Advanced approach for active and durable proton exchange membrane fuel cells: Coupling synergistic effects of MNC nanocomposites
Abstract
Atomically dispersed metal and nitrogen co-doped carbon (MNC) is a promising oxygen reduction reaction (ORR) catalyst for electrochemical energy storage and conversion applications but typically suffers from low durability and activity under the acidic conditions of practical polymer electrolyte exchange membrane fuel cells (PEMFCs). Recently, the performance of MNC nanocomposites under acidic ORR conditions has been enhanced by exploiting the synergistic coupling effects of their constituents (single-atom sites, nanoclusters, and nanoparticles). The unique geometric structures formed by the coupling of diverse sites in these nanocomposites provide optimal electronic structures and efficient reaction pathways, thus resulting in high activity and long-term durability. This work provides an overview of MNC nanocomposites as ORR electrocatalysts under practical PEMFC conditions, focusing on activity and durability enhancement methods and highlighting the strategies used to prepare electrocatalytically efficient MNC nanocomposites containing no or low amounts of platinum group metals. Progress in the development of advanced MNC nanocomposites as acidic ORR catalysts is discussed, and the pivotal role of synergistic effects resulting from the coupling sites within the nanocomposites is explored together with the characterization methods used to elucidate these effects. Finally, the challenges and prospects of developing MNC nanocomposites as next-generation electrocatalysts are presented.
1 INTRODUCTION
Greenhouse gas emissions due to the rapid industrialization and increasing energy demand cause climate change, which is manifested by global warming and other environmental and socioeconomic problems. Consequently, considerable efforts have been made to decrease fossil fuel use and CO2 emissions. The realization of a carbon-neutral society requires the development of renewable energy sources to reduce our dependence on the limited fossil resources.1 In this regard, electrochemical reaction technologies have drawn considerable attention because of their high conversion efficiency and ecofriendliness.2
The oxygen reduction reaction (ORR) is crucial for the operation of energy conversion and storage devices, such as fuel cells and Zn–air batteries. Given their high energy density and short refueling time, proton exchange membrane fuel cells (PEMFCs) have been extensively researched as power sources for electric vehicles but have not yet been commercialized because of the difficulty in realizing high membrane electrode assembly (MEA) performance and low production costs.3 In particular, large amounts of expensive Pt-based catalysts are needed to achieve high MEA performance and reduce the overpotential of the sluggish ORR.4 Among the various ORR catalysts, those based on atomically dispersed metal and nitrogen co-doped carbon (MNC) feature isolated single-atom active sites and hold considerable promise. In particular, these catalysts utilize earth-abundant metals, such as iron, cobalt, or nickel, and offer a cost-effective solution for scaling up electrochemical applications, particularly compared with traditional platinum group metal (PGM)-based catalysts. Nanoparticle catalysts containing PGM or non-PGM compounds, such as oxides and nitrides, often suffer from low atom utilization efficiencies, as numerous atoms embedded within the particle core are not accessible for catalytic reactions. In contrast, MNC catalysts exhibit high atom utilization efficiencies, as their single-atom active sites are distributed on the surface and efficiently participate in the reaction. Furthermore, the ORR active sites of PGM-based catalysts can be poisoned by air contaminants or direct fuel (e.g., alcohol and NH3) crossover. In contrast, MNC catalysts demonstrate a high tolerance to contaminants, such as NH3, acetonitrile, and SO2, and are resilient under practical operating conditions. Despite these advantages, MNC catalysts are less intrinsically active and durable than commercial Pt/C under practical PEMFC acidic conditions.5
The coupling of MNC and atomic-metal nanocomposite catalysts to synergistically enhance catalytic activity has been achieved by introducing additional single-metal sites, nanoparticles (NPs), and atomic clusters (ACs) in the vicinity of single-atom active sites.6 Interactions between the different sites in nanocomposite catalysts can increase intrinsic activity (by regulating the d-band center energy and spin state of single-atom active sites) and durability (by stabilizing the active sites against leaching or demetallation).7 Furthermore, low-Pt MNC catalysts feature enhanced PEMFC MEA activity and durability and can therefore meet the technical targets of PEMFCs more effectively than commercial Pt/C catalysts.8
In this review, we provide a brief background on acidic ORR catalysts in practical PEMFCs and then discuss advanced MNC nanocomposite catalysts, which can be classified into dual-atom catalysts, catalysts featuring NPs or ACs combined with MNC, and low-PGM MNC catalysts. The synergistic effects of coupling MNC and nanocomposite sites in practical PEMFC applications are discussed to shed light on the synergistic mechanisms underlying structure–performance relationships and thus deepen our understanding of the potential properties of MNC nanocomposite catalysts. Physical and electrochemical characterization methods used to confirm the origins of these synergistic effects are explained, and the challenges and prospects of developing MNC nanocomposite ORR catalysts for high-performance low-cost PEMFCs are discussed.
2 ORR CATALYSTS: BACKGROUND
2.1 PEMFCs and Acidic ORR Mechanism
PEMFCs convert the chemical energy of hydrogen and oxygen into electrical energy through spontaneous electrochemical reactions, namely the anodic hydrogen oxidation reaction (HOR) and cathodic ORR, both of which require catalysts to reduce their overpotentials (η) and establish efficient reaction pathways. PGMs supported on high-surface-area carbon have been commonly used for this purpose. The use of PGM catalysts, such as Pt NPs and Pt alloys, substantially accelerates the HOR and reduces its kinetic overpotential under the acidic conditions of PEMFCs.9 However, despite the use of PGM-based catalysts, the kinetic overpotential associated with the sluggish ORR remains a major contributor to performance losses.10 For this reason, the related research has focused on developing ORR electrocatalysts and investigating their reaction mechanisms to enhance the kinetics and overall performance of PEMFCs.
As a multi-electron-proton reaction, the ORR features complex elementary steps, which hinders the understanding of the intrinsic ORR mechanism and, hence, the design of PGM-based and PGM-free MNC electrocatalysts. The complete four-electron pathway (Equations 1, 2, and 3) is desirable, whereas the two-electron pathway (Equations 4 and 5) is not, as its final byproduct is the strongly oxidizing H2O2, which can destroy the polymer membrane and oxidize the carbon support to cause cell degradation.11
The ORR properties (pathway, mechanism, activity and selectivity) are determined by the oxygen adsorption (Griffiths, Pauling, or Yeager) model and intermediate interaction/sorption energy or dissociation barrier of the catalyst surface.12 ORR pathways are mainly classified into dissociative (Equation 2) and associative (Equation 3) ones. In the associative pathway, O2 molecules are adsorbed according to the Pauling and Griffiths models to form MOO structures, with subsequent electron and proton addition affording *OOH. The addition of an extra electron and proton results in the formation of H2O + *O or (in the case of the two-electron reaction) H2O2. In the case of the direct four-electron reaction, *O is reduced to *OH by the addition of a proton and electron, and the reduction of *OH by further proton and electron transfer affords H2O as the final product. H2O can also be produced via an indirect four-electron reaction, which involves the two-electron reduction of H2O2. In the case of the dissociative pathway, O2 is adsorbed on the active sites in Yeager mode to form MOOM bridge-on-site structures. Subsequently, the OO bond is broken upon the addition of two protons and electrons to afford 2(M*OH), and the following addition of two protons and electrons affords H2O (O2 + 4H + 4e− → 2H2O). Compared with the associative pathway, the dissociative pathway features rapid one-step four-electron transfer and facilitates the ORR. Traditional close-packed metal surfaces, such as those of Pt and Pt alloy NPs, favor the dissociation of O2 molecules under the action of two neighboring active metal sites and therefore result in high ORR efficiency and rate.12
2.2 Adsorption energy and electronic structures of active sites
The use of appropriate catalytic reaction descriptors allows one to effectively demonstrate activity, durability, and selectivity trends and establish catalyst design guidelines.13 In particular, the energy of oxygen atom and intermediate adsorption on the catalyst surface are used to evaluate catalyst efficiency.
