Recommended practice for measurement and evaluation of oxygen evolution reaction electrocatalysis
Abstract
The Oxygen evolution reaction (OER) is a pivotal technology driving next-generation sustainable energy conversion and storage devices. Establishing a robust analytical methodology is paramount to fostering innovation in this field. This review offers a comprehensive discussion on measurement and interpretation, advocating for standardized protocols and best practices to mitigate the myriad factors that complicate analysis. The initial focus is directed toward substrate electrodes and gas bubbles, both significant contributors to reduced reliability and reproducibility. Subsequently, the review focuses on intrinsic activity assessment, identification of electrochemical active sites, and the disentanglement of competing process contributions. These careful methodologies ensure the systematic delivery of insights crucial for assessing OER performance. In conclusion, the review highlights the critical role played by precise measurement techniques and unbiased activity comparison methodologies in propelling advancements in OER catalyst development.
1 INTRODUCTION
The oxygen evolution reaction (OER) serves as a fundamental process in electrochemistry, playing a pivotal role in various energy conversion and storage technologies.1-4 It is essential in water electrolysis and serves as a counter-reaction in processes like CO2 reduction and direct NH3 production, highlighting its importance in advancing sustainable energy solutions.5-9 However, the practical implementation of these technologies hinges upon the precise measurement and interpretation of OER performance. Achieving reliable insights demands not only a nuanced understanding of experimental best practices but also a keen consideration of the myriad factors that influence the electrochemical response.
In this comprehensive review, we will unravel the intricacies of OER experimentation and analysis, focusing on two essential dimensions. Firstly, we will discuss a meticulous examination of best practices for precisely measuring OER, with a laser focus on the impact of substrate electrodes and the complex dynamics of bubble effects during measurement. The selection of substrate (backing) electrode emerges as a cornerstone of OER experimentation, profoundly influencing the behavior of catalyst materials. The choice of substrate introduces a complex interplay of factors, including corrosion resistance, electrical conductivity, and electrochemically inertness. A nuanced understanding of these substrate properties is paramount for ensuring the reliability and reproducibility of OER measurements.
Additionally, we confront the formidable challenge posed by the presence of bubbles at the electrode-electrolyte interface. Indeed, the seemingly innocuous presence of bubbles at the electrode-electrolyte interface can profoundly affect the accuracy and reliability of OER measurements. These bubbles, often formed during electrolysis due to gas evolution reactions, introduce complexities in mass transport dynamics that can distort precise electrochemical measurements. Recent strategies to mitigate bubble effects, ranging from optimizing experimental conditions to leveraging advanced electrode configurations and employing sophisticated sonication methods will be discussed.
Secondly, we explore methodologies for extracting intrinsic values and pinpointing electrochemical active sites in oxide materials, recognizing the indispensable role of robust analysis in unlocking the full potential of OER research. We unravel the complexities of interpreting OER catalyst performance, navigating methodologies for determining electrochemical active sites, evaluating intrinsic activity metrics such as mass and specific activity, and disentangling the contributions of oxygen evolution from competing processes.
Incorporating existing knowledge and emerging trends, our comprehensive review aspires to furnish researchers with a robust resource for conducting and interpreting OER experiments meticulously. Ultimately, it seeks to propel OER research forward and facilitate the development of efficient electrocatalysts for renewable energy applications, thereby contributing to the global pursuit of a greener, more sustainable future.
2 HOW TO MEASURE THE PERFORMANCE OF OER CATALYST
2.1 Substrate (backing electrode) for proper measurement
- Electrochemical inertness: this is the primary requirement for a substrate, as the main focus of the analysis is on the electrochemical features attributed to the active material. For most electrocatalyst or photoelectrode evaluations, the best approach is to choose a substrate that approximates an ideally inert support as closely as possible under the given testing conditions.
- High electrical conductivity: this is an important criterion for a substrate since low electrical conductivity may hinder the true kinetic analysis of active materials.
- Electrochemical and chemical stability: stability is also crucial, especially when the reaction potential window is corrosive. The stability of the substrate affects the electrochemical stability measurement, thereby obstructing the opportunity for stability analysis of active materials.
In general, glassy carbon (GC) is considered the standard substrate (backing electrode) for evaluating electrocatalytic activity due to its high conductivity, electrochemically inertness (only for capacitance), and relative stability within the potential window for reactions (such as hydrogen oxidation/evolution, oxygen reduction, alcohol oxidation, etc.). However, carbon corrosion is significant in the OER region, which hinders its long-term operation as a standard substrate. Therefore, selecting a suitable substrate can be a challenge, as it necessitates the experimentalist to weigh numerous substrate material requirements, with the relevant properties fluctuating depending on the testing parameters. In this chapter, we aim to discuss two aspects: the limitations of carbon-based supports due to carbon corrosion and alternative selections for an appropriate substrate.
During current measurements, the current recorded during polarization is commonly attributed solely to oxygen evolution, with carbon oxidation assumed or shown to be negligible. Therefore, carbon corrosion cannot be directly monitored by electrochemical measurement. As a result, real-time gas analysis has been performed through the direct detection of CO and CO2.
In addition to soluble components, insoluble organic products of carbon oxidation, such as graphite oxides and surface oxygen functional groups, are formed. The structural and chemical changes of the GC electrode due to oxidation were confirmed through various spectroscopic techniques. Yi et al.15 investigated the corrosion behavior of the GC electrode in three different pH environments (acidic, neutral, and alkaline) at 1.8 V (vs. RHE) for 24 h. Several distinct features were observed in the Raman spectrum. Firstly, an increase in the intensity of the G band (at 1620 and 1580 cm−1) was observed after prolonged potential holding under acidic and neutral electrolytes, indicating molecular vibrations in the spectra. Secondly, the intensity at 1500 cm−1, between the D and G bands, originated from amorphous carbon entities, such as organic molecules, fragments, functional groups, and/or defects. Notably, at low pH levels of the electrolyte during carbon oxidation, the band intensity at approximately 1500 cm−1 increased significantly, suggesting that the carbon electrode oxidized under these low pH conditions contained the aforementioned entities. X-ray photoelectron spectroscopy (XPS) studies also supported the Raman results. After potential holding, the oxygen content increased to 20.46 at.% (in acidic conditions) and 19.47 at.% (in alkaline conditions), indicating surface oxidation of carbon. It is worth noting that the oxygen content of pristine GC before the reaction was 9.12 at.%. Further analysis through deconvolution of the carbon 1s XPS spectra revealed that the major component for the oxidized form was CO bond in acidic conditions, while alkaline conditions led to CO (carbonyl) as the dominant species.
