The impact of ketogenic diet on the frequency of psychogenic non-epileptic seizures (PNES): A feasibility randomized pilot study
Abstract
The potential of dietary interventions, particularly the use of the ketogenic diet in patients with Psychogenic Non-Epileptic Seizures (PNES), remains underexplored. This study aimed to assess the feasibility of a 6-week ketogenic diet (Modified Atkins Diet, MAD) intervention in adult patients with PNES and to compare its effects on PNES frequency and other variables against a control healthy diet (CD). A feasibility pilot randomized controlled trial was conducted at a tertiary neurology hospital, enrolling outpatients diagnosed with PNES and assigning them to either MAD or CD. Baseline and follow-up assessments (at 2, 4, and 6 weeks) included evaluation of mental health, PNES frequency, and metabolic measures. Descriptive and inferential methods, including repeated measures ANOVA, were used for statistical analysis. Seventeen patients (mean age 28.23 ± 7.1) were randomly allocated to receive either MAD (n = 12) or CD (n = 5). The entire sample exhibited a significant decrease in monthly PNES frequency (p = 0.01, Hedges ES = 0.618) without differences between groups. The MAD group showed significant improvement in PNES frequency, depression, and anxiety at week six. Results demonstrate that the implementation of MAD is feasible in patients with PNES and suggest that it may reduce seizure frequency and symptoms of depression and anxiety. These findings warrant further investigation in larger, powered studies to demonstrate efficacy.
Plain Language Summary
This study explored the potential benefits of the Modified Atkins Diet (MAD) in reducing the frequency of psychogenic non-epileptic seizures (PNES). The results showed that the diet is safe, well-tolerated, and may decrease the occurrence of PNES, as well as symptoms of depression and anxiety. These findings suggest that dietary modifications could be a helpful complement to PNES treatment, though larger studies are necessary to confirm these outcomes.
Key points
- Seventeen outpatients with PNES were randomly assigned to a 6-week Modified Atkins Diet (MAD) or a control healthy diet.
- All participants exhibited a decrease in monthly PNES frequency, with no significant differences between the groups.
- Patients on the MAD showed significant improvement in PNES frequency, depression, and anxiety.
- The implementation of MAD is feasible in patients with PNES.
- Dietary modifications could be a helpful complement to PNES treatment, though larger studies are necessary to confirm these outcomes.
1 INTRODUCTION
Psychogenic nonepileptic seizures (PNES) are paroxysmal episodes characterized by subjective changes in consciousness and involuntary movements that resemble epileptic seizures but are not associated with epileptic activity.1 Despite its prevalence and impact, the diagnosis and management of PNES remain complex, contributing notably to neurology referrals, particularly in emergency and first-seizure settings, with an annual incidence ranging from 1.5 to 6.17 cases per 100 000.2, 3 PNES are often complicated by comorbid medical and psychiatric conditions, underscoring the need for an integrated management approach.4, 5
Although there is growing evidence supporting the benefits of ketogenic diets (KDs) in neurological and psychiatric disorders,6, 7 their effects on dissociative disorders, such as functional neurological disorders, remain unexplored. The ketogenic diet aims to mimic the state of starvation by inducing ketosis through a high-fat, low-carbohydrate diet. Ketone bodies produced include acetoacetate, beta-hydroxybutyrate, and acetone.7 The classic ketogenic diet, used in people with drug-resistant epilepsy, consists of a 4:1 ratio of fat to protein and carbohydrates combined.8 However, less restrictive forms, such as the modified Atkins diet (MAD), are used.6 MAD is based on a ratio of approximately 1:1 and includes 10–30 g of carbohydrate per day with no restriction of fluids, calories, or protein.8 The positive effects of KD in neurological and psychiatric conditions seem to be related to potential anti-inflammatory mechanisms of ketone bodies (e.g., beta-hydroxybutyrate),9 enhancement of gut microbiota genomic diversity, and reduction in reactive oxygen species (ROS).6
The aim of this study was to determine whether a 6-week MAD intervention is feasible for adult patients with PNES, whether the diet can be maintained and ketosis achieved, and to compare its effects against a reduced-calorie diet (CD) on PNES frequency and other mental health variables.