Experimental and density functional theory (DFT) studies have shown that ORR activity is determined by specific energy changes corresponding to the protonation of oxygenated species in each step. The step with the highest increase in energy, forming high energy barriers and overpotentials, is the rate-determining step (RDS), governing the reaction rate in its entirety. To achieve high catalytic activity, one should decrease the energy barrier and overpotential of the RDS.14 The Sabatier principle explains the relationship between catalytic activity, catalytically active sites, and intermediate adsorption energy in heterogeneous catalytic reactions, such as the ORR.15 Overly high adsorption energy can hinder the removal of adsorbed oxygenated species occupying the active sites and thus interfere with subsequent steps, whereas overly low adsorption energy disfavor the adsorption of reactants, such as O2 molecules, and lead to the formation of H2O2 by favoring two-electron reduction over four-electron reduction. The electronic structure descriptors of active sites, such as d-band center energy and spin state, are widely used to explain structure–ORR activity relationships. The variation in the adsorption energy of oxygenated species arises from changes in the electronic structure of catalytic sites due to those in their geometric structure. Therefore, by modifying the environment of catalytically active metal sites (e.g., through strain, ligands, defects, and heteroatom doping), one can alter their charge distribution and spin state and thus enhance activity.16
2.2.1 Charge distribution and d-band center
Considerable progress has been achieved in adjusting the electronic structure of active sites, primarily in d-band center and charge distribution optimization. The d-band theory of catalysis, elucidated by Nørskov et al., forms the foundation for linear scaling relationships and volcano plots, providing valuable insights into electrocatalytic reactions.17 The d-band configuration is directly correlated with the adsorption strength of oxygenated intermediates. When oxygen molecules or oxygenated intermediates interact with metal active sites, the adsorbed oxygenated species form both bonding and antibonding states within the narrow d-band of the metal surface. The proportion of antibonding orbital filling and d-band width/shape of active metal sites determine the extent and direction (upward/downward) of d-band center shifting. Low and high d-band center energy indicates the weak and strong adsorption of oxygenated intermediates, respectively.18 The modification of the local coordination environment of active sites influences the d-band center shift.19 Heteroatom doping, heterojunction formation, alloying, metal–support interactions, and other catalyst design techniques can be used to alter the charge distribution and adsorption properties of electrocatalysts.20
2.2.2 Spin state and eg orbital filling
The spin state or magnetic moment is an important descriptor for rationalizing catalytic activity trends.21 Local spin and magnetic moments arise from the unsaturated bonding on the surface of catalysts and transition metal sites. O2 molecules and oxygenated intermediates have triplet and singlet electronic configurations, respectively. Interactions between metal sites and triplet/singlet oxygenated species with spin-related electron transfer play an important role in determining ORR kinetics.22 Therefore, the spin configurations of active sites influence molecular adsorption/desorption behavior. Owing to the above interactions, the five d-orbitals split to form lower-energy t2g bonding orbitals (dxy, dyz, dxz) and higher-energy eg antibonding orbitals (dx2–y2, dz2). Although metal atoms typically favor low-spin states, their electronic structures can be manipulated to induce intermediate-spin and high-spin states characterized by the presence of one or two unpaired electrons in the eg orbitals. The filling of these orbitals depends on the number of d-electrons and affects the spin state of transition metal cations. Generally, low filling corresponds to the low electron occupancy of antibonding orbitals for a small-molecule model, resulting in excessively strong bonding. Conversely, higher eg filling leads to weaker interactions.23 Numerous strategies for enhancing catalytic activity by manipulating the spin state and electronic configuration of the eg orbitals have been explored. Geometric and electronic structure changes of active sites due to dopants, defects, and surface structures can affect the filling of the metal eg orbitals and thus help achieve optimal intermediate adsorption behavior.
2.3 PGM-based ORR catalysts
Pt-based electrocatalysts, comprising Pt NPs loaded on porous carbon supports with high electrochemically active surface areas, exhibit outstanding stability and superior activity but are not widely used because of the scarcity and high cost of Pt.4, 5, 24 To meet the cost target of $35 kW−1 required for the global commercialization of fuel cell systems, the US Department of Energy (DOE) has set some technical goals for PGM catalysts to be realized in 2025, aiming for a mass activity of 0.44 A mgPGM−1 and total PGM content of <0.1 g kW−1 in PEMFCs, with the recommended electrode loading lying below 0.125 mg cm−2.25 Accordingly, the development of catalysts based on Pt and other PGMs primarily aims to diminish PGM content while boosting the intrinsic activity and durability of ORR active sites. Pt-based catalysts featuring modified morphologies, alloys, and intermetallic structures have been engineered to date. Pt-based alloy and intermetallic catalysts are particularly promising, as they allow one to substantially decrease Pt loading by incorporating other transition metals and thus increase mass activity.26 These catalysts are also intrinsically more active than their Pt counterparts because of the complex metal–Pt interactions. The use of Pt alloys or intermetallic catalysts provides electronic and geometrical synergies and thus enables the alteration of critical reaction descriptors, such as the d-band center energy, adsorption energy, and reaction energy barrier.27 By adjusting the types and contents of transition metals in the employed alloy, one can fine-tune the effects of strain and ligands on Pt active sites. The engineering of lattice mismatch–induced strain helps adjust the adsorption/desorption energy and thus influence catalytic performance. Compressive strain decreases interatomic spacings, broadening the d-band and weakening adsorbate–surface interactions, while tensile strain has the opposite effect. Theoretical research indicates that the strong binding of Pt to oxygenated intermediates makes compressive strain beneficial for enhancing the catalytic performance of Pt active sites.28 The ligand effect occurs at the interfaces between atoms with different electronegativity and d-band centers, facilitating electronic charge transfer.29 Ligand and strain effects are entwined during the alloying of Pt with other transition metals. Despite the favorable performance of Pt alloy and intermetallic catalysts, their stability is compromised by the corrosion of carbon supports and NP dissolution/agglomeration.29 Further research is needed to address these challenges and fully exploit the potential of Pt-based catalysts for practical use in fuel cells and electrochemical devices.
2.4 Atomically dispersed MNC catalysts
The need to reduce the cost and facilitate the large-scale application of PEMFC cathode catalysts has inspired extensive research on PGM-free and inexpensive transition metal–based catalysts.30 Among the various PGM-free alternatives, MNC catalysts have drawn considerable attention because of their promising acidic ORR performance, comprising individual metal-atom active sites within porous nitrogen-doped carbon supports. These catalysts are prepared by high-temperature annealing, which results in the carbonization and nitrogen doping of the organic precursors to form a carbonaceous matrix with embedded metal atoms. Nitrogen atoms act as coordinating ligands for the embedded metal atoms, preventing their aggregation and promoting their atomic-scale dispersion within the carbon matrix. The synthesis method, precursor species, pyrolysis temperature, and metal precursor concentration strongly influence the composition, morphology, and performance of the resulting MNC catalysts.31 PGM-free MNC catalysts offer the benefits of high theoretical metal-atom utilization efficiencies and low production costs. Additionally, the electronic structures of MNC catalysts can be tailored to enhance catalytic activity and selectivity.32 The incorporation of heteroatoms, such as nitrogen, sulfur, or phosphorus, into the local coordination environment of metal active sites can modify their geometric and electronic structures and enhance their activity by optimizing the intermediate adsorption/desorption energy and changing the reaction pathway.33, 34 Among the MNC catalysts, those based on Fe and Co promote the acidic ORR. However, given that CoNC catalysts promote the undesired two-electron reduction because of weak oxygen adsorption and therefore suffer from the generation of H2O2, the related research has extensively focused on FeNC catalysts, which have a relatively low selectivity for H2O2 in the direct four-electron reaction and high ORR activity.35 Various methods for refining the electronic structure of active sites and adjusting the catalyst morphology and pore structure have been used to obtain catalysts suitable for practical PEMFCs.36 Despite the substantial progress in and potential of this field, the development and commercialization of atomically dispersed FeNC catalysts for PEMFCs remain challenging. For example, although these catalysts demonstrate performance