.
Although the 15N mass conservation method has been used to simultaneously estimate the denitrification N loss in our pot experiment, this approach does have the inherent limitation that 15N may escape through other pathways. Until now, direct measurement of denitrification N losses with technology remains difficult (Lan et al., 2015), and in our study unrecovered 15N was used to estimate denitrification N losses. There is uncertainty in estimating denitrifying N losses using unrecovered 15N. Added 15N escapes through other pathways and processes, such as N leaching, emissions of N2O and NO from nitrification, and NH3 volatilization throughout the incubation experiment. In this study, ammonia volatilisation could be ignored due to the depth of 15N fertiliser application into the soil of 3–5 cm, based on the results of previous studies (Liu et al., 2015; Wang et al., 2017; Zhu et al., 2013). In addition, N leaching was prevented during the entire duration of the pot experiments. Thus, the main N loss pathway could be denitrification losses. As mentioned above, nitrification does occur in rhizosphere soils during the rice growth stages, and therefore, there are emissions of N2O and NO during nitrification. Moreover, N2, N2O, and NO produced during denitrification have a significant environmental impact (Yang et al., 2019). Our results represent denitrification N loss in soils during the fallow period and may differ from in situ processes. Therefore, in future studies, there is a need to measure NO and N2O emissions and to identify their production processes.
5 CONCLUSIONS
Using the pot experiment and 15N trace technique, the relationship between net nitrification rates of rice rhizosphere soil and NUE, denitrification N loss was estimated in the rice growth period. The results showed that rhizosphere nitrification played a dominant role in decreasing NUE and increasing denitrification. Nitrification in rice rhizosphere not only initiates competition for -N between rice and nitrifiers but also produces -N for denitrification, leading to denitrification N loss. Therefore, rhizosphere nitrification rates are a critical factor in determining the NUE of rice plant and denitrification N loss in paddy soil. The mechanisms of nitrification on NUE and denitrification loss could be partly due to the rhizosphere community structure of AOB. It should be noted that these results were based on pot experiments where only mineral N fertiliser was applied. Field investigations, the use of organic N fertilisers, and the effect of rhizosphere nitrification rate on N2O emission in paddy fields under different rice growth stages should be explored in future studies for assessing the environmental impacts of rhizosphere nitrification in paddy soils.
ACKNOWLEDGMENTS
This study was financially supported by grants from the National Natural Science Foundation of China (31901447, 31872179, and 41701329), the Key R&D Plan Program of Jiangsu Province (BE2020319), the Innovation and Entrepreneurship Program of Jiangsu Province (JSSCBS20211062), the Pilot Project of National Cooperative Promotion Plan of Major Agricultural Technologies (2020-SJ-047-04-01), the Excellent Doctor in Lvyangjinfeng of Yangzhou City (YZLYJFJH2021YXBS155), the Priority Academic Program Development of Jiangsu Higher Education Institutions, and the Blue Project of Yangzhou University.
CONFLICT OF INTEREST
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
Open Research
DATA AVAILABILITY STATEMENT
The data presented in this study are available from the corresponding author upon request.