2 METHODS
2.1 Subjects
This randomized controlled clinical trial included outpatients with PNES who had a diagnostic level of certainty classified as either “clinically stablished” or “documented,” according to the ILAE proposed levels.10 This was irrespective of coexisting epilepsy, provided the epilepsy was under control. Participants were recruited from the National Institute of Neurology and Neurosurgery “Manuel Velasco Suárez” (INNN-MVS) in Mexico City. Inclusion criteria included age over 17 years, a monthly PNES frequency greater than three, and if epilepsy was present, the patient had to have been seizure-free for the past year. Exclusion or elimination criteria included metabolic or hemodynamic instability, liver failure, intolerance to oral intake, acute pancreatitis, pregnancy, rare metabolic disorders, and withdrawal of informed consent or for missing the first follow-up session after having started the diet. Participants who were undergoing psychotherapy or pharmacotherapy with antidepressant drugs or antiseizure medications were allowed to continue their treatments, provided they had been stable with no changes in the last 6 weeks. During the intervention, changes to their treatment were not allowed unless deemed necessary, which would result in the withdrawal of the patient from the study.
2.2 Procedures
Participants who wished to participate and met the inclusion criteria were referred from the epilepsy and neuropsychiatry outpatient clinics. Participants were allocated to receive either MAD or the CD, using simple random sampling of consecutive cases with an Excel table of random numbers. They were blinded to their assigned diet, while evaluators and medical/nutritional staff were aware. Initial psychiatric and nutritional assessments were conducted to obtain baseline data. The CD was based on recommendations for a normal distribution of macronutrients, with an emphasis on the consumption of complex carbohydrates over added sugars. Follow-up appointments occurred at 2, 4, and 6 weeks. Baseline psychiatric evaluation included collecting sociodemographic data, administering the Montgomery-Asberg Depression Rating Scale (MADRS) and Hamilton Anxiety Rating Scale (HAM-A), conducting laboratory tests and general urine examination, and documenting daily PNES frequency. To ensure adherence to the KD and maintain blinding, all patients, including those in the CD group, were provided with urine test strips, which they were required to use and send a photo of to the investigators three times a week. Nutritional visits involved guiding patients on adhering to either MAD or CD. Blinding was maintained by the Nutritional Support Department, and diet adherence was assessed through food frequency diaries and urine ketone monitoring.
2.3 Measures
Demographic variables, mental health measures (MADRS, HAM-A), seizure frequency, physiological measures (fasting glucose levels, blood lipid analysis), and metabolic indicators were assessed. More details on measures and their timing are provided in Figure 1 (Appendix S1).
2.4 Statistical analysis
Descriptive analysis and baseline comparisons were carried out. Repeated measures analysis of variance (ANOVA) was performed with an intention-to-treat design. Effect sizes (Hedges) of diet on seizure frequency, MADRS, and HAM-A scores were calculated. SPSS v28 for Windows was used for all analyses (IBM, 2021).
2.5 Protocol approval and informed consent
The study received approval from the ethics and research committee of INNN-MVS (registration no. 8/21), adhering to the principles of the Helsinki Declaration. Registered as a clinical trial (identifier NCT05219006 on ClinicalTrials.gov), participant enrollment occurred between July 15, 2021, and May 20, 2023. Written informed consent was obtained from all adult participants before inclusion.
3 RESULTS
Of the 23 patients initially evaluated, the final sample included 17 participants: 16 females and one male. Twelve participants were assigned to the MAD group, while five, including the male participant, were in the CD group (see flowchart in the Appendix S1). Participant ages ranged from 17 to 48 years, with a mean age of 28.23 ± 7.1 years.
All 17 participants (17/17) were on a stable dose of antidepressants, and seven participants (7/17) were on a stable dose of antiseizure medications, with six of these in the MAD group (6/12) and one in the CD group (1/5). No patients were undergoing psychotherapy during the treatment. Additionally, four participants (4/16) had a diagnosis of epilepsy, all of whom were in the MAD group.
3.1 Adverse events
Only mild adverse effects were reported, mainly at the beginning of the diet, in both groups, but none required any pharmacological intervention or were a reason to discontinue the diet. Nausea, constipation, and headache were the most frequently reported adverse effects in patients in the MAD group, but they resolved on their own when the diet was continued.