comparable with that of commercial Pt/C in half-cell tests, their intrinsic activity remains lower than that of Pt/C. Additionally, FeNx sites have been synthesized using low metal loadings to prevent the formation of agglomerated compounds, such as metals and oxides, which can affect the electronic structure of active sites. The low intrinsic activity and metal loading of FeNC catalysts necessitate the use of substantial catalyst loadings (>2 mg cm−2) to achieve high performance in practical PEMFC MEAs, which results in higher mass transport resistance and lower fuel cell performance compared with those observed for Pt/C.37 Consequently, efforts are being made not only to enhance intrinsic activity by controlling the local coordination environment of active sites but also to increase the number of active sites participating in the reaction by developing synthesis methods compatible with high loadings (>5 wt%) and adjusting the pore structure to enhance the accessibility of active sites.38 Moreover, FeNC catalysts suffer from low durability and rapid degradation in acidic environments because of carbon corrosion, demetallation, and nitrogen protonation.39 The Fenton-like reaction between Fe3+ active sites and H2O2, a byproduct of the two-electron ORR pathway, generates reactive oxygen species (ROS), such as OH˙, leading to the marked corrosion and oxidation of the carbon support and thus inducing carbon framework disintegration, pore structure alteration, and active site agglomeration/loss. Carbon support corrosion not only increases mass transport resistance but also reduces electron conductivity. Moreover, the collapse of the carbon support around the FeNx active sites indirectly facilitates their demetallation. Under acidic ORR conditions, the coordinating nitrogen atoms of the FeNx active sites are protonated to form NH bonds. This protonation favors FeN bond cleavage, leading to the detachment of the metal center from the nitrogen coordination sites and subsequent leaching during the ORR.40 The durability of FeNC catalysts has been increased by developing various forms of FeNx active sites with geometric and electronic structure adjustments and thus promoting the four-electron ORR and suppressing H2O2 generation. Additionally, the introduction of radical scavengers can be used to eliminate ROS.41
3 MNC NANOCOMPOSITE CATALYSTS WITH ENHANCED ACIDIC ORR PERFORMANCE
3.1 Classification of MNC nanocomposite catalysts
The interactions between single-atom active sites and diverse compounds (single atoms, ACs, NPs) in MNC nanocomposite catalysts exert synergistic effects on various electrochemical reactions.7 Recently, various MNC nanocomposites have been investigated and tested in the cathodes of PEMFC MEAs, demonstrating high performance (Tables 1 and 2). Based on the configurations of their metal atoms, MNC nanocomposite catalysts can be grouped into three categories. The first category encompasses dual-atom catalysts (DACs, M1M2NC), which feature two types of single-atom metal active sites and exhibit two active site arrangements (separated individual metal sites without bonding and bonded configurations defined by the bonding mode between the two metals (MM)). DACs exhibit apparent synergy through the interatomic modulation of the geometric and electronic structures of their single-atom active sites. The intermediate adsorption energy and reactant binding modes of the active sites can be optimized by modulating the electronic structure of DACs to favor the desired reaction pathway. Current research on DACs focuses on the use of ternary and high-entropy single-atom strategies to increase the number of active sites by incorporating additional metal species alongside dual-metal active sites.58 Furthermore, this approach enables the fine-tuning of catalyst performance through heteroatom doping in the vicinity of active sites to enhance intrinsic activity and durability.59
| Catalyst | Half-cell test | H2O2 PEMFC | H2air PEMFC | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Catalyst loading (mg cm−2) | Half-wave potential E1/2 (V) | Catalyst loading (mg cm−2) | Backpressure | Maximum peak power density (W cm−2) | Current [email protected] ViR-free (mA cm−2) | Catalyst loading (mg cm−2) | Backpressure | Maximum peak power density (W cm−2) | ||
| FeMnN-C | - | 0.79 | 2.0 | 2.5 bar | 1.048 | - | - | - | - | 42 |
| FeSACuSA/NC | 0.6 | 0.86 | 1.0 | 2.0 bar | 0.912 | - | - | - | - | 43 |
| Spa-SFe, Co/NC | 0.635 | 0.846 | 2.5 | 2.0 bar | 0.663 | - | 2.5 | 2.0 bar | 0.299 | 44 |
| Fe/ZrNC | 0.8 | 0.872 | 2.0 | 1.5 bar | 1.08 | 4 | 2.0 | 2.0 bar | 0.72 | 45 |
| FeRuNC | 0.8 | 0.86 | 4.0 | 1.0 bar | 0.7 | 165 (0.75 V) | - | - | - | 46 |
| D(CoNP/CoSANC) | 0.8 | 0.83 | 4.0 | 1.0 bar | 1.060 | 105.3 (0.8 V) | 4.0 | 1.0 bar | 0.454 | 47 |
| FeSA/AC@NC | 0.5 | 0.8 | 4.0 | 1.0 bar | 0.8 | 20 | - | - | - | 48 |
| Fen@FeNpyrrC | 0.1 | 0.81 | 4.0 | 2.0 bar | 0.804 | 41.6 | 4.0 | 2.0 bar | 0.33 | 49 |
| FeSA/FeAC2DNPC | 0.4 | 0.81 | 1.5 | 1.0 bar | 0.8 | 15 | 1.5 | 1.0 bar | 0.34 | 50 |
| FesaCunc/NC | 0.6 | 0.83 | 3.0 | 2.0 bar | 0.971 | - | - | - | - | 51 |
| Co4/Fe1@NC | 0.76 | 0.835 | 3.0 | 2.0 bar | 0.840 | 33.4 | - | - | - | 52 |
| FeNC/PdNC | 0.6 | 0.87 | 3.0 | 20 psi | 0.920 | - | - | - | - | 53 |
- Abbreviations: ORR, oxygen reduction reaction; PEMFCs, proton exchange membrane fuel cells.
| Catalyst (Pt wt%) | H2O2 PEMFC | PEMFC MEA durability | Ref. | |||
|---|---|---|---|---|---|---|
| Cathode Pt loading (mg cm−2) | Backpressure | Maximum peak power density (W cm−2) | Mass activity @0.9 V iR-free (A mgPt−1) | |||
| PtFe/FeNC (10 wt%) | 0.05 | 1.5 bar | 2.49 | 1.87 | 8 mV voltage loss at 0.80 A cm−2 and no ECSA loss after 60 k cycles | 54 |
| Pt1.Ni1-x/Ni-NC (8 wt%) | 0.05 | 1 bar | 1.52 | 0.7 | 26.3% mass activity loss after 30 k cycles | 54 |
| PtA@FeSANC (13.1 wt%) | 0.13 | 1 bar | 1.13 | 0.45 | 24.4% mass activity loss after 10 k cycles | 54 |
| Pt@FeNOMC-2 (12.95 wt%) | 0.1 | 1 bar | 1.05 | 0.25 | - | 54 |
| LP@PF1 (2.72 wt%) | 0.033 | 1 bar | 1.05 | 1.08 | 36% mass activity loss after 30 k cycles | 55 |
| LP@PF2 (2.81 wt%) | 0.035 | 1 bar | 1.2 | 1.77 | 85% mass activity loss after 30 k cycles | |
| PtFeNC (1.7 wt%) | 0.015 | 1 bar | 1.08 | 0.77 | 3% mass activity loss after 100 k cycles | 56 |
| PtFeFeNC (0.64 wt%) | 0.012 | 1 bar | 0.87 | 1.5 | 12.5% power density loss after 30 k cycles | 57 |
| PtNPMnSA/C (4 wt%) | 0.06 | 20 psig | 1.214 | 1.77 | - | 8 |
| Catalyst | H2air PEMFC | PEMFC MEA durability | ||||
|---|---|---|---|---|---|---|
| Cathode Pt loading (mg cm−2) | Backpressure | Maximum peak power density (W cm−2) | Mass activity @0.9 V iR-free (A mgPt−1) | |||
| Pt/FeN4C | 0.1 | 150 kPa | 0.9 | 0.45 | 20% mass activity decay after 30 k cycles | 54 |
| Pt3Co/FeN4C | 0.1 | 150 kPa | 0.95 | 0.72 | 38% mass activity decay after 30 k cycles | |
| Pt@MnSANC | 0.1 | 150 kPa | 1.2 | 0.63 | 22.2% mass activity decay after 30 k cycles | 8 |
| L12Pt3Co@MnSANC | 0.1 | 150 kPa | 1.58 | 0.91 | 50% mass activity decay after 30 k cycles | |
- Abbreviations: MEA, membrane electrode assembly; ORR, oxygen reduction reaction; PEMFCs, polymer electrolyte membrane fuel cells.
The second category of MNC nanocomposite catalysts are those combining single-atom active sites in MNC with ACs and NPs. The strong electronic coupling between single atoms and other nanocomposite sites enhances the ORR kinetics by lowering the energy barrier for the reduction of oxygen molecules and oxygenated intermediates to afford an efficient reaction pathway.60
Additionally, the synergistic effects of coupling can extend beyond catalytic activity. By adjusting the size of NPs and ACs, as well as the metal type and distance from single atoms, one can regulate the interaction between the single atoms and NPs/ACs. Moreover, the charge distribution alterations due to this interaction can impact the length and strength of MN bonds in MNx active sites to inhibit demetallation, a major contributor to the low durability of MNC catalysts.61 Furthermore, the coupling of MNC catalysts with metal compounds capable of removing ROS, which can be generated via the incomplete MNC-promoted two-electron reduction and Fenton-like reactions, may prevent catalyst performance degradation resulting from carbon oxidation and demetallation.62
As mentioned above, the scarcity and high cost of Pt hinder the commercialization of PEMFCs. Although atomically dispersed MNC catalysts are promising alternatives to Pt-based ones for the acidic ORR, the former are inferior to the latter in terms of activity and stability under practical PEMFC conditions. The drawbacks of both MNC and Pt-based catalysts can be addressed through the fabrication of MNC-supported Pt-based electrocatalysts. Thus, MNC-supported low-Pt catalysts are the third category of MNC nanocomposite catalysts. Similar to NP/AC-containing MNC nanocomposite catalysts, MNC-supported low-Pt catalysts prevent the dissolution of Pt and demetallation of single-atom sites through the strong electron–metal support interaction involving Pt-based catalysts and MNx single-atom sites in MNC supports. Furthermore, this interaction enables a more delicate tuning of the adsorption energy of oxygenated species and intrinsic ORR activity of Pt and/or MNx active sites. Both Pt-based catalysts and MNx active sites demonstrate ORR activity under acidic conditions, which suggests that ORR performance can be enhanced by increasing the number of active sites and coupling nanocomposite and electronic structure modification under practical PEMFC conditions even at very low Pt loadings.54 The design and synthesis of MNC nanocomposite catalysts, which exhibit high ORR performances while featuring low PGM contents, is expected to help meet the DOE's 2025 technical targets for the global commercialization of PEMFCs.