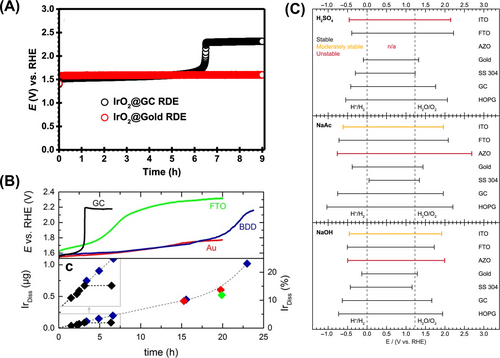
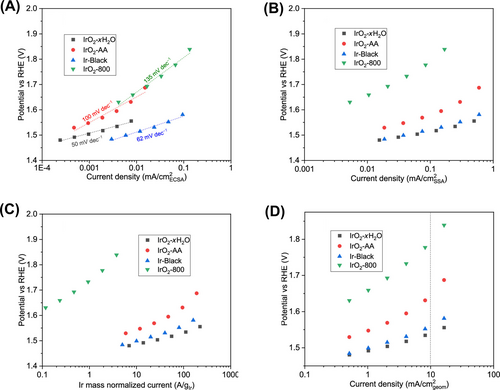
The oxidation of carbon, leading to the production of both soluble and insoluble byproducts, induces changes in conductivity and surface area. Not surprisingly, these factors significantly impact the electrochemical performance of the OER. Khan et al.16 discussed the limitations of the chronopotentiometry test protocol depending on the substrate (Au and GC). The initial activity of IrO2 is identical on both Au and GC substrates. However, during chronopotentiometry at 10 mA cm−2, the effect of the electrode on OER stability becomes apparent. The potential response of the GC electrode remained steady and comparable to that of the Au electrode until 6 h, at which point a sudden rise in potential from 1.66 to 2.13 V (vs. RHE) was observed, followed by a steady state at high potential for the remainder of the experiment (Figure 1A). In contrast, no such jump in electrode potential was observed during the chronopotentiometry experiment using an Au electrode, with only a slight increase in overpotential observed initially. By comparing the chronopotentiometry results, the increase in potential after 6 h (reaching 2.13 V vs. RHE) resembles the conditions observed when bare GC was used as an electrode without IrO2. This suggests that the sudden loss of connectivity between the catalyst layer (including the top layer) and the bulk disk leads to the observed jump in potential during the stability measurement, as all the current is then generated by the corrosion of the GC. Consequently, the electrocatalyst becomes electronically isolated and hence inactive.
Similarly, Edgington et al.14 proposed the oxidation effect of GC electrode as a substrate for OER in acidic media. Through various analyses, the oxidation of GC was confirmed under OER conditions, resulting in the formation of an insulating oxide surface layer. This layer increases in resistance as it grows, eventually leading to significant losses in electrode performance. The decrease in current density and electrochemically active surface area (ECSA) observed during stability testing corresponds with a notable increase in electrode charge transfer resistance, primarily caused by the formation of the oxidized GC surface layer. In essence, the stability of GC-based electrodes during a chronoamperometric stability test does not necessarily reflect the stability of the catalyst material.
The importance of backing electrode for stability analysis was well suggested by Serhiy group, when comparing four different backing electrodes (Au, GC, Boron doped diamond; BDD, and F doped Tin Oxide; FTO) (Figure 1B).17 In the OER activity of commercial Ir-black on these backing electrodes, the initial activity on gold and GC is higher, most likely due to higher contact resistance and low electrical conductivity. Regarding stability, the GC electrode experiences an abrupt increase in overpotential, leading to insulation of the catalyst around 3 h into the experiment. Conversely, lower passivation and a better interconnection of the catalyst and the backing electrode are observed on Au and BDD. However, this does not ensure an ideal backing electrode, as there is still ongoing degradation caused by metal dissolution and insulation.18 In the case of the Au backing electrode, two adverse effects impede the accurate measurement of OER. Firstly, there is a significant increase in Au dissolution below 1.23 V (vs. RHE), likely resulting in surface roughening and destabilization of active materials. Additionally, operating below 1.23 V (vs. RHE) leads to Au redeposition on active materials, further reducing the ECSA, especially during longer accelerated stress tests. The behavior of the BDD electrode resembled that of GC, decreasing the conductivity of BDD and affecting activity. Based on the results of this work, there is no ideal substrate for the OER. Therefore, careful selection of the backing electrode is essential, as deactivation varies depending on materials (such as dissolution/redeposition, corrosion, insulation, etc.).
Ti felt and Ni foam electrodes are widely recognized as the optimal alternative to carbon-based substrates for studying OER processes.19-22 These substrates are particularly valued for their high stability in both acidic and basic conditions and their resistance to highly oxidizing environments. Ti and Ni substrates also offer new avenues for enhancing electrocatalyst activity through catalyst-support interactions. Recent research has demonstrated the significant influence of substrate morphology on the activity and stability of OER catalysts.23, 24 Amano et al. compared the performance of amorphous IrO₂-Ta₂O₅ on Ti plates and Ti felts.25 Their findings indicated that Ti felts, compared to Ti plates, exhibited an increased ECSA and required 0.27 V less overpotential to achieve a current density of 10 mA cm−2. Similarly, Zhang et al. enhanced the activity of Fe₂OF₄ catalysts by leveraging the dynamic surface reconstruction of Ni foam supports.26 Compared to carbon cloth, Ni foam demonstrated superior performance, with an overpotential of 0.238 V at 10 mA cm−2. Moreover, the optimized reconstituted Ni foam electrode maintained stability for 125 h at a high current density of 250 mA cm−2, outperforming conventional carbon cloth in both activity and stability. In Fe-based catalysts, the formation of FeNi-oxyhydroxides is associated with increased activity, driven by the dynamic surface reconstruction of the Ni foam support during the OER process. Recently, Pt-coated titanium (in acidic conditions) and nickel (in alkaline conditions) have been considered the best solutions for applications, although their cost and conductivity remain bottlenecks.
