NORSE secondary to anti-GAD65 antibody-positive encephalitis treated with novel adjunctive rapid titration VNS protocol
Abstract
New Onset Refractory Status Epilepticus (NORSE) is a rare and severe condition characterized by refractory seizures in individuals without a prior history of epilepsy. This case report describes a 37-year-old woman diagnosed with anti-glutamic acid decarboxylase 65 (anti-GAD65) antibody-positive encephalitis-related NORSE. Her seizures were refractory to multiple interventions, including anti-seizure medications, anesthetics, immunotherapies, a ketogenic diet, and electroconvulsive therapy. Seizures recurred twice during the tapering of anesthetic medications. However, after 32 days of treatment, the seizures were successfully controlled. To maintain seizure control and facilitate the weaning of anesthetics, a Vagus Nerve Stimulator (VNS) was implanted using a novel rapid titration protocol. This allowed for the successful tapering of anesthetics by day 50, with no recurrence of seizures. At her 9-month follow-up, the patient remained seizure-free and had an improved quality of life. This case highlights that early initiation of immunosuppressive treatment may lead to a favorable prognosis. The novel application of VNS therapy assisted seizure control in NORSE, thus encouraging further research investigating the potential role of VNS in this condition.
Plain Language Summary
New Onset Refractory Status Epilepticus (NORSE) is a rare and severe condition characterized by relentless seizures in individuals without a prior epilepsy history. This report shares the case of a 37-year-old woman with NORSE, associated with a high anti-glutamic acid decarboxylase 65 antibody titer. Her seizures were super-refractory, requiring multiple anti-seizure medications, anesthetics, immunotherapies, a ketogenic diet, and electroconvulsive therapy. Seizures recurred twice during the tapering of anesthetic medications. However, by hospital day 32, the seizures were successfully controlled with these interventions. To further stabilize seizure control and enable the successful discontinuation of anesthetics, a Vagus Nerve Stimulator (VNS) was implanted. The patient had no further seizures and gradually recovered back to her pre-disease baseline. This case suggests that a novel rapid VNS titration protocol could be a promising treatment option for NORSE, warranting further investigation.
Key points
- A 37-year-old woman presented with NORSE related to anti-GAD65 antibody-positive encephalitis.
- Her seizures were super-refractory, requiring multiple therapies. (ASMs, anesthetics, immunotherapies, ketogenic diet, and electroconvulsive therapy).
- A VNS was implanted using a novel rapid titration protocol to maintain seizure control and facilitate anesthetic weaning.
- The novel use of VNS therapy shows promise in managing NORSE.
INTRODUCTION
New onset refractory status epilepticus (NORSE) is a rare syndrome characterized by refractory status epilepticus in patients with no previous history of epilepsy. It can manifest as refractory status epilepticus (RSE), defined by seizures persisting despite the appropriate use of benzodiazepines and other parenteral anti-seizure medications (ASMs). NORSE can also present as super-refractory status epilepticus (SRSE), characterized by seizures lasting at least 24 h after initiation of anesthesia that occur continuously or recur after attempts to stop anesthesia. NORSE is a syndrome rather than a specific diagnosis.1 An autoimmune cause is identified in up to 36.2% of cases, with the majority (19.7%) being non-paraneoplastic. Despite treatment with multiple ASMs and anesthetics, NORSE carries a high mortality rate of 22%.2 Early initiation of immunotherapies, such as intravenous immunoglobulin (IVIG) or Plasma exchange (PLEX), is recommended for cases with suspected autoimmune-mediated or cryptogenic etiologies. However, due to suboptimal responses, second-line immunosuppressants are often required, though strong evidence-based guidelines for NORSE management remain limited.3 We present a case with NORSE secondary to encephalitis with anti-glutamic acid decarboxylase 65 (anti-GAD65) antibody positivity. The patient experienced breakthrough seizures despite treatment with ASMs, immunotherapies, a ketogenic diet, and electroconvulsive therapy (ECT). A vagus nerve stimulator (VNS) was used as adjunctive therapy with a novel rapid titration protocol, resulting in successful tapering of anesthetics and sustained seizure freedom.