3.2 Dual-atom active sites with MNC catalysts
DACs can potentially overcome the limitations of single-atom catalysts (SACs) and are characterized by the presence of two adjacent metal atoms as dispersed active sites, thus offering potential for new catalytic mechanisms and enhanced structural tunability compared with SACs.63
3.2.1 Bonded DACs
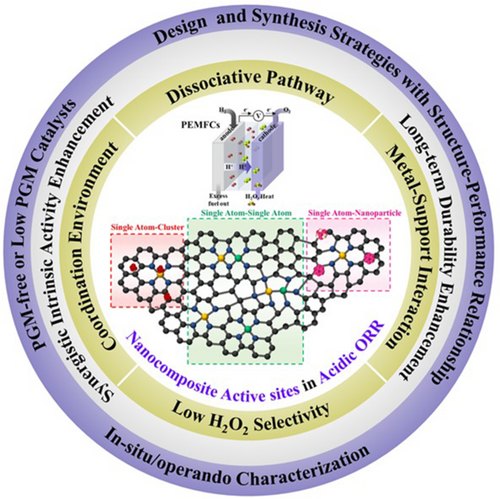
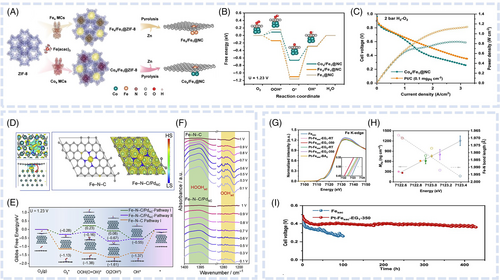
Bonded DACs feature a catalyst matrix comprising two distinct single-atom metal active sites chemically bonded to each other, enabling the creation of metal–metal interactions exhibiting electronic structure coupling effects. Huang et al. synthesized a dual-site FeMn catalyst featuring FeN4MnN3 moieties, showing that the formation of FeMn bonds induced a strain effect substantially optimizing the FeMnNC electronic structure.42 The electronic structure change of Fe induced by the incorporation of MnN3 weakened the adsorption of oxygenated intermediates and thus facilitated the desorption of *OH. Moreover, the FeMn dual site cooperatively adsorbed *OOH to almost entirely suppress the two-electron ORR and achieve a low yield of H2O2 (Figure 1A,B). Considering the excellent ORR activity of FeMnNC and its low H2O2 yield, excellent performance was confirmed at the MEA level. Under H2O2 conditions, the power peak density of an FeMnNC-containing PEMFC reached 1.048 W cm−2 at 2.9 A cm−2, and the current density at 0.65 V reached 0.95 A cm−2. Zhu et al. prepared FeMo sites coordinated by a nitrogen-doped carbon support (FeMoNC) using a general host–guest strategy.64 Forming the FeMoN6 active moiety induced a downward shift in the d-orbital center of Fe. Two different adjacent active site configurations resulted in different reaction mechanisms for the bridge-cis adsorption of oxygen molecules. This adsorption induced the elongation of the OO bond and promoted its cleavage, thus considerably increasing the ORR efficiency (Figure 1C). As the adsorption method changed, the OO bond of the FeMoN6OH site became more stretched than that of the FeN4 site, which favored the *OOH + H + e− → *O + H2O step (Figure 1D). As a result, the achieved ORR activity was comparable with that of commercial Pt/C catalysts in both half-cell and PEMFC MEA tests.
3.2.2 Nonbonded DACs
In comparison to bonded DACs, nonbonded ones feature distinct single-atom metal sites randomly distributed on the support and not bonded to each other. Zhang et al. prepared ORR electrocatalysts with atomically dispersed Fe and Cu species on porous nitrogen-doped polyhedral carbon by combining post-adsorption and two-step pyrolysis methods.43 This structure was characterized by the co-anchoring of atomically distributed FeN4 and CuN4 sites on the NC framework (FeSAN4 & CuSAN4). The FeSAN4 & CuSAN4 dual-atom geometric structure affected the electronic configuration, which resulted in the electron redistribution of Fe sites in FeSACuSA/NC (Figure 1E). The increased electron accumulation on the central Fe atom of FeSACuSA/NC weakened its ability to adsorb ORR intermediates. This enhanced activity notably lowered the energy barrier of the final electron-transfer step of *OH protonation during the ORR and improved the catalytic performance of FeSACuSA/NC. Compared with the single-atom FeSA/NC and CuSA/NC catalysts and the benchmark Pt/C catalyst, the FeSACuSA/NC catalyst exhibited excellent ORR performance, featuring a half-wave potential of 0.86 V in 0.1 M HClO4 and PEMFCs. The peak power density of an FeSACuSA/NC-based MEA under a backpressure of 2.0 bar reached 0.912 W cm−2, approaching that of a commercial Pt/C-based MEA (1.010 W cm−2) under identical conditions (Figure 1F). Yang et al. synthesized a double metal atom–dispersed Fe, Mn/NC electrocatalyst by incorporating MnN sites into an FeNC framework via prepolymerization and pyrolysis.65 The dual-site dispersion in the N-doped defective carbon originated from the adsorption of Mn salts and their interaction with adjacent FeN4 centers (Figure 1G). The introduction of MnN moieties and high electron affinity of Mn(III) altered the electronic structure of Fe(III), inducing a low-spin (t2g5eg0) to intermediate-spin (t2g4eg1) change (Figure 1H,I) and thus facilitating the penetration of oxygen into the antibonding π-orbital. The results of DFT calculations showed that the bond length and bond energy interactions with oxygen in Fe, Mn/NC were more optimized than those in FeNC and MnNC, which reduced the dissociation energy barrier. Moreover, these interactions resulted in the efficient capture of oxygen-containing intermediates and rapid MOH bond cleavage, facilitating the regeneration of *O and *OH (Figure 1J,K). This process effectively suppressed peroxide production, contributing to excellent ORR activity and durability. It has been reported that nonbonded DACs with Fe and secondary metals, such as Cu,67 Co,44 Sn,68 Zr,45 and Ru,46 can induce charge distribution and spin state modulation.
In addition to adjusting the electronic structure of the active site, the dual-atom strategy can exhibit various synergistic effects. ROS not only impact the oxidation of carbon and indirect demetallation of MNC catalysts but also contribute to polymer membrane degradation.39 To address this ROS-related durability issue, Xie et al. introduced TaTiOx NPs as radical-scavenging promoters on a Ketjenblack substrate.69 The TaO2OH site in these scavengers exhibited a hydroxyl radical (˙OH) affinity and H2O2 adsorption energy higher than those observed for FeNC catalysts. Consequently, during the acidic ORR in PEMFC cathodes, TaTiOx increased the durability of FeNC catalysts by preventing carbon support oxidation through ROS removal. Similarly, Chu et al. reported an Fe, CeNC catalyst with radical-scavenging Ce single-atom sites (Figure 1L)66 that exhibited superior oxygen and H2O2 reduction properties and lower H2O2 selectivity than the related FeNC catalyst (Figure 1M). The former catalyst featured a peak power density loss of only 10% after 30 000 V cycles in accelerated stability tests (ASTs) under H2–air conditions. The introduction of adjacent Ce single-atom sites not only increased the intrinsic activity of Fe single-atom sites but also protected the catalyst from ˙OH and H2O2.
As an example of a different synergistic effect, Bae et al. synthesized an Fe0.5NCPt dual-atom catalyst by introducing Pt single-atom sites into an FeNC catalyst.70 Although the Fe0.5NCPt catalyst exhibited an ORR activity similar to that of the Fe0.5NC catalyst, it showed marked differences in Fe leaching without discernible Pt dissolution, as determined by online inductively coupled plasma-mass spectrometry (ICP-MS). In PEMFC testing, the Fe0.5NC-Pt catalyst demonstrated excellent stability, maintaining performance after 50 h of operation at 0.5 V, unlike the Fe0.5NC catalyst. This enhanced stability was attributed to the introduction of isolated Pt sites, which induced the stabilization and prevented the demetallation of FeN4 sites.
3.3 Coupling of NPs/ACs with MNC nanocomposite catalysts
3.3.1 NPs/ACs-modified MNC nanocomposite catalysts
The synthesis of MNC catalysts involves high-temperature annealing, which can reduce catalyst performance by inducing the aggregation of metal atoms and formation of inactive clusters and NPs to decrease the density of single-atom active sites.71 However, regarding the electronic structure of single-atom active sites, interactions with ACs or NPs result in synergistic activity and durability enhancement. Therefore, numerous works have focused on designing ORR catalysts by incorporating NPs/ACs without notably impacting the density of single-atom active sites. Despite these efforts, research has been predominantly conducted under limited alkaline conditions because of the instability and dissolution of NPs/ACs (which weakly interact with support materials) under acidic conditions.72 However, recent studies have tried to harness this synergy even under the acidic conditions present in PEMFCs.
NPs–modified MNC nanocomposite catalysts
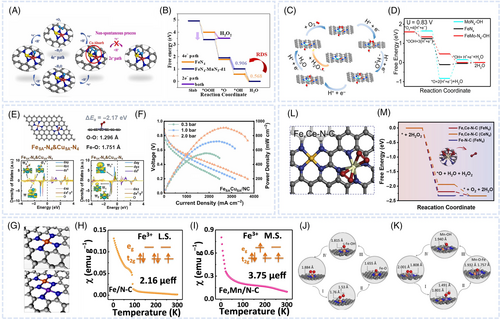
The encapsulation of NPs by MNC catalysts can not only change the geometric structure of the latter, for example, promote the formation of concave surface structures, but also stabilize NPs under acidic conditions. Furthermore, the hybrids of metal compounds and MNC catalysts exhibit reaction mechanisms different from those observed for MNC catalysts alone. Cheng et al. utilized metalorganic gaseous doping based on two-step pyrolysis to create CoNP and single-atom (SA) CoN4 composite sites and effectively increase the total CoN4 site density (Figure 2A).47 The d-(CoNP/CoSANC) composite catalyst, featuring CoNP encapsulated by a CoSANC shell, exhibited a certain degree of local curvature and distortion at the outer layers. Theoretical studies suggested that both the CoNPCoN4 composite and deformed CoN4 sites induced the side-on adsorption of O2 molecules and decreased the energy barrier for the dissociative pathway (Figure 2B,C). Hence, the CoNPCoN4 sites inhibited H2O2 formation and efficiently promoted the direct four-electron pathway to improve ORR activity and catalyst durability. As a result, d-(CoNP/CoSANC) exhibited an outstanding PEMFC peak power density of 1.207 W cm−2. Pan et al. employed an in-situ doping–adsorption phosphatization strategy to realize a well-defined coupling of atomically dispersed FeN4 and FeP sites in FeSA-Fe2P NPs anchored on N, P codoped carbon frame (NPCF) catalysts (FeSA-Fe2PNP/NPCF).73 DFT calculations further demonstrated that the strongly electron-donating doped P atoms increased the energy of Fe 3d orbitals and facilitated O2 adsorption. The reaction mechanism in acidic media was investigated through x-ray absorption spectroscopy (XAS), in-situ Raman spectroscopy, and attenuated total reflection surface-enhanced infrared absorption spectroscopy (ATR-SEIRAS). The FeP site promoted the formation of a PFeOH* intermediate, the OH group of which could be cleaved to form spillover H species that combined with an N4FeOH* intermediate, leading to rapid ORR kinetics (Figure 2D).