As discussed, the substrate should be chosen based on three criteria: electrochemical inertness, conductivity, and electrochemical stability under each experimental condition. Regarding inertness, following a criterion determined by a threshold current density of 50 μA cm−2 is helpful for selection (Figure 1C).27 However, this criterion does not guarantee electrochemical stability within the potential window (Note: the criterion is based on the inertness of the electrode, not its stability). Regarding conductivity, it is crucial not only for laboratory-based analysis but also for real applications. Therefore, there is a high demand for developing materials with high conductivity and electrochemical stability under reaction conditions. For stability analysis, proper comparison of OER electrocatalyst stability in comparative benchmarking studies is highly necessary. This includes quantitatively monitoring the dissolution rate during reaction conditions and expecting the life-time analysis.5, 18, 28-31
2.2 Bubble issues during oxygen evolution reaction
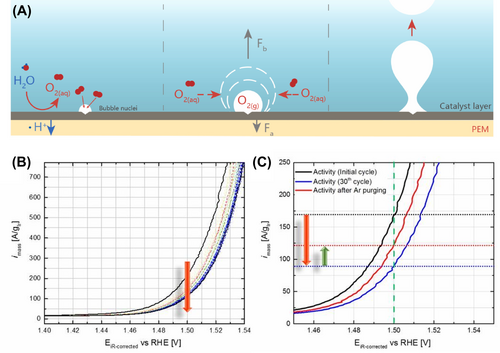
Bubble formation is a common occurrence in electrochemical systems, particularly during water electrolysis processes involving OER and hydrogen evolution reaction.32, 33 The evolution of bubbles at a gas-evolving electrode typically follows a distinct life cycle characterized by four stages: nucleation, growth, detachment, and coalescence (Figure 2A).34-38 Nucleation marks the initial phase, where gas molecules spontaneously form clusters within a solution supersaturated with dissolved gas. These clusters serve as the nuclei for bubble formation. Subsequently, the initial bubble continues to expand as it assimilates additional dissolved gas molecules from the surrounding medium. During the growth phase, the bubble steadily increases in size, driven by the continued influx of gas molecules. As the bubble expands, the buoyant force acting upon it intensifies. Eventually, this force surpasses the adhesion force that binds the bubble to the electrode surface, prompting its ascent and detachment. Coalescence, another pivotal stage, occurs when two bubbles converge and merge, either while attached to the electrode or within the solution itself. This process serves to minimize the system's overall surface energy by reducing the number of individual bubble interfaces.
In essence, this cyclic progression—from nucleation to growth, coalescence, and detachment—dictates the behavior and dynamics of bubbles at gas-evolving electrodes, significantly impacting the accuracy and reliability of performance analysis, particularly in activity measurement and stability assessment. In electrochemical research, accurately determining the activity of catalyst materials is important for evaluating their effectiveness in promoting desired reactions. However, bubble formation at the electrode surface during electrochemical testing can disrupt local mass transport dynamics and increase the effective impedance of the electrode. This interference distorts current density readings, leading to inaccuracies in assessing the true catalytic activity of the material under investigation. Moreover, bubble formation poses challenges to the evaluation of electrocatalyst stability over prolonged operation periods. As bubbles accumulate on the electrode surface, they can induce mechanical stress and promote catalyst detachment or degradation. Additionally, the blockage of active sites by gas bubbles can lead to fouling of the catalyst, resulting in a decline in its catalytic performance over time. Understanding the mechanisms underlying bubble-induced degradation is crucial for predicting the long-term durability of electrochemical devices and ensuring their reliable operation under real-world conditions.
The Gasteiger group investigated the impact of bubbles on OER measurements.39 Figure 2B illustrates the continuous potential cycling for OER measurement over 30 cycles using an Ir/ATO catalyst on an Au disk, operating at 2500 RPM with a scan rate of 10 mV s−1. The observed continuous decrease in OER current during potential cycling could be attributed to either catalyst degradation or the gradual accumulation of oxygen gas bubbles within the pores of the catalyst layer. This accumulation of bubbles may obstruct electrolyte access to a portion of the OER active sites. To differentiate between the effects of catalyst degradation and bubble accumulation, the rotating disk electrode (RDE) setup was purged with argon (Ar) for 30 min immediately after the OER testing (as depicted in Figure 2C). Subsequently, the electrode was switched to open circuit potential (OCP) mode, followed by the acquisition of another polarization curve under an oxygen atmosphere. The experimental design was based on the hypothesis that if the decrease in activity was primarily due to the blocking of active sites by oxygen bubbles, then activity could be recovered through Ar purging. Conversely, if catalyst degradation stemming from processes such as reconstruction, dissolution, and detachment was the dominant factor, activity recovery would not be observed. The results indicate that Ar purging partially restores the activity lost after 30 cycles of OER testing, suggesting that nano- and micro-bubbles within the pores of the catalyst layer contribute to the observed decrease in current during potential cycling. Indeed, the influence of bubbles on electrochemical measurements can vary significantly depending on the experimental conditions, such as the thickness of the electrode and the specific potential holding or cycling protocols employed.40 These factors can affect the formation, behavior, and impact of bubbles on the performance of electrocatalysts. For instance, the thickness of the electrode can influence the rate at which bubbles form and accumulate, as well as their ability to detach from the electrode surface. Thicker electrodes may impede the release of bubbles, leading to increased accumulation and potential blockage of active sites. Consequently, the reliability of activity measurements is compromised, hindering our ability to develop efficient electrocatalysts for applications.
To address these challenges, researchers have developed various strategies to mitigate the adverse effects of bubble formation on performance analysis in electrochemical reactions. One common approach is to use RDE setups with high rotating speeds to facilitate mass transport and enhance the reliability of electrochemical measurements. While this method effectively promotes efficient mixing of the electrolyte and reduces mass transfer limitations, it does not entirely eliminate the influence of bubbles on the electrode surface.41, 42 Despite high rotation rates, bubbles can still form and adhere to the electrode, particularly during gas evolution processes such as the OER. The presence of bubbles can lead to a decrease in the effective electrode area available for catalysis, thereby affecting the accuracy of activity measurements. To effectively identify and remove residual oxygen bubbles, researchers employ a pretreatment technique known as constant reductive potential (CRP) at 0.05 V.43 This potential is low enough to facilitate the reduction of any remaining oxygen through the oxygen reduction reaction (ORR). By subjecting the electrode to a constant potential treatment within the ORR reductive potential range (e.g., 0.05–1.0 V), both visible and invisible bubbles can be eliminated. This elimination occurs because ORR is intentionally triggered at the interface between the electrode and electrolyte, leading to the depletion of oxygen gas within the bubbles and their subsequent removal. As a result, there is a significant difference not only in OER activity but also in capacitance between samples directly measured (without CRP) and those conditioned with CRP. This difference underscores the impact of bubble removal on the accuracy of electrochemical measurements and the importance of employing appropriate pretreatment techniques for reliable analysis.