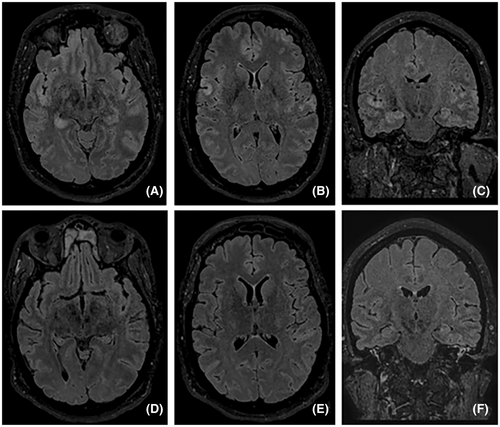
A 37-year-old, previously healthy, right-handed woman was transferred to our facility due to worsening cognitive function and was diagnosed with NORSE. An electroencephalogram (EEG) upon arrival confirmed non-convulsive status epilepticus originating from the right temporal and left frontal lobes. To achieve seizure control, therapeutic burst suppression was induced. She required multiple ASMs, including levetiracetam, phenytoin, lacosamide, topiramate, perampanel, phenobarbital, and clobazam, as well as various anesthetics such as ketamine, midazolam, propofol, and pentobarbital. A brain magnetic resonance imaging (MRI) revealed hyperintensity in bilateral frontal and temporal lobes on T2-weighted fluid-attenuated inversion recovery (FLAIR) sequence (Figure 1). A cerebrospinal fluid (CSF) study was inconsistent with an infectious etiology, showing a white blood cell count of 4/mm3, red blood cell count of 99/mm3, glucose of 84 mg/dL, protein of 26 mg/dL, and a negative viral PCR panel. She had a transient fever of 38.4 C and tested positive for influenza A PCR on admission. Autoimmune encephalitis was suspected based on the criteria proposed by Graus et al., further supported by a high antibody prevalence in epilepsy and encephalopathy 2 (APE2) score of 5.4, 5 Treatment for autoimmune-mediated NORSE was initiated on hospital day 5 with IVIG at 30 g for 5 days and methylprednisolone at 1 g for 5 days. Other treatments throughout the 55-day hospitalization are summarized in Figure 2. A CSF autoimmune encephalitis panel (Mayo Clinic Laboratories, ENC2) later confirmed a positive anti-GAD65 antibody level of 4.67 nmol/L (normal ≤0.02 nmol/L). The remainder of the panel, including antibodies against the N-methyl-d-aspartate receptor (NMDAR), the gamma-aminobutyric acid receptor (GABAR), and the leucine-rich, glioma-inactivated glycoprotein (LGI1), were all negative. CT scans of the chest, abdomen, and pelvis were negative, ruling out the malignancy. The patient underwent plasma exchange (five sessions) and rituximab infusions (1 g on day 24 and day 31, followed by 500 mg on day 38 and day 46). Concurrently, she received two sessions of electroconvulsive therapy and a ketogenic diet. Despite brain MRI improvement and a concurrent anti-GAD65 antibody titer reduction, the ictal-interictal continuum (IIC) and non-convulsive status epilepticus recurred twice during attempts to wean anesthetic medications. Although seizures stopped on day 32, a vagus nerve stimulator (VNS) was implanted on day 35 to ensure sustained seizure control, given the previous unsuccessful weaning attempts. A novel rapid titration protocol was initiated, concurrently increasing the output current to 2.0 mA with a 51% duty cycle under normal mode within 4 days and further adjusting to 2.5 mA with a 58% duty cycle over 9 days (Table 1). Pentobarbital and midazolam were tapered once the normal output mode reached 1.5 mA on day 37. All infusions were successfully discontinued by day 50. The patient was discharged 5 days later to a rehabilitation facility on six ASMs (phenobarbital, lacosamide, levetiracetam, topiramate, perampanel, and clobazam). At her 9-month follow-up visit, she remained seizure-free and returned to her previous job as a paramedic. She is currently on four ASMs (levetiracetam, lacosamide, perampanel, and phenobarbital) with the same VNS settings. She is also on maintenance rituximab every 6 months. Before immunosuppression therapy, her serum anti-GAD65 antibody level was at 2444.2 U/mL (normal <5 U/mL). Her Montreal-cognitive-assessment (MoCA) score was 27/30, with mild impairment in executive function, calculation, and delayed recall. Her Patient Health Questionnaire-9 (PHQ-9) score was 3, and her Generalized Anxiety Disorder-7 (GAD-7) score was 0, indicating minimal depression and no anxiety. Additionally, her Quality of Life in Epilepsy (QOLIE-31) score was 88, reflecting a significant improvement in her overall condition.