Radical-scavenging NPs–modified MNC nanocomposite catalysts
When radical scavengers are not within the diffusion distance, catalyst surface oxidation may occur before they are completely consumed because of the high reactivity of ˙OH and H2O2. Hence, radical scavenging sites adjacent to Fe single atoms are necessary for instant ROS scavenging. Cheng et al. confirmed the effectiveness of CeO2 NPs anchored in locations adjacent to FeN4 sites (FeNC/Scaad-CeO2) for scavenging ROS and chemically decomposing H2O2 (Figure 2E).74 The strategy of instant radical elimination through the adjacency of the CeO2 NPs to the FeN4 sites reduced the survival time of radicals and localized the area of radical-induced damage. Compared with that prepared with FeNC, the fuel cell prepared with FeNC/Scaad-CeO2 exhibited a smaller power density decay after 30 000 cycles, as determined by the US DOE–relevant AST (69% vs. 28%, respectively) (Figure 2F,G). Luo et al. designed a similar catalyst structure combining radical-scavenging NPs and single-atom sites.75 The authors developed a sol–gel method to synthesize a new aerogel with a TiN-interpenetrated FeNC structure for the ORR in fuel cells. The cell with FeNC/TiN exhibited a peak power density of 0.88 W cm−2 and voltage loss of 17 mV at 0.80 A m−2 after 10 000 V cycles during AST. This high performance was attributed to the high radical-scavenging efficiency of TiN and its strong adsorptive interaction with H2O2 and free radicals.
ACs–modified MNC nanocomposite catalysts
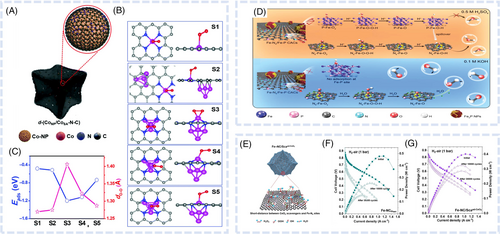
Coupling with adjacent acid-stable small ACs modulates the electronic structure of single-atom active sites. Chen et al. synthesized a structure featuring Fe single atoms with adjacent Fe-atom clusters by annealing a mixture of Fe-doped zeolitic imidazolate frameworks (ZIFs) and graphitic carbon nitride (gC3N4) at a high temperature (900°C).48 Few-Fe-atom clusters markedly impacted the spin state of the adjacent Fe single-atom sites and enhanced ORR activity and fuel cell performance. Specifically, the cluster-free FeSA@NC catalyst primarily featured a high-spin eg2t2g3 Fe(III) configuration with a relatively low splitting energy. However, the introduction of Fe ACs increased the splitting energy and decreased the electron filling in the eg orbital of the Fe single-atom sites in FeSA/AC@NC, resulting in electron rearrangement and an eg1t2g5 Fe(II) configuration (Figure 3A,B). This modification of the single-Fe-atom spin state resulted in an optimized energy of oxygenated intermediate sorption. The MEA fabricated with FeSA/AC@NC exhibited a peak power density of 0.80 W cm−2 under H2O2 conditions. Xue et al. developed a novel approach involving the construction of Fen ACs and satellite single-atom FeN4 active sites.49 The high activity of FeN4 (S1) sites was achieved through an α-Fe2O3 atomization process assisted by the pyrrolic N of polyvinylpyrrolidone. The Fen clusters effectively stabilized the FeN4 (S1) active center, preventing oxidation and inhibiting the protonation of N atoms in the FeN4 moiety (Figure 3C). This process alleviated the Fenton reaction and demetallation, resulting in enhanced catalyst durability and activity. The H2O2 fuel cell with Fen@FeNpyrrC delivered a maximum power density of 0.80 W cm−2 and demonstrated a higher durability during 10 000 V cycles than the cell with FeNpyrrC. Wan et al. fabricated a similarly structured FeNC catalyst with Fe clusters or NPs around FeN4 active sites for the ORR in PEMFCs, demonstrating that the ORR activity of these (FeSA/FeAC2DNPC) catalysts was superior to that of other FeNC catalysts (Figure 3D).50 In particular, the FeSA/FeAC2DNPC catalysts were characterized by an enhanced intrinsic activity of Fe single-atom active sites and long-term durability. According to DFT calculations, the Fe clusters induced the formation of OH ligands at the FeN4 sites; hence, the electronic structure modulation of FeN4 active sites decreased the ORR energy barrier and increased intrinsic activity (Figure 3E,F). A PEMFC MEA with FeSA/FeAC2DNPC showed a high maximum peak power density of 0.8 W cm−2 under H2O2 conditions (Figure 3G). Furthermore, a decay of only 0.11% after a 150 h chronoamperometry (0.5 V) stability test was observed for FeSA/FeAC2DNPC, whereas a higher value of 50% was observed for FeSA-2DNPC after 100 h (Figure 3H). Fe clusters exerted a pinning effect and promoted incoherent vibrations between FeAC and FeN4, which led to FeN bond shortening and thereby suppressed demetallation and enabled long-term stability.
Heteronuclear NPs/ACs–modified MNC nanocomposite catalysts
The hybridization of two different metal-based ACs with MNC nanocomposites can result in more pronounced synergistic effects and different electronic structures of single-atom sites than the introduction of homonuclear ACs. Liang et al. introduced ultrasmall copper nanoclusters into porous N-doped carbon containing isolated FeN4 sites (FeSACuNC/NC).51 The FeSACuNC/NC nanocomposite was synthesized using a two-step vacuum gas diffusion method. Initially, Fe(acac)3 was in-situ encapsulated in ZIF-8, and the resulting material (Fe(acac)3@ZIF-8) was subjected to copper precursor (Cu(acac)2) deposition followed by high-temperature annealing. The thus synthesized FeSACuNC/NC composite exhibited a synergistically enhanced intrinsic ORR activity and durability at all tested pH values. According to DFT calculations, when Cu nanoclusters and Fe single atoms were within a certain distance from each other, the former donated electrons to the latter and thus regulated their spin state. The modified electronic structure of Fe single atoms accelerated electron transfer in the RDS of the ORR under acidic, neutral, and alkaline conditions. The FeSACuNC/NC catalyst was concluded to hold promise for practical electrochemical applications, delivering a peak power density of 0.971 W cm−2 in a PEMFC operated under H2O2 conditions. Han et al. fabricated a Co4@/Fe1@NC catalyst through a preconstraining strategy using Co4 molecular clusters and Fe(acac)3-implanted ZIF-8 (Figure 4A).52 This synthesis ensured the controllable preparation of atomically dispersed Co4/Fe1 and Fe4/Fe1 polymetallic centers. The combination of Co4 or Fe4 ACs with Fe SACs substantially optimized the free energy of the oxygen-containing intermediate states of the FeN4 configuration (Figure 4B), leading to improved intrinsic activity and PEMFC performance (peak power density = 0.84 W cm−2) (Figure 4C).
Precious metal NPs/ACs with MNC nanocomposite catalysts
Pt-based catalysts have long been regarded as ideal for the ORR. However, non-Pt precious metals, including Ru, Ir, Rh, Pd, and Au, have electronic structures and d-band center energy different from those of Pt. These differences result in varying adsorption/desorption energy of oxygenated intermediates and inferior ORR activity compared with those of Pt-based catalysts. Similar to transition metal ACs and NPs, precious metal clusters exhibit synergistic effects when coupled with MNC catalysts. Wei et al. synthesized hybrid catalysts comprising single Fe atoms and Pd clusters.53 Although the Pd clusters individually exhibited lower ORR activity than FeNC, the coupling of FeNC with Pd clusters in the FeNC/PdNC hybrid markedly enhanced the ORR activity of Fe active sites, resulting in a half-wave potential of 0.87 V in the half-cell test. This enhancement was attributed to the interaction between Pd clusters and single Fe atoms, which induced a transition from low-spin Fe in FeNC to medium-spin Fe in FeNC/PdNC (Figure 4D). Generally, FeNC catalysts favor the indirect four-electron associative pathway because of the high energy barrier of O2 dissociation. In the medium-spin state induced by the Pd NC introduction, Fe(II) adsorbed O2 in a side-on fashion, thereby favoring the direct four-electron dissociative pathway over the indirect four-electron associative pathway (Figure 4E). The dissociative pathway of FeNC/PdNC was confirmed by the detection of different oxygenated intermediates on the catalyst surface (Figure 4F). Furthermore, the partially occupied dz2 orbitals of single Fe atoms alleviated the strong adsorption of OH*, consequently decreasing the ORR overpotential. The enhanced ORR stability was attributed to the strong metal–support interactions, electronic interactions between Fe and Pd, and low production of H2O2, which helped avoid the agglomeration and demetallation of metal sites. In the PEMFC MEA test, FeNC/PdNC delivered a peak power density of 0.92 W cm−2.