To enhance bubble removal efficiency, Hartig-Weiss et al.42 proposed the use of a sonication method. They employed a modular cup horn ultrasonicator with adjustable power, positioning it directly into the electrolyte near the iridium electrode (as depicted in Figure 3A). The researchers conducted constant current experiments at a current density of 10 mA cm−2geo, both with and without sonication. It is worth noting that the percentage indicates different levels of sonication power. The results showed that samples with higher rotating speeds (2500 rpm) and ultrasonication started at lower potentials, indicating immediate oxygen bubble accumulation upon the application of constant current (as shown in Figure 3B). It was observed that strong ultrasonication combined with electrode rotation facilitated the removal of microscopic bubbles formed on the Ir-disk surface. However, even with this method, the potential increased within the first 100 s of the experiment before stabilizing, suggesting that the applied sonication was not entirely effective in preventing bubble accumulation. To address this, the sonication power was increased to 30%. As a result, the potential remained constant at a very low value until the end of the experiment. This stability implied that the rate of oxygen bubble formation and removal on the Ir-disk surface reached a steady state without further accumulation of bubbles.
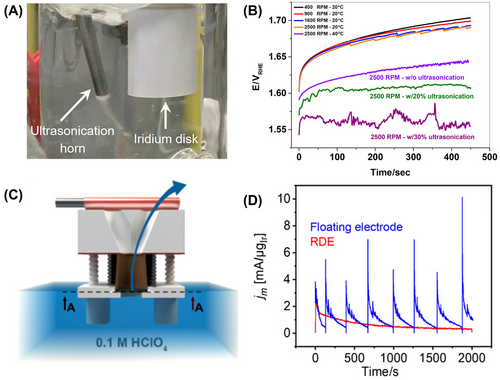
Similarly, Karimi et al.44 proposed a combination of experimental setups to mitigate bubble formation during the OER. Their strategies included altering the orientation of the working electrode, applying external agitation through rotation of the working electrode, and/or utilizing an ultrasonic field. Each method was explored individually as well as in combination. Following experimentation, the researchers pinpointed the optimal conditions that produced promising outcomes. These conditions effectively bolstered both charge- and mass-transfer resistance. Consequently, they achieved a remarkable 164% enhancement in mass activity. In addition to RDE, Jovanovič et al.45 proposed using floating electrodes to mitigate the effects of bubbles during electrochemical measurements. A gas diffusion (porous) electrode was prepared as the working electrode, and the back contact was treated with vacuum suction to remove gas bubbles (as illustrated in Figure 3C). Interestingly, under chronoamperometric conditions, the OER activity of the two electrode types exhibited remarkable differences. Specifically, the mass-normalized current densities were generally higher in the case of the floating electrode (as shown in Figure 3D). This improvement in the utilization of the catalyst layer in the floating electrode was attributed to the continuous removal of gas bubbles, a process absent in the RDE setup. The presence of vacuum suction during electrochemical treatment had a clear effect on the current response. Particularly noteworthy was the enhancement of repetitive current oscillations when the vacuum was applied, while these oscillations ceased in the absence of vacuum suction. These findings highlight the effectiveness of employing floating electrodes to enhance the performance of electrochemical systems by facilitating the continual removal of gas bubbles, thereby improving the utilization of the catalyst layer and ultimately increasing OER activity.
Furthermore, designing electrodes with tailored surface properties, such as nanostructured morphologies or hydrophobic coatings, can promote the efficient release of gas bubbles and prevent their adhesion to the electrode.46-52 Additionally, optimizing electrolyte composition and flow rates can help control bubble formation and improve mass transport kinetics, thereby ensuring more accurate and reproducible performance analysis.53 By implementing these strategies, researchers aim to enhance the reliability and accuracy of performance assessments in electrochemical systems, facilitating the development of more efficient and durable electrocatalysts for a wide range of energy conversion applications.
3 HOW TO EVALUATE THE PERFORMANCE OF OER CATALYSTS
Establishing fundamental criteria for quantitatively evaluating the activity of a specific catalyst is a cornerstone for correctly identifying the optimal catalyst. Typical OER catalysts are loaded onto diverse substrates and studied under versatile operating environments, including electrochemical activation cycling, electrolyte composition, pH, and electrochemical cell configuration.54, 55 These diverse experimental environments role as a major obstacle to comparing the characteristics of developed catalysts. Currently, a protocol for the performance evaluation of catalysts is presented and used as an indicator for OER catalyst development. To clearly compare the performance of various catalysts, it is crucial to establish a clear and comprehensive definition of a common activity concept. In this chapter, we briefly describe how to quantitatively measure OER catalyst performance using electrochemical methods. It is emphasized that extrinsic activity and intrinsic activity, which have often been vaguely misused, must be clearly distinguished and applied according to the purpose of research.
3.1 Evaluation of electrochemical activity
3.1.1 Electrochemical methods for measuring activity
In general, electrochemical protocols for determining the performance index of a catalyst can be divided into two methods: (1) comparing current densities at the same fixed overvoltage, and (2) comparing overvoltage that achieves a constant current density. The comparison of voltage–current characteristics among various catalysts is conducted within restricted, identical voltage and current ranges. This method is preferred in much of the research as it offers an intuitive means to succinctly compare the activities of target catalysts.56-60 However, it is essential to exercise caution when comparing activity at overpotentials and high current densities to mitigate the effects stemming from oxygen bubble. Gas bubble formation can lead to underestimates of activity by blocking the active sites of the catalyst, reducing the active surface area, and in the case of non-conducting gasses it promotes uncertainty by increasing the error in compensated resistance. Electrochemical methods to measure the OER activity of a catalyst include linear sweep voltammetry (LSV), cyclic voltammetry (CV), chronoamperometry (CA), and chronopotentiometry (CP). Although these electrochemical measurement methods show clear differences, they are often overlooked and used interchangeably.
Yang's group reported that when contrasting measurement methods for CV and CP, there is a probability of overestimating activity in CV depending on the specific characteristics of the catalyst.61-63 This is exemplified by comparing the differences in activity measured by CV and CP in ScCOO3−δ and LaCoO3. In SrCoO3−δ, it is observed that CV measurements show a 3-fold increase in activity compared to CP. However, in the case of LaCoO3, the activity overlapped in the two electrochemical measurement profiles. This discrepancy arises because the current contributed by the O2 intercalation effect of the SrCoO3−δ catalyst is involved, leading to higher performance in CV measurements. The results suggest that, depending on the catalyst, activity figures may not present consistent results across different electrochemical methods. Therefore, to enhance the accuracy of activity evaluation, it is crucial to determine of the properties of the catalyst before selecting the electrochemical potential profile to be applied. It is also advisable to measure and report both CV and CA to provide a comprehensive assessment.