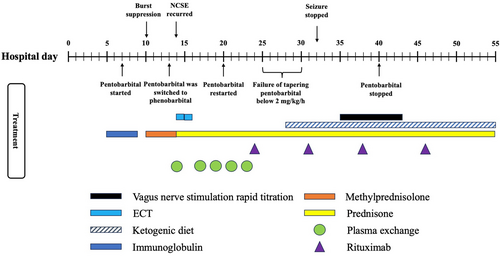
| Hospital day | Normal mode | Autostim mode | Magnet mode | Pentobarbital | Midazolam | Seizure activity | |
|---|---|---|---|---|---|---|---|
| Current | Duty cycle | ||||||
| 35 | 0.5 mA | 16% | 0.5 mA | 0.75 mA | 2 mg/kg/h | 40 mg/h | No |
| 36 | 1 mA | 25% | 1 mA | 1.25 mA | 2 mg/kg/h | 40 mg/h | No |
| 37 | 1.5 mA | 35% | 1.5 mA | 1.75 mA | 1.5 mg/kg/h | 40 mg/h | No |
| 38 | 2 mA | 51% | 2 mA | 2.25 mA | 1.0 mg/kg/h | 40 mg/h | No |
| 39 | 2 mA | 51% | 2 mA | 2.25 mA | 0.5 mg/kg/h | 40 mg/h | No |
| 40 | 2 mA | 51% | 2 mA | 2.25 mA | 0 mg/kg/h | 40 mg/h | No |
| 41 | 2 mA | 51% | 2 mA | 2.25 mA | 0 mg/kg/h | 40 mg/h | No |
| 42 | 2 m | 51% | 2 mA | 2.25 mA | 0 mg/kg/h | 20 mg/h | No |
| 43 | 2.5 mA | 58% | Off | 2.75 mA | 0 mg/kg/h | 0 to >20 mg/h | Right-hand twitching, no EEG correlation |
| 44 | 2.5 mA | 58% | Off | 2.75 mA | 0 mg/kg/h | 20 mg/h | No |
| 45–50 | 2.5 mA | 58% | Off | 2.75 mA | 0 mg/kg/h | 20 to >0 mg/h | No |
1 DISCUSSION
Glutamic acid decarboxylase is a neuronal enzyme responsible for synthesizing the neurotransmitter gamma-aminobutyric acid. The anti-GAD65 antibody has been associated with various neurological conditions, including epilepsy, limbic encephalitis (LE), stiff-person syndrome (SPS), and cerebellar ataxia (CA).6 Anti-GAD65 antibody-positive encephalitis presenting as NORSE is a rare occurrence. In our case, the patient met the diagnostic criteria for possible autoimmune encephalitis, and an APE2 score of 5 supported an autoimmune etiology. Predictive models like the APE2 and RITE2 scores demonstrated reasonable sensitivity and specificity, helping guide clinical evaluation and predict the response to immunotherapy in patients with suspected autoimmune causes.4, 5 Therefore, IVIG and steroids were promptly initiated after the infectious workup returned negative. PLEX and a total of 3 g of rituximab were also administered due to the suboptimal response to prior therapies. Although managing her seizures remained clinically challenging, the CSF study and MRI showed signs of improvement, and her overall outcome was excellent. This case underscores the importance of early and aggressive immunosuppressive therapy in managing anti-GAD65 antibody-positive encephalitis-related NORSE.
VNS therapy has been widely used over the past few decades for patients with drug-resistant epilepsy (DRE), demonstrating a significant reduction in seizure frequency.7 However, only a few case reports have documented its application in adult patients with NORSE. A 2019 systematic review on using VNS in treating RSE and SRSE found that VNS successfully interrupted RSE and SRSE in 74% of patients. The median duration of RSE/SRES before VNS implantation was 18 days (range 3–1680 days), and the median time to cessation of RSE/SRSE after implantation was 8 days (range 3–84 days).8 It is worth noting that some studies in this review included patients with known epilepsy etiologies, which do not align with the diagnostic criteria for NORSE. As of this submission, only six cases with relatively high-quality data have been reported.9-14 The average age of onset for the patients in these case studies was 40 years (range 24–54). One patient was diagnosed with anti-NMDAR antibody encephalitis, another with anti-GluRε antibody encephalitis, and the remaining four cases were cryptogenic. The timing of VNS implantation following the onset of status epilepticus varied significantly, ranging from 5 days to over 14 months. Most cases applied faster titration of VNS settings than the standard recommendations.15 Among the six patients, the time from VNS implantation to the cessation of status epilepticus ranged from approximately 2 to 14 days. Three cases (3/6, 50%) have favorable outcomes with mild to moderate cognitive impairment.9, 12, 14 Among these three cases, one was diagnosed with anti-NMDAR receptor antibody encephalitis and underwent several courses of immunotherapy; VNS was implanted after 110 days in NCSE.9 The other two cases were cryptogenic and also received immunotherapy with VNS implantation on day 30 and day 42, respectively.12, 14 Two patients experienced complications and eventually died after termination of NORSE.11, 13 The last patient underwent a corpus callosotomy before VNS implantation and became seizure-free but suffered from severe cognitive impairment.10 Given the diverse range of etiologies, variations in treatments, and associated complications, it is difficult to determine the precise role VNS played in the treatment of NORSE cases. In our case, although the seizures ceased 3 days before VNS implantation, multiple failed attempts of tapering anesthetics and ASMs suggest that the VNS may have contributed to the subsequently successful weaning of anesthetic medications after its placement.