Concerning the relationship between the durability of FeNC catalysts and interactions with precious metal NCs, Gao et al. elucidated the durability enhancement mechanism and monitored Fe dissolution using online ICP-MS and DFT calculations.76 Metal NPs/ACs were shown to act as electron donors (Figure 4G), increasing the electron density at FeN4 sites and therefore strengthening the FeN coordination bonds, inhibiting the electrochemical dissolution of Fe, and enhancing catalyst stability (Figure 4H). This mechanism remained consistent across various metal particle types, forms, and contents. Consequently, a stable particle/cluster-modified Fe-based acidic ORR catalyst capable of reliably operating for up to 430 h in direct methanol fuel cells was identified (Figure 4I).
3.3.2 Low-PGM catalysts with MNC supports
With the increasing commercialization of PEMFCs, research has focused on reducing Pt usage and enhancing the intrinsic activity of Pt-based catalysts. However, decreasing the Pt loading of catalysts increases the mass transport resistance of reactants at low voltages and thus hinders efficient oxygen reduction. This problem can be mitigated by hybridizing PGMs with SACs.77 The utilization of MNC as supports for PGM NPs addresses the individual weaknesses of these materials through various synergistic effects when applied to practical PEMFCs. The MNx sites in MNC supports provide additional ORR active sites and enhance the efficiency of Pt utilization. The strong interaction with MNC supports increases the activity and stability of Pt-based catalysts. Additionally, given that PGMs and single-atom active sites have different energy barriers for each step of the four-electron ORR, an efficient and complete four-electron ORR is achieved through a relay or cascade reaction pathway. Furthermore, this synergistic reaction pathway helps prevent carbon oxidation in the MNC support and polymer membrane degradation by hindering H2O2 generation.
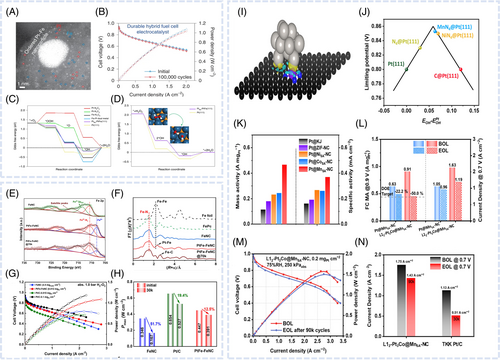
Chong et al. developed a hybrid nanocomposite catalyst with an ultralow Pt loading (2–3 wt%) consisting of PtCo alloy NPs supported on CoNC, achieving a mass activity of 1.77 A mgPt−1 at 0.9 V.55 Similarly, Huang et al. fabricated CoNCsupported hybrid nanocomposite catalysts, revealing that the PtCo@CoNC, NCNT, and rGO hybrid (PtCo@CoNC/NTG) substantially outperformed commercial Pt/C because of its multiple (e.g., PtCo, CoN, and CN) active sites.78 DFT calculations suggested that H2O2 generated at the CoNC sites migrated to the adjacent PtCo sites for the further reduction to water. This synergistic pathway enhanced catalytic efficiency. As a result, PtCo@CoNC/NTG demonstrated a high mass activity (1.52 A mgPt−1 at 0.9 V) and durability (1.3% decay after 30 000 V cycles). Xiao et al. synthesized a hybrid nanocomposite ORR catalyst with an ultralow Pt loading (1.7 wt%) and an FeNC support (Figure 5A).56 This hybrid catalyst, featuring Pt and Fe single-atom sites and PtFe alloy NPs, achieved a mass activity of 0.77 A mgPt−1 at 0.9 V and high durability, retaining 97% of its activity after 100 000 V cycles (Figure 5B). Multiple active sites (PtN1C3, FeN1C3, and PtFe@Pt) induced synergistic ORR activity despite the low Pt loading, as confirmed by DFT simulations (Figure 5C). The enhanced durability of the hybrid catalyst was attributed to the reduced H2O2 formation and resulting mitigation of membrane and ionomer degradation (Figure 5D). Yin et al. prepared FeN4 active sites electronically coupled with PtFe alloys using vapor deposition, thus obtaining a hybrid catalyst with an ultralow Pt loading (0.64 wt%).57 X-ray photoelectron spectroscopy and Fourier-transform extended x-ray absorption fine structure analyses indicated that the oxidation state of Fe in PtFe–FeNC was minimally lower than that in FeNC, and the construction of the PtFe bond suggested strong electronic coupling between the Fe single-atom sites and PtFe alloy (Figure 5E,F). This interaction shifted the Pt d-band center of PtFe NPs, hindering OH adsorption on the Pt surface and enhancing ORR activity. A PtFeFeNC-based PEMFC MEA cathode demonstrated a peak power density of 1.087 W cm−2 at a Pt loading of 0.012 mgPt cm−2 under H2O2 conditions (Figure 5G). After 70 000 potential cycles, the PtFeFeNC catalyst experienced no notable performance degradation (Figure 5H). Zeng et al. explored the synergistic effect between single-atom sites and Pt-based catalysts applied in PEMFCs for heavy-duty vehicles (HDVs), which require high power density and long-term durability.8 The authors experimentally and theoretically demonstrated the effects of coupling between Pt and single metal (Ni, Co, Mn)-rich carbon, ZIF-derived carbon, and Ketjenblack as supports, revealing that the integration of MnSANC and Pt enhanced interactions and weakened OH adsorption (Figure 5I–K). Among the single metal–rich carbons, MnSANC was an ideal support for improving PEMFC activity. Pt and ordered L12Pt3Co NPs on the MnSANC support were designed as cathode ORR catalysts, featuring initial mass activity of 0.63 and 0.91 A mgPt−1, respectively. After 30 000 AST cycles, the mass activity remained higher than the DOE target (0.44 A mgPt−1) under the light duty–vehicle condition (Figure 5L). Additionally, L12Pt3Co@MnSA–NC exhibited a minor power density loss and retained 82% of its current density at 0.7 V after 90 000 AST cycles under HDV conditions (at a higher Pt loading of 0.2 mgPt cm−2 and backpressure of 250 kPaabs) (Figure 5M,N). This catalyst showed minimal particle coarsening after AST, probably because of the strong chemical coupling between the MnSANC support and PGM NPs and the physical hosting effect of the curved surface and porous structure of MnSANC support. Wei et al. fabricated a high-performance ORR catalyst (PtNPMnSA/C) composed of rich PtNPMnSA pairs.79 The interaction between Pt NPs and MnSA enhanced OO bond cleavage and the dissociative ORR pathway, resulting in lower H2O2 production through electronic polarization. The synergistic interactions between Pt and MnSA weakened the PtO interaction, thus accelerating intermediate desorption. PtNPMnSA/C exhibited a high ORR activity, achieving a peak power density of 1.214 W cm−2 in the MEA cell test. Regarding durability, the low H2O2 and radical production, combined with strengthened structural stability, resulted in only a 5% decline in mass activity after 80 000 AST cycles.
4 IN-SITU/OPERANDO CATALYST CHARACTERIZATION
The performance of catalysts primarily depends on active sites, which highlights the importance of accurately identifying these active components and monitoring their changes. Conventional ex-situ characterization techniques used in past studies often expose catalysts to air during experiments, potentially causing irreversible changes in their state and leading to results that may not accurately reflect the true state of the catalyst and risk misinterpretation. Additionally, catalysts undergo dynamic structural changes under actual operating conditions, which can alter the active sites and complicate their precise identification. In-situ characterization techniques allowing one to monitor the structural changes of the catalyst in real time are important for solving this problem. This approach can not only identify the active site, but also provide important directions for evaluating the rationality of the catalyst design and developing high-performance catalysts. In particular, designing a catalyst with abundant active sites is essential for increasing the efficiency of the ORR. However, the specific reactions occurring during catalyst synthesis are not yet fully understood, which often results in complicated synthetic procedures and high costs. Therefore, visually tracking the structural and conformational changes occurring during catalyst synthesis is important for simplifying these procedures and reducing the synthesis time.