3.2 Effect on PNES frequency
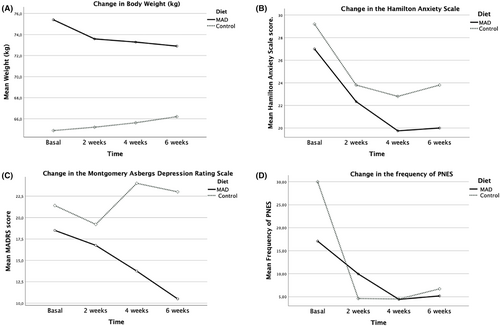
Repeated measures linear regression revealed no significant differences between the groups (F[1,15] = 0.006, p = 0.939). Both groups experienced a reduction in the number of seizures at 6 weeks (see Table 1), but this reduction was statistically significant only in the MAD group (p = 0.04) and for the entire sample (p = 0.01). The Hedges' effect size (ES) was 0.5 (95% CI: −0.73 to 1.0) for the MAD group and 0.58 (95% CI: 0.09–1.13) for the entire sample (see Table 1).
| Measure | RCD (n = 5) | MAD (n = 12) | WS (n = 17) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline Mean ± SD | 6 weeks Mean ± SD | p < 0.05, h (95%CI) | Baseline Mean ± SD | 6 weeks Mean ± SD | p < 0.05, h (95% Cl) | Baseline Mean ± SD | 6 weeks Mean ± SD | p < 0.05, h (95% CI) | |
| PNES | |||||||||
| Monthly | 60 ± 67.1 | 11.2 ± 6.7 | 0.08, 0.58 (−0.2–1.4) | 40.6 ± 58.3 | 9.6 ± 14.8 | 0.044, 0.5 (−0.73–1.0) | 46.3 ± 59.5 | 10 ± 12.8 | 0.011, 0.58 (0.08–1) |
| Weekly | 15 ± 16.7 | 3.35 ± 1.9 | 0.09, 0.55 (−0.26–1.3) | 10 ± 14.5 | 2.5 ± 4.5 | 0.045, 0.5 (−0.76–1.0) | 11.5 ± 15 | 2.8 ± 3.7 | 0.012 0.57 (0.07–1) |
| MADRS | 21.4 ± 10 | 23 ± 13.7 | 0.33, −0.17 (−0.8–0.54) | 18.5 ± 9.7 | 10.5 ± 8.2 | 0.005, 0.83 (0.19–1.4) | 19.3 ± 9.6 | 14.1 ± 11.3 | 0.01, .52 (.02–1.0) |
| HAM-A | 29 ± 10.9 | 23.8 ± 17 | 0.082, 0.76 (−0.2–1.3) | 27 ± 12.7 | 20 ± 14.3 | 0.02, 0.58 (0–1.1) | 27.6 ± 12 | 21 ± 13.7 | <0.001, 0.623 (0.11–1.1) |
| Weight (kg) | 64.8 ± 13 | 66.2 ± 11 | 0.16, −0.4 (−1.1–0.36) | 75.4 ± 15 | 72.9 ± 15 | <0.001 1.7 (0.65–2.2) | 72.3 ± 15 | 70.9 ± 14 | 0.02, 0.5 (0.01–.98) |
| Glucose (mg/dL) | 86.4 ± 4 | 83.3 ± 4 | 0.21, 0.32 (−0.42–1.0) | 100.7 ± 52 | 98 ± 53 | 0.11, 0.37 (−0.22–0.95) | 96.5 ± 44 | 93.9 ± 44 | 0.06, 0.37 (−0.10–0.83) |
| Total cholesterol (mg/dL) | 167 ± 40 | 180 ± 61 | 0.24, −0.26 (−0.97–0.46) | 178.1 ± 26 | 194 ± 53 | 0.19, −0.42 (−1–0.2) | 174.3 ± 31 | 189 ± 54 | 0.06, −0.41 (−0.9–0.11) |
| Triglyce-rides (mg/dL) | 147.6 ± 112 | 153 ± 104 | 0.24, −0.27 (−0.97–0.46) | 138 ± 106 | 96.5 ± 27 | 0.10, 0.40 (−0.22–1.1) | 141 ± 104 | 116.8 ± 68 | 0.12, 0.32 (−0.22–0.85) |
- Note: There were no statistically significant differences between the groups. Intragroup and total sample effects are shown. Statistically significant results are shown in italics and bold (𝜶 <0.05).
- Abbreviations: h, hedge's g, HAM-A, Hamilton Anxiety Rating Scale; MAD group, Modified Atkins diet, MADRS, Montgomery Asberg Depression Rating Scale, PNES, psychogenic non-epileptic seizures, RCD group, reduced calorie diet, SD, Standard deviation, WS, whole sample.
3.3 Effect on anxiety and depressive symptoms
No significant differences were found between the groups in HAM-A and MADRS scores. However, both the MAD group and the full sample analysis showed significant improvements in depression and anxiety scores (see Table 1).
3.4 Effect on weight and metabolic variables
The MAD group was the only group to show a statistically significant reduction in weight (F[1,11] = 22.6, p < 0.001). No significant differences, either between or within groups, were observed for other metabolic variables (see Table 1).