Conclusions drawn about the kinetic properties of OER based solely on catalyst voltage–current data involve logical deficiency. To more accurately assess the faradaic kinetic performance of OER, the Tafel slope is often reported within a specific OER potential range.64-66 However, presenting OER activity through the Tafel slope requires consideration of two key perspectives. In the high potential region, not all active sites are engaged due to the generation of O2 bubbles, and the iR compensation problem cannot be clearly addressed due to varying resistance. On the other hand, in the low potential region, the proportion of non-faradaic currents is large. Therefore, careful observation is necessary to establish the potential region representing a linear tendency where kinetic behavior predominates.
3.1.2 Metric for activity comparison and its limitations
- Geometric activity (normalized to the geometric area of the electrode),
- Mass activity (normalized to the loading mass of the catalyst),
- Specific activity (normalized to the electrochemical active site area of the catalyst),
- Turnover frequency (TOF) (number of electrons generated per active site per hour).
- Overpotential required to achieve a certain current density (for example, 10 mA cm−2)
Figure 4A provides an intuitive depiction of the difference between geometric, mass, and specific activity for each activity.72 The factors that determine the activity of an OER catalyst are the area of the active site and the specific activity of each active site. Geometric activity holds industrial significance as both volume and area must be considered during the design process of water electrolysis equipment. In addition, mass activity is significant as a reliable matrix to prove the economic feasibility of the developed catalyst by linking the usage amount and price of the catalyst. Specific activity and TOF are related to the active site for reaction, making them valuable for active site engineering for high activity. Finally, overpotential is also a simple yet valuable metric for the discussion of the activity of a system.
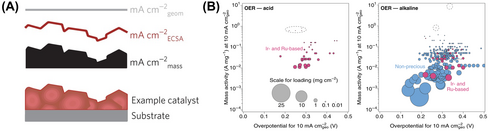
The most careful consideration is whether each value is intrinsic or extrinsic. Based on the concept, researchers conjectured that all values are intrinsic; however, they are significantly affected by the condition of measurement. In a study by Kibsgaard et al.,73 statistical data showed that geometrical activity varies with the loading mass of the catalyst. Figure 4B illustrates two general trends: (1) higher loading results in lower overpotential, and (2) lower loading exhibits higher mass activity. This highlights the significant limitations of using activity metrics to objectively compare the activities of different catalysts. Although mass activity is normalized by the loading mass, it is still influenced by the mass loading. There is an underlying assumption for normalization that all catalysts in the electrode exhibit identical activity (utilization) with respect to loading. However, as the loading increases, the utilization decreases due to mass transport limitations in thick electrodes. In the case of overpotential, researchers often overlook that the observed (measured) overpotential is a material intrinsic property; however, the observed value is affected by the mass loading and surface area.74, 75 Even if it is calibrated by the mass and area, the value is still affected by the measurement conditions. Therefore, normalization does not guarantee that the value is intrinsic.73
Most studies bridge various activity metrics to analyze OER activity based on the experimental environment and the catalyst sample. This approach connects the engineering research for industrial application of highly active OER catalysts with the understanding of their fundamental properties. To objectively compare the activities of different types of catalysts, it is recommended to report both extrinsic and intrinsic activities. Gao et al.59 analyzed OER activity according to ECSA, specific surface area, mass, and geometric area of the electrode to compare the properties of four types of Ir-based catalysts. By comparing geometric activity, they observed different trends in ECSA-normalized specific activity (Figure 5A–D). Additionally, significant differences were observed in mass activity compared to other metrics. The comparative report of the activity results of four catalysts clearly highlights the necessity for multifaceted analysis in evaluating catalyst activity.
To effectively demonstrate the high activity of a synthesized catalyst, it is essential to analyze not only its extrinsic activity but also its intrinsic activity. The study by Lee et al.76 demonstrated the excellent activity of rutile RuO2 nanoparticles by presenting two sets of activity evaluation data. By comparing mass-normalized OER activity with actual surface area, which establishes activity descriptors from volcanic trends and actual device performance, respectively, they highlighted the benefits of OER activity for rutile IrO2, commercial Ir, and Ru nanoparticles supported on carbon. Presenting both activities simultaneously ensures the universality and reliability of the high activity claims for the catalyst. Grimaud et al.77 investigated the OER activity of model catalysts La2LiIrO6 and IrO2 to understand the high activity observed in Ir-based catalysts. They compared the mass activity and specific activity of the two catalysts. While the mass-normalized activity was similar, the specific activity of La2LiIrO6 was found to be 50 times higher than that of commercial IrO2. The study tracked the evolution of pseudocapacitive charge over 50 cycles to analyze the high surface activity of La2LiIrO6. The disparity in activities between the two catalysts provides insight into the OER mechanism, particularly concerning the specific activity of the catalyst.
Ambiguously accepting and indiscriminately suggesting a clear difference between extrinsic activity and intrinsic activity is a root cause of the immaturity of quantitative protocols for activity evaluation. Analyzing the activities of both standards objectively compares the performance of catalysts in various environments, ensures the reliability of the excellent activity of the developed catalyst, and provides important clues for mechanism research to identify factors that determine the unique characteristics of the catalyst. To assess the performance of OER catalysts effectively, careful consideration must be given to analyzing and presenting activity criteria that are appropriate indicators according to the researcher's objectives.
3.2 The importance of electrochemical active surface area and regarding specific activity
In the case of specific activity and TOF, often considered intrinsic activity, careful quantification of ECSA is important. The meticulous determination of ECSA stands as a paramount concern for optimizing both the overall electrode performance and the intrinsic characteristics of catalysts.71 Despite its significance, the dynamic reconstruction of surfaces and the definitive identification of active sites remain elusive, leading to a conspicuous absence of a universal protocol for precise ECSA measurements.78, 79 Many studies are making concerted efforts to improve the accuracy of ECSA measurements through judicious application of well-founded techniques for assessing the surface area of OER catalysts.65 Common ECSA measurement methodologies can be categorized into two main types: electrochemical-based and non-electrochemical-based approaches. This chapter delves into the procedural intricacies of each method, shedding light on their inherent uncertainties, and advocates for electrocatalysis researchers to be mindful of the diverse ambiguities associated with ECSA determination methods. Ultimately, we emphasize the need to diversify appropriate ECSA measurement methods depending on catalyst properties. We suggest that the reliability of ECSA measurements should be strengthened by verifying results using a combination of multiple measurement methods.