4.1 Real-time monitoring of active site dynamics via in-situ/operando XAS
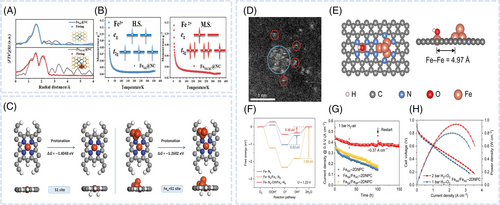
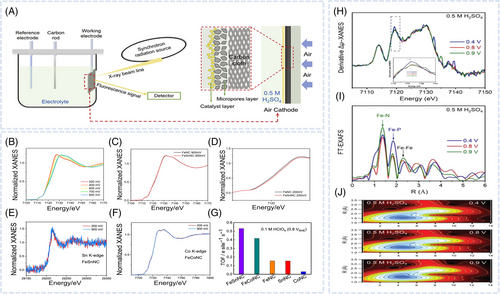
The accurate analysis of the reaction mechanism and changes in the geometric and electronic configurations of the active sites is important for identifying the origin of activity enhancement in nanocomposite catalysts. XAS is a versatile method for studying fuel cell catalysts, measuring the x-ray absorption of a material across a range of photon energy. The obtained spectra comprise two key regions, namely x-ray absorption near edge structure (XANES) and extended x-ray absorption fine structure (EXAFS). Single and multiple scattering approaches are used to analyze oscillations in EXAFS spectra and determine the local geometric structure, including the coordination numbers (CNs) and interatomic distances around the active-site atoms of catalysts.80 In the XANES region, core electrons transition to unoccupied states, which results in strong absorption and edges sensitive to the oxidation state of the probed element. Moreover, the type of ligand or local coordination environment of active sites influences the shape of XANES spectra and position of the absorption edge. During the ORR, the electronic and atomic structures of active sites may change. In-situ XAS enables the monitoring of active-site structural changes in real environments and thus contributes to the understanding of catalytic mechanisms and design of robust electrocatalysts with high activity and optimized selectivity. Generally, operando XAS tests are conducted in fluorescence mode using a three-electrode system where a gas diffusion electrode serves as the cathode, while the air working electrode is composed of a catalyst layer, microporous layer, and carbon paper (Figure 6A).81 Luo et al. investigated the geometric and electronic structures of the active sites and interactions between the two metals in a bimetallic MNC catalyst synthesized by doping a secondary metal (Sn, Co) into FeNC.68 Secondary metal–doped FeNC showed a higher ORR activity than FeNC. Operando XAS analyses of Fe, Co, and Sn K-edges were conducted in N2-saturated electrolytes to investigated structural and reactivity effects. The change in the threshold energy of Fe K-edge XANES spectra in the 0.2–0.9 VRHE region indicated a Fe(III)/Fe(II) redox transition and the structural modification of the FeNx site (Figure 6B). The Fe K-edge XANES spectra of FeSnNC and FeNC showed no difference at 0.9 and 0.6 VRHE (Figure 6C), although a minimal shift to higher energy was observed at 0.4 and 0.2 VRHE (Figure 6D). This indicated that FeSnNC had a higher average Fe oxidation state than FeNC in the low-potential region. The same behavior was observed for Co, suggesting that Fe sites in secondary metal–doped FeNC had a higher oxidation state than those in FeNC. The Sn and Co K-edge XANES spectra showed no dependence on potential, consistent with results obtained for SnNC and CoNC (Figure 6E,F). After additional NH3 treatment, the catalysts exhibited higher ORR activity and Fe K-edge oxidation states than FeSnNC and FeCoNC. This indicated that the SnNx and CoNx sites did not undergo changes in structure and oxidation state within the ORR potential range and implied that changes in the actual oxidation state of Fe were related to the variations in ORR performance. Consequently, the enhanced intrinsic catalytic activity was related to the higher mean oxidation state of FeN4 sites in the bimetallic catalysts (Figure 6G). As reported in another paper, the combination of Fe2P NPs with single-atom FeN4 sites can result in the formation of atomically coupled FeN4/FeP active centers (CACs) and thus facilitate synergistic ORR electrocatalysis.73 This synergistic effect arises from the combined catalytic activity of FeP and FeN bonds, resulting in improved ORR activity, particularly in acidic environments. Operando XAS revealed changes in the electronic and atomic structures of CACs during the ORR in O2-saturated 0.5 M H2SO4 and 0.1 M KOH. In 0.5 M H2SO4, an upward shift in the Fe K-edge was observed upon a potential increase from 0.4 to 0.8 V in the ORR region, indicating an increase in the oxidation state of Fe centers due to O or OH adsorption (Figure 6H). The results of Fourier transform EXAFS spectrum fitting demonstrated that the changes in the FeP bond and CN had a larger contribution to the increased ORR activity than those in the FeN bond with increasing potential. However, in the non-ORR region at 0.9 V, the CN of FeP remained unchanged (Figure 6I,J). In 0.1 M KOH, in the ORR region at 0.6 and 0.9 V, the operando Fe K-edge XANES spectra showed minimal changes, while the corresponding first-derivative Δμ-XANES spectra exhibited a minimal shift to higher energy as the potential increased from 0.6 to 0.9 V. This shift suggested an increase in the oxidation state of Fe, indicating the adsorption of O or OH on the Fe centers. However, the CN of FeP sites remained nearly unchanged, indicating that these sites were not involved in the alkaline ORR. In conclusion, regardless of electrolyte pH, the FeN4 sites in FeN4/FeP CACs act as the primary active centers. Structural analysis via in-situ XAS revealed changes in FeN and FeP bonding and CN. Under acidic conditions, both FeN4 and FeP were involved in the ORR, with FeP facilitating H species spillover to the N4FeOH* intermediate, which indicated a novel reaction mechanism for rapid ORR kinetics (Figure 2D). In contrast, under alkaline conditions, FeP remained unchanged, and only FeN4 experienced an electronic structure change for the optimal adsorption of oxygenated intermediates, which led to high ORR performance.
4.2 In-situ/operando Raman and infrared spectroscopy for ORR mechanism understanding during ORR process
The detection and monitoring of intermediates and products during reactions are crucial for mechanistic studies, particularly for processes involving various oxygenated intermediates, such as the ORR. One of the most effective on-site characterization methods for monitoring oxygenated species is vibrational spectroscopy, including electrochemical Raman and infrared spectroscopy.82 In situ/operando Raman spectroscopy enables the monitoring of real-time changes on catalyst surfaces and identification of intermediates during the ORR.
Wei et al. demonstrated that PdNC incorporation resulted in a reaction pathway different from that observed for pristine FeNC, as revealed by in-situ Raman spectroscopy, leading to improved ORR performance.53 When voltage was applied, OO stretching peaks corresponding to OOH* (around 1260 cm−1) and HOOHad (around 1396 cm−1) appeared in the in-situ ATR-IR spectra of FeNC (Figure 4F). However, in the case of PdNC/FeNC, the intensity of both peaks was markedly reduced, indicating a reduction in the association pathway and the dominance of the dissociation pathway. The dissociation pathway (direct four-electron process) featured a lower OO bond cleavage barrier and faster O2 dissociation than the association pathway (indirect four-electron process), thereby enhancing ORR kinetics. Consequently, the ORR reaction pathway in PdNC/FeNC was altered, which prevented H2O2 formation and improved both activity and durability. Liang et al. determined the RDS in various media using operando Raman spectroscopy.51 In acidic media, a band attributed to OH intermediates (1085 cm−1) was observed for both FeSACuNC/NC and FeSA/NC (Figure 7A,B). However, no Raman signal was detected in neutral and alkaline media, which indicated that oxygen-containing intermediates were not involved in the RDS under these conditions. Consequently, in acidic media, the RDS involved the desorption of *OH (*OH + e− + H+ → H2O + *), while in neutral and alkaline media, the RDS was considered to involve oxygen adsorption and electron transfer (* + O2 + e− → *O2−) (Figure 7C,D).
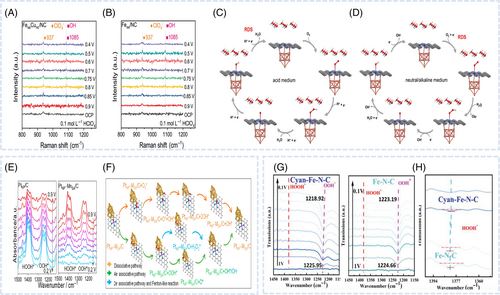
In-situ Fourier transform infrared spectroscopy enables the real-time tracking of groups formed on the catalyst surface during the ORR, with spectral frequency or intensity corresponding to the types or amounts of homogeneous materials, respectively.84 Wei et al. reported that the introduction of MnSA facilitated the construction of PtNPMnSA pairs and provided a dissociative pathway that enhanced OO bond cleavage because of a stronger polarization effect.79 In-situ ATR- Fourier transform infrared spectroscopy was used to analyze the intermediates formed during the ORR, offering more definitive evidence. The intensity of the *OOH peak of PtNP/C markedly increased with the decreasing potential (Figure 7E), which indicated that PtNP/C favored the associative pathway to generate *OOH, inevitably producing H2O2 as a byproduct. In contrast, PtNPMnSA/C showed the opposite trend, which strongly suggested that the associative pathway was suppressed, and the dissociative pathway was more dominant. By assembling the PtNPMnSA pair with an optimal electronic structure, the pathway was switched in PtNPMnSA/C, preventing the formation of H2O2 (Figure 7F).
Compared with other ATR or external reflection techniques, ATR-SEIRAS is more sensitive to the interfacial region. Wang et al. developed a catalyst comprising stable Fe(pyrrolic N)4 active sites with axial Fe4C ACs and investigated the related catalytic mechanism.83 The Fe4C clusters induced the delocalization of the Fe d-orbitals, enhancing the charge distribution toward the N bonds of the Fe(pyrrolic N)4 sites, which not only optimized the activity of the Fe single-atom sites but also stabilized them. In-situ ATR-SEIRAS was used to elucidate the reaction mechanism and oxygenated intermediates formed on cyanFeNC (with Fe4C) and FeNC. The two peaks at 1225 and 1380 cm−1 in the ORR potential region were ascribed to the OO stretching mode of surface-adsorbed superoxide (OOHad) and the OOH bonding mode of surface-adsorbed H2O2 (HOOHad), respectively (Figure 7G). The *OOH peak shift for cyanFeNC exceeded that observed for FeNC. The shift of the *OOH peak suggested a weak interaction with intermediates rather than stable chemical bonds, thereby confirming the associative mechanism. Additionally, the *HOOH peak position and intensity changes indicated a strong interaction between the catalysts and H2O2. The negligible change in the *HOOH peak position and notable change in the peak intensity were observed for both cyanFeNC and FeNC (Figure 7H). The result obtained for the *HOOH peak indicated that Fe-based clusters and particles on the catalyst surface further adsorbed H2O2, which explained the low H2O2 yield.