4 DISCUSSION
Considerable evidence supports the efficacy of the KD in treating people with epilepsy (PWE), particularly in drug-resistant cases,11 as well as other neurologic conditions. Additionally, a growing number of reports suggest that this metabolic approach may also benefit some psychiatric disorders.12 However, there is limited evidence for the use of the KD in dissociative disorders as PNES.
The assessment and treatment of functional neurological disorders is an interdisciplinary process that may involve various approaches, including healthy lifestyle changes, psychotherapeutic interventions, psychoeducation, treatment of psychiatric comorbidities, and, in select cases, physical rehabilitation. In this pilot feasibility study, we found that the MAD, a less restrictive ketogenic diet with better adherence, could be implemented and was well tolerated by patients with PNES. Moreover, despite the limited sample size, patients in the MAD group showed a statistically significant reduction in the number of seizures and improvements in depression and anxiety scores. A reduction in seizure frequency was also observed in patients in the low-calorie diet group, although this was not statistically significant, likely due to the limited number of participants.
Although it is feasible to think that the improvement in the patients was because they received more attention during the study, this does not seem to be the case, since the change in the frequency of crises and in the depression and anxiety scales was greater and significant only in the patients on the MAD.
The KD has shown positive effects on depression and anxiety, mediated through multiple biological pathways, including its anti-inflammatory properties, enhanced mitochondrial energy production, improved insulin signaling, neuroprotective effects, and the induction of brain-derived neurotrophic factor gene expression.13 However, studies exploring dietary interventions for dissociative disorders, including PNES, are virtually nonexistent. This study hypothesizes that the KD may benefit PNES through mechanisms such as reducing inflammation and modulating the gut-brain axis. Specifically, patients with PNES have been reported to exhibit elevated levels of proinflammatory factors distinct from those found in PWE,14, 15 suggesting that the KD's anti-inflammatory effects could help mitigate PNES symptoms. Additionally, the ketone bodies generated by the KD could positively influence gut microbiota (GM) composition. Since GM plays a critical role in central nervous system function through immune, circulatory, and neural pathways—often referred to as the “gut microbiota-brain axis”—these changes may enhance neurotransmitter balance, such as by increasing gamma-aminobutyric acid or glutamate decarboxylase enzyme production.16 Further research is needed to validate these hypotheses and uncover the full potential of the KD for PNES.
5 LIMITATIONS AND STRENGTHS
This pilot study has notable strengths but also several limitations that warrant consideration. While the small sample size and unequal distribution between the MAD and control diet groups limit the generalizability of the findings, the study demonstrates the feasibility of implementing the MAD in patients with PNES, a population for which dietary interventions remain underexplored. Adherence to the control diet could not be objectively verified, and potential confounders such as prior dietary habits, physical activity levels, and socioeconomic factors were not assessed. Additionally, while patients were blinded to their diet assignment, meal plans may have allowed them to infer their group, introducing potential bias. Mechanistic pathways, such as inflammatory biomarkers and gut microbiota changes, were not evaluated, which limits understanding of the biological underpinnings of the observed effects. Despite these challenges, the study provides preliminary evidence that the MAD may reduce seizure frequency and improve depression and anxiety symptoms. The structured protocol, regular follow-ups, and use of validated scales ensured reliable data collection and high adherence rates, with no dropouts due to adverse events. Future studies should address these limitations by including larger sample sizes, double-blind designs, objective adherence measures, and assessments of mechanistic variables to confirm and expand upon these promising findings.
6 CLINICAL RELEVANCE
The main objective was met: demonstrating that it is feasible to implement a dietary intervention and perform longitudinal follow-up in patients with PNES. All patients tolerated the diet and the adverse effects that occurred during the first days. The results suggest that the Modified Atkins Diet (MAD) may improve the frequency of PNES and symptoms of anxiety and depression, provided these findings can be replicated in a double-blind, controlled study designed with sufficient power to demonstrate efficacy.
AUTHOR CONTRIBUTIONS
Conception and design of the study: C.M.D., J.A.R., M.J.I., C.M.H. Patient evaluation and diet implementation for each patient: M.H.C., A.L.G., A.H.A., G.P.J., G.C.K., H.P.M. Data acquisition, statistical analysis, and manuscript revision: C.M.D., J.A.R., M.J.I.
ACKNOWLEDGMENTS
This research has not received external or governmental funding. Open access funding provided by UNAM.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
Approval for this study was granted by the ethics and research committee of the INNN-MVS. We confirm that we have read the journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
CONSENT
All patients provided verbal and written consent for this work.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author, C.M.D., upon reasonable request.