3.2.1 Electrochemical measurement to determine ECSA
In general, the most reasonable solution for deriving ECSA is through electrochemical methods.71 These can be divided into two approaches: measurement from electrochemical double-layer capacity, which involves a non-faradaic reaction, and estimation from faradaic reactions (such as CO stripping, Hg UPD (upper potential deposition), metal surface adsorption resulting from surface redox reactions).80, 81 The electrochemical double-layer capacity can be obtained by utilizing scan rate-dependent CV graphs or by measuring the frequency-dependent impedance for the equivalent circuit of the target system and then processing the data.82 The measured electrochemical double-layer capacitance (DLC) is proportional to ECSA, representing the charge capacity of the catalyst surface. Therefore, ECSA can be obtained by dividing by the specific capacitance, as is often reported in typical values. Jaramillo's group proposed a general protocol to measure the surface area of several metal oxide catalysts in a base solution.56 To quantify the electrochemical DLC, they selected a potential range spanning 0.1 V relative to the OCP. This range ensures that faradaic reactions are effectively suppressed, facilitating the accurate measurement of DLC. An essential assumption is made that all non-faradaic currents in this region contribute to the double-layer charge. Afterward, current-potential graphs were created while changing the scan speed of CV. A plot of current versus scan speed appears as a linear relationship, with the slope representing the electrochemical DLC (Cdl) (Figure 6A). Additionally, the Cdl was validated using electrochemical impedance spectroscopy in the same non-faradaic region. In the high-frequency and non-faradaic range, the electrochemical system is approximated by a Randles circuit, as inserted in the Nyquist plot in Figure 6B. It is observed that the two methods have an error of ±15% in Cdl, demonstrating the reliability of the electric layer method for ECSA calculation.
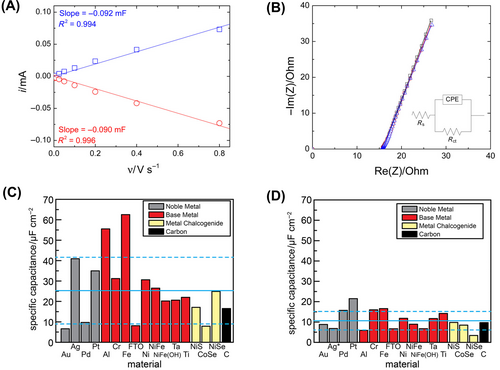
However, general electrochemical measurements have limitations that cannot be universally applied to all types of catalysts. Firstly, these methods assume that the current generated in the limited potential window region, where only non-faradaic reactions occur, fully contributes to the electric double-layer charging.56 In various catalysts, specific adsorption and insertion of electrolyte ions, as well as the generation of additional current due to ion transfer reactions at the interface, must be considered.83-85 When complex non-faradaic reactions occur on the surface, it is challenging to distinguish the Cdl. Surendranath's group proposes DLC measurements in polar aprotic electrolytes as an alternative to alleviate these limitations.86 The influence of the protic electrolyte on DLC measurements was observed by comparing the specific capacitances of 0.15 M NaClO4 aqueous electrolyte and 0.15 M KPF6 in CH3CN electrolyte. Aqueous electrolytes exhibited significant variations in specific capacitance due to differences in surface adsorption equilibrium across the catalysts. In aqueous electrolytes, unlike in polar aprotic electrolytes, the influence of strongly adsorbed OHx species is assumed to contribute additionally (Figure 6C,D). DLC measurement in a polar aprotic electrolyte was confirmed to have the potential to be a powerful method for quantifying ECSA, as it minimizing ion transfer reactions while maximizing the kinetics of electrolyte rearrangement. Overall, DLC measurements in polar aprotic electrolytes yield more uniform specific capacitance values across different catalyst materials.
Similarly, the simple Randles circuit, widely applied in EIS methods, cannot cover the system in all catalysts, especially for overlapping adsorption and pseudo-capacitance (contribution from the bulk). To address this limitation, various equivalent circuit models have been proposed. Watzele et al.87 proposed a new equivalent electric circuit that includes the adsorption capacity generated from oxygen evolution intermediates at low overpotentials. Impedance spectroscopy was used to clearly distinguish the value of Ca (OER adsorbate capacitance) and validated its applicability by investigating various oxide materials. Similarly, Jeon et al.88 used EIS to measure the OER adsorbate capacitance generated by OER intermediates for ECSA measurement of a non-conductive Ni-based hydroxide catalyst in the non-faradaic potential region. The study analyzed the Ca of NiFe LDH and Ni(OH)2 across various electrode potentials, identifying an optimal potential range for precise ECSA evaluation. By excluding high current regions prone to bubble accumulation and potentials where capacitances overlap, a steady-state range was determined. Results from activity measurements at varying NiFe LDH loadings indicated consistency, endorsing the accuracy of ECSA measurements derived from the proposed equivalent circuit.
Secondly, a key limitation in comparing electrochemical surface area quantifications arises from normalizing Cdl to surface area. The aim of analyzing ECSA is to normalize activity to attain intrinsic activity. To derive surface area from Cdl, specific areal capacitance is required. For instance, in calculating the ECSA of Pt, the charge involved in hydrogen underpotential deposition (Hupd) is normalized by a constant,89 typically an average value of 210 mC cm−2 for the charge associated with a hydrogen adsorption/desorption monolayer formed on smooth polycrystalline platinum. This value varies with material and facet, making it challenging to convert the actual value across different materials due to the absence of universally valid constants. Hence, while Cdl serves as a useful metric for double-layer comparison, careful consideration is necessary as it does not directly imply ‘surface area’.