The VNS titration protocol used in our study represents the most rapid approach reported so far, involving simultaneous adjustments of both output current and duty cycle. The normal mode output current was rapidly ramped up to 2 mA with a 51% duty cycle over 4 days and further escalated to 2.5 mA with a duty cycle of 58% in 9 days. During anesthetic tapering, the patient experienced transient stereotypical right-hand twitching without EEG correlation and otherwise remained seizure-free. In the chronic drug-resistant epilepsy (DRE) population, the literature supports rapid titrations to an output current of 1.625 mA in less than 3 months, which can lead to a faster response without significant increases in side effects.16 Given the critical and high-morbidity nature of NORSE, a more aggressive treatment strategy may be warranted. Our case suggests rapid concurrent titration of VNS output current and duty cycle may support treatment efforts in SRSE in the setting of NORSE. It is also important to mention that NORSE patients are often sedated and closely monitored for side effects in the intensive care units. In the cases we reviewed, including ours, no patient experienced adverse side effects from rapid VNS titration. We recommend further investigation into protocols that involve simultaneous output and duty cycle adjustment in normal mode. Such approaches may reduce neurological damage caused by prolonged status epilepticus and facilitate earlier tapering from anesthetics.
VNS has been proposed to involve several physiological pathways, including neurotransmitter regulation, anti-inflammation effects, adjustment of cerebral blood flow, and EEG rhythm desynchronization. However, its exact mechanism of treating epilepsy remains unclear.17 Overproduction of proinflammatory cytokines in serum and CSF has been observed in patients with SE. The same phenomenon was noticed in patients with NORSE, especially cryptogenic, compared to other forms of RSE.18 Following a novel rapid titration protocol, we speculate that the VNS might elicit anti-inflammatory effects faster in NORSE. Additionally, VNS has also been shown to increase the GABAA receptor density (GRD) in patients with drug-resistant partial epilepsy.19 A rapid titration protocol could expedite this regulatory process because SE is associated with decreased inhibitory GABA-A receptors and increased excitatory NMDA receptors.
In conclusion, this is the first reported case of using VNS with a novel rapid titration protocol in anti-GAD65 antibody-positive encephalitis-related NORSE. Early and aggressive immunotherapy, guided by evidence-based prediction models, is essential for achieving favorable outcomes. Currently, there is no standardized treatment protocol utilizing VNS in NORSE patients, and this case provides valuable and detailed data that may serve as a foundation for future large-scale studies.
AUTHOR CONTRIBUTIONS
Mingyu Li: Writing—original draft preparation (lead), writing—review and editing (lead), and resources (equal). Nilufer Yalcin: Writing—review and editing (lead) and resources (equal). Danielle Weiss: Writing—review and editing (lead) and resources (equal). Leila A. T. Hill: Writing—review and editing (equal). Klepper Alfredo Garcia: Writing—review and editing (equal) and resources (equal). Manan Shah: Writing—review and editing (equal) and resources (equal). Fernando Vale Diaz: Writing—review and editing (equal), resources (equal), and investigation (lead). Luis Rueda Carrillo: Writing—review and editing (equal). Hunter Smith: Writing— review and editing (equal). Debra Moore-hill: Writing—review and editing (lead), conceptualization (lead), supervision (lead), investigation (lead), and resources (equal).
FUNDING INFORMATION
No funding to disclose.
CONFLICT OF INTEREST STATEMENT
Dr. Fernando L. Vale Diaz and Dr. Debra T. Moore-Hill: speakers bureau for LivaNova. The rest of authors have no conflict of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
ETHICS STATEMENT
The studies involving human participants were reviewed and approved by the hospital ethic committee. The author provided the written informed consent to participate in this study.
Open Research
DATA AVAILABILITY STATEMENT
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.