4.3 In-situ/operando analysis for elucidating the demetallation mechanism of MNC catalysts
The long-term stability of electrocatalysts during electrochemical redox reactions is a major challenge. In the case of MNC-based catalysts, the demetallation of single-atom active sites and corrosion of carbon supports are the principal degradation factors. However, the corresponding degradation mechanisms have not been sufficiently precisely analyzed. ICP-MS can detect elements dissolved in the electrolyte down to ppt (part per trillion, 10−12) levels. Thus, by performing online ICP-MS measurements while altering electrochemical reaction parameters such as time, temperature, and potential one can analyze the demetallation or dissolution behavior of catalysts.
Choi et al. investigated the in-situ demetallation mechanism of FeNC catalysts using online SFC/ICP-MS.85 In a 0.1 M HClO4 solution at room temperature, the real-time ICP-MS signal for the Fe mass released per mass of FeNC catalyst was observed below 0.8 VRHE, with the release rate peaking at 0.4 VRHE, indicating that leaching began at relatively low voltages (Figure 8A). Online DEMS analysis combined with SFC was carried out under chronoamperometric conditions, revealing the cause of the oxidation current. The increasing DEMS signals for CO2 and CO suggested that the substantial demetallation of FeNx sites is due to the corrosion of carbon producing CO2 and leads to substantial activity degradation (Figure 8B). Gao et al. monitored Fe dissolution and confirmed inhibition behavior induced by the interaction between metal-cluster Fe single-atom sites.76 Fe dissolution from FeNC catalysts and FeNC catalysts with hybrid catalyst precious-metal (Au, Pt) clusters was quantified using online ICP-MS at different potentials (Figure 8C). The combined results of online ICP-MS and XANES spectroscopy showed that the amount of Fe dissolution depended on the Fe oxidation state (Figure 4G,H). This trend indicated e higher integrated crystal orbital hamilton population (ICOHP) values strengthen the FeN bond, resulting in reduced Fe dissolution (Figure 8D). Notably, Pt exhibited a greater inhibition potential than Au. The reduction in Fe dissolution due to FeN bond strengthening exhibited a similar trend across various metal types (Figure 8E).
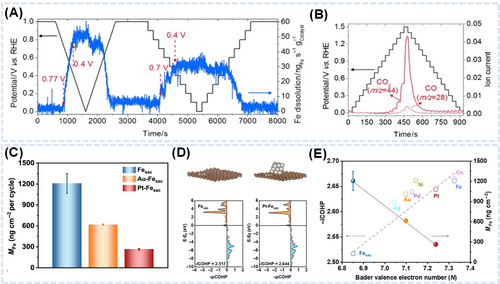
Bae et al. argued that one should consider the presence of Pt single atoms instead of Pt NPs with FeNC and the effects of Pt single-atom sites on the enhanced stability of FeNC catalysts.70 After synthesizing Fe0.5NCPt with PtN4 sites, the authors assessed its stability using an online ICP-MS instrument connected to a gas-diffusion electrode–type electrochemical flow cell at 0.6 VRHE and 353 K under O2 flow. Although its initial ORR activity was close to that of Fe0.5NC, Fe0.5NCPt exhibited a considerably better activity retention during a 2 h potentiostatic hold. The Fe loss of Fe0.5NC–Pt was about three times less than that observed for Fe0.5NC under Ar flow, while no discernible Pt dissolution was observed. The stability diagram of Fe0.5NCPt showed an interrelation of current density (j) loss with a decrease in turnover frequency, suggesting that the enhanced stability resulted from the Pt-induced stabilization of FeN4 sites. This enhanced stability was confirmed in a PEMFC operation test, with Fe0.5NCPt exhibiting high stability during 50 h of operation. The postmortem characterization of the Fe0.5NCPt cathode showed no discernible changes in Pt species distribution, ruling out the formation of highly active Pt nanoclusters or particles. The fundamental origin of enhanced stability was identified as the isolated Pt–induced stabilization of catalytic FeN4 sites against demetallation. This proof-of-concept study validated the various synthetic approaches used to avoid Fe demetallation, a critical degradation path during PEMFC operation.
5 CONCLUSIONS AND FUTURE PERSPECTIVES
- Structure–performance relationships: as the performance of a catalyst is optimized when it has appropriately structured active sites, a comprehensive understanding of catalyst structure–performance relationships is essential. In the case of MNC nanocomposite catalysts, which feature enhanced performances due to the coupling of synergistic effects, one can finely adjust the geometric and electronic structures of active sites through various forms of coupling and metal compositions. However, predicting the appropriate structure among numerous compositions and formations is challenging, as is the synthesis of the desired form of active sites at the atomic level. To address these challenges, one should systematically combine experimental and theoretical approaches to design high-performance electrocatalysts. Additionally, new conventional synthesis methods allowing one to precisely determine the optimal form and finely adjust the structure of active sites are needed.
- PGM-free or low-Pt catalysts: the development of PGM-free or low-Pt catalysts is essential for commercializing PEMFCs and requires sustainable and economical synthesis strategies that can meet the DOE targets for activity and durability, as described in the introduction. A promising approach involves combining ACs/NPs with MNC nanocomposite catalysts. The ACs/NPs offer the advantages of the efficient use of metal resources and high catalytic activity because of their high surface area, while the nanocomposite catalysts provide enhanced durability and stability. This combination achieves both high activity and durability, thereby increasing the commercial viability of PGM-free or low-Pt catalysts.
- Dynamic active site analysis: the ORR mechanism of MNC composite catalysts is still not fully understood, particularly when multiple metal centers and clusters are involved. The complex interaction between the active sites and oxygenated intermediates makes it difficult to control the selectivity between the two- and four-electron pathways. To overcome this challenge and identify the exact role of each component in the reaction, more in-depth mechanistic studies are needed. The identification of electrocatalyst structures and active site evolution during reactions is a matter of high practical significance. In-situ/operando analysis helps track catalyst surface changes and monitor active site reorganization in real time, revealing key performance determinants and facilitating the design of efficient structural characteristics. The integration of various analytical techniques will enhance the understanding of electrocatalyst principles and contribute to the design of innovative catalysts with improved efficiency and stability.
- Overcoming the limitations of in-situ analysis: despite the substantial progress in in-situ studies, challenges remain, such as the development of more realistic in-situ cell designs, improving temporal resolution, and advancing experimental techniques for studying the full lifecycle of fuel cells. Current infrared and Raman analyses do not adequately reflect the actual conditions of fuel cells, necessitating improved sensitivity. Enhancing temporal resolution is crucial for capturing short-lived intermediates during reactions. Moreover, one should develop multiscale in-situ techniques capable of the real-time monitoring of the entire fuel cell, including the catalyst, membrane, and gas diffusion layer. Theoretical methods must also evolve to comprehensively address the interactions among the catalyst, support, solvent, and gas molecules, while the integration of machine learning is essential for efficiently handling large datasets.
- While MNC composite catalysts with various clusters can be synthesized and tested at the lab scale, scaling up their production while maintaining their structure and performance is a formidable challenge. Ensuring the reproducibility of the active site density and distribution across large-scale production batches is essential for the commercial viability of these catalysts. Although MNC catalysts are based on nonprecious metals, the inclusion of various metal clusters and complex synthesis methods can increase production costs. If the processes involved in the fabrication of these advanced materials become overly costly or intricate, their potential for large-scale industrial application diminishes. The challenge lies in developing cost-effective synthesis methods delivering high-performance ORR catalysts.
This research supports the development of efficient and stable electrocatalysts for the acidic ORR with high practical application potential.
AUTHOR CONTRIBUTIONS
Y. Jang and S. Y. Yi contributed equally to the investigation, writing, and editing of the manuscript. Jinwoo Lee supervised the project.
ACKNOWLEDGMENTS
The authors acknowledge financial support through a National Research Foundation of Korea (NRF) grant funded by the Korean government (NRF-2019M3D1A1079306, 2022M3I3A108190213).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Biographies
Yeju Jang is currently a doctoral candidate at the Department of Chemical and Biomolecular Engineering, Korea Advanced Institute of Science and Technology (KAIST), Republic of Korea, under the supervision of Prof. Jinwoo Lee. She received her MS degree from the Department of Chemical and Biomolecular Engineering of KAIST in 2023. Her current research interests focus on the synthesis and application of electrochemical catalysts for fuel cells.
Dr. Seung Yeop Yi received his PhD degree from the Department of Chemical and Biomolecular Engineering at KAIST in 2024 and is currently a postdoctoral researcher at the same department under the supervision of Prof. Jinwoo Lee. His research interests mainly focus on the synthesis of atomically dispersed electrocatalysts and understanding the operation and degradation mechanisms of oxygen reactions in proton exchange membrane fuel cells.
Prof. Jinwoo Lee obtained his BS (1998) and PhD (2003) degrees from the Department of Chemical and Biological Engineering of Seoul National University (SNU), Republic of Korea. After postdoctoral research at SNU (with Prof. Taeghwan Hyeon) and Cornell University (with Prof. Ulrich Wiesner), he joined the Department of Chemical Engineering at POSTECH (2008–2018). In 2018, he joined the Department of Chemical and Biomolecular Engineering at KAIST. Prof. Lee is interested in the synthesis and application of nanofunctional materials for energy conversion and storage devices.