Methods that have been verified for quantitative ECSA measurement utilizing surface redox reactions are based on monolayer surface adsorption, such as Hupd and CO stripping.62 However, these methods are less applicable to metal oxides, which constitute most OER catalysts, and are more sensitive to the electrode history. They primarily target catalyst materials that adsorb H and CO in a single layer, limiting their use to Pt, Ru, and Ir metal catalysts. Determining ECSA poses challenges when dealing with oxide materials due to their limited adsorption capabilities. For instance, in evaluating an iridium based OER catalyst, it is crucial to note that iridium undergoes oxidation typically when the potential exceeds 0.5 ~ 0.6 V (vs. RHE). This phenomenon leads to considerable variability in the OER activation voltage region, often resulting in an underestimation of the ECSA. A proposed strategy to overcome the constrained environment typically associated with hydrogen deposition or CO stripping involves leveraging the redox reactions of surface metals. To utilize the concept of monolayer deposition for ECSA calculation, Zhao et al.90 exploited Zn2+ adsorption to calculate the ECSA of iridium oxide in an operating proton exchange membrane electrolyzer. The Zn2+ solution was adsorbed onto IrOx by electrodeposition, and the amount of zinc ions adsorbed on the metal oxide surface was quantified using UV–Vis spectroscopy. ECSA was then measured based on the correlation between ex-situ pseudocapacitive charge and the ECSA of iridium oxide. Although the experimental correlation showed good linearity, differences depending on loading and material type, as well as discrepancies with the results calculated by BET, imply that the proposed zinc adsorption process is different depending on the type of catalyst. Alia et al.91 proposed a mercury low-potential deposition method as an alternative solution to the challenges faced in applying oxides. They calculate the ECSA of Ir and Ir oxide by integrating the anodic peak at 0.35 V, where mercury layer desorption occurs. Comparing these results with BET values, they confirmed reasonable and comparable ECSA results. Advantages of the mercury low-potential deposition method include its applicability to Ir oxides and the consistency of ECSA results in the OER active region. Despite extensive efforts, resolving inaccuracies in electrochemical-based ECSA methods remains challenging due to dynamic alterations influenced by factors like catalyst morphology and applied potential. Addressing these issues requires ongoing research efforts, highlighting significant tasks anticipated in this field.
3.2.2 Non-electrochemical techniques to determine ECSA
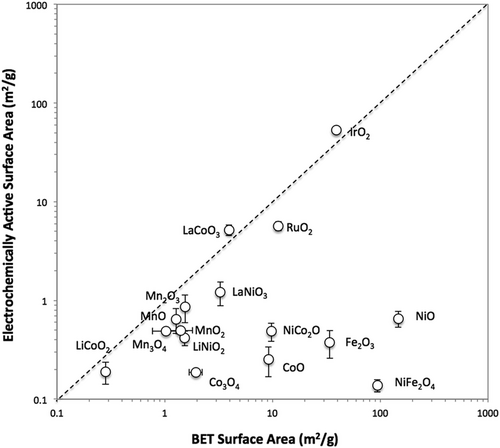
BET (Brunauer–Emmett–Teller) and AFM (atomic force microscopy), as non-electrochemical ECSA measurement techniques, broaden the scope of potential catalysts for evaluation, particularly those inaccessible to electrochemical methods. The BET method is mainly a method of measuring the physical surface area of a powder sample by utilizing the physical adsorption of probe gas molecules on the solid surface.92 AFM, on the other hand, is mainly applied to thin film electrodes with a well-defined surface and low density. AFM images and surface contour results form the basis for calculating ECSA.93 Contrary to the traditional assumption of a flat electrode surface in geometric area calculations, roughness coefficient analysis through AFM confirms that the specific surface area of the film exceeds the cross-sectional area of the electrode. These methods, however, do not distinguish between active and inactive parts of the investigated material and include all surface areas, such as those where gas is adsorbed or visible under a microscope. Consequently, the measured surface area may be overestimated because it includes the contributions from all surface areas rather than only the actual electrochemically active sites. Another drawback is that these methods cannot replicate the catalyst-electrolyte interfacial environment occurring during OER. For instance, BET measurements are taken at the solid–gas interface and cannot be completely identical to the system in which OER occurs. Despite these shortcomings, BET analysis offers a distinct advantage by providing generally reasonable surface area data for both non-metal and metal oxide catalysts, thus serving as a suitable alternative when electrochemical methods may have limited applicability depending on catalyst characteristics. However, it is crucial to note that the values obtained from BET analysis often deviate from those obtained through electrochemical methods. The study conducted by Jung et al.94 presented a comparative analysis between BET and electrochemical ECSA methods concerning crystalline metal oxide OER catalysts.
Additionally, it deliberated on the appropriateness of each system for assessing such catalysts. In the case of BET, a powder-type nitrogen adsorption–desorption isotherm was used. The electrochemical measurement was performed by loading powder-based ink by drop casting onto a GC electrode and measuring CV at varying scan speeds in 1 M NaOH to measure the Cdl. In general, the two surface area measurement methods did not derive similar values (Figure 7). High surface area, small particles of IrO2-(i) and NiO-(i), and the low surface area, large particles of IrO2-(ii) and NiO-(ii) were selected for comparison. The high activity of NiO-(i) is captured in BET, a characteristic not evident in ECSA measurements. On the other hand, for IrO2, the high surface area suggested by both ECSA and BET measurements is consistent. The inaccuracy of ECSA measurements in NiO is likely due to its highly insulating oxide nature. This reflects that the oxide capacitance is smaller than the Cdl for high surface area systems due to dielectric behavior.
To effectively analyze the unique performance of electrocatalysts, we mention the diversification of approaches that can measure the active surface area of catalysts and present their pros and cons to provide the appropriate measurement direction for evaluating OER catalysts. Ultimately, as the suitability of each measurement method for a particular sample is uncertain, we recommend reporting both ECSA and BET surface areas comprehensively. This allows for a full consideration of the possible surface area and range of activity. By utilizing the fundamental disparity between the two surface area measurement methods, researchers can pinpoint and analyze the underlying cause of changes in intrinsic activity.
3.3 The need for selectivity analysis
The current density observed in the OER region comprises the cumulative currents of all redox reactions transpiring at that potential. Voltammetry encounters limitations when attempting to closely analyze the origin of oxidation currents. Imperfections in the data lead to cases where it may be mistaken to attribute the current density in the OER region solely to oxygen production. In the oxidation potential range exceeding 1.23 V, processes marked by anodic currents take place, including the activation or decomposition of both the catalyst and its supporting material. In order to specifically measure only the OER activity of the corresponding catalyst, it is essential to exclude the contributions of other side reactions to the oxidation current. Indeed, careful attention to detail and the adoption of innovative measurement techniques are the solutions to avoid these errors.
We propose two techniques to achieve this objective. The rotating ring disk electrode (RRDE) can distinguish between products by simply adjusting the potential of the ring electrode.65, 95 The Faradaic efficiency of O2 is mainly determined by measuring the reduction current of oxygen by applying the potential of the ORR region to the ring electrode. An electrochemical mass spectrometer (EC-MS) is mainly used for gas product analysis. By measuring gasses generated in real-time within the OER region,61 it becomes feasible not only to verify the potential occurrence of other reactions but also to quantify them, thereby enabling accurate measurement of the Faradaic efficiency of oxygen. This chapter discusses the application of additional measurement techniques, emphasizing that extracting the current density by the OER from the total current in the OER is an essential protocol that must be performed before discussing the activity of the catalyst.
3.3.1 Faradaic efficiency of oxygen using RRDE
The use of RRDE provides valuable insight into distinguishing between catalytic and side reaction currents. Scholz et al.96 investigated the OER activity of smooth epitaxial (100)-oriented La0.6Sr0.4MnO3 (LSMO) films grown on Nb:SrTiO3 (STNO) as a model electrode using the rotating ring disk method (Figure 8A). The CV was run from 1.1 V to 1.75 V, with the ring electrode held constant at 0.4 V to probe O2. Due to insufficient evidence for other reducible species of the catalyst, the onset of OER catalysts was distinguished by utilizing the ring current, which depends only on oxygen concentration. Wang et al.97 by adding gas chromatography data along with RRDE to clearly demonstrate that O2 production current had the largest contribution. OER activity was measured using CoOOH nanorods and nanosheets as models. Furthermore, employing gas chromatography in a cell environment continuously purged with N2 revealed that O2 as the primary product, reaffirming that the oxidation current of the catalyst predominantly contributes to oxygen production. Vos et al.98 used RRDE voltammetry in an iridium-based double perovskite catalyst to separate OER and chlorine evolution reaction (CER) currents, which proceed in parallel. To selectively measure Cl2 generated in the disk, the potential of the Pt ring was fixed at 0.95 V to isolate the CER current contribution. The obtained ring current, directly proportional to the CER rate, was corrected for the ring collection coefficient of chlorine to yield iCER. Excluding this from the current generated by the disk electrode allowed the remaining current to be measured as the full contribution of the OER. By referring to the Pourbaix diagram, the ring current was checked to ensure that there was no interference from reactions other than OER and CER. In addition, serious problems were ruled out by verifying through inductively coupled plasma mass spectrometry (ICP-MS) measurements that no catalytic metal dissolved ions were re-oxidized and precipitated in the ring. This confirmed the absence of additional reduction reactions at 0.95 V.
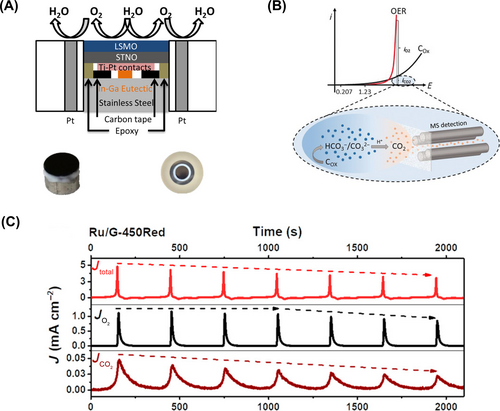
Caution should be exercised when using RRDE due to potential deviations in collection efficiency during gas-evolving reactions. Typically, the collection efficiency (N) of RRDE depends solely on the distance between the disk and ring electrodes and is often quantified using the redox reaction of potassium ferricyanide (K3Fe(CN)6). However, some works suggested that RRDE experiments involving gas formation on the disk may encounter reliability issues, including significant ring collection failure.5, 95, 99, 100 During gas evolution, both nano- and macro-sized bubbles may form, nucleating directly on the electroactive surface. These bubbles can transiently isolate active surface sites or block transport through pores in porous electrodes, leading to irregularities in the current-potential response. The accumulation of bubbles at the interface between the disk and ring strongly affects the N by forming a physical barrier that can decrease N.
3.3.2 Analysis of gaseous products in the OER region using mass spectrometry
Mass spectrometry provides a useful tool to isolate the currents contributing to gaseous and volatile product formation reactions in actual OER reactions. The strength of in-situ analysis not only possible contributes to clearly distinguishing the current due to oxygen evolution, but also provides certain evidence to clearly establish the onset potential of OER through the onset of the O2 signal. Abril et al.101 used an EC-MS to show that catalytic decomposition causes a CO2 burst before oxygen production begins. The early progression of the CO2 signal indicates the potential decomposition of picolinic acid in the catalyst, suggesting that a significant portion of the organic component of the catalyst complex has undergone degradation during the initial oxidation process. However, it should be noted that the current shown by chronoamperometry at 1.9 V is negligible compared to this trace of CO2. Therefore, it can be sufficiently approximated that after the organic portion is momentarily oxidized in the first cycle, the oxidation current contributes to the oxidation reaction of water in subsequent cycles.
Möller et al.11 employed differential electrochemical mass spectrometry (DEMS) to validate that carbon corrosion within carbon-supported catalysts can indeed contribute to anodic current (Figure 8B). For most OER measurements performed on carbon-supported catalysts, it is generally assumed that the current due to carbon oxidation is marginally small. However, for NixB catalyst supported on Vulcan, it was confirmed that O2 generation current is dominant at high current densities. This suggests that corrosion of the carbon support at the onset of OER may influence the oxidation current. Huang et al.102 highlighted the importance of in-situ separation of the measured current into carbon oxidation current, catalytic oxidation current, and OER current to reasonably evaluate OER performance (Figure 8C). For this purpose, a chip-based EC-MS was utilized to monitor changes in O2 products and other gaseous products. The study identified and quantified the contribution of graphene-supported Ru-based catalysts to the OER anode current. Using a chip EC-MS system, it was quantitatively demonstrated that catalytic side reactions are the dominant factors affecting the Faradaic efficiency of oxygen evolution. While MS is tailored for analyzing gaseous products, it is not conducive to elucidating oxidation currents arising from liquid-phase reactions or reactions involving dissolved ions. Nonetheless, MS excels at monitoring all gas generation reactions within the OER region, enabling precise determination of O2 generation potential and distinguishing the contribution of oxidation current to gas generation reactions. This capability underscores MS as a specialized tool for investigating OER selectivity.
4 CONCLUSION
In our exploration of OER measurement and evaluation, precision in measurement and careful consideration in activity comparison emerge as foundational elements for progress and innovation. Throughout our discussion, it becomes clear that achieving accurate measurements and conducting careful comparisons are critical for extracting meaningful insights and advancing the field. The first step toward progress is to ensure precise measurement of electrochemical performance.62, 103-110 Across all aspects of OER catalysis, from characterizing catalyst morphology to evaluating reaction selectivity, attention to detail is essential to ensure reliable results. Moreover, the importance of careful activity comparison cannot be overstated. Our review highlights the challenges associated with comparing the performance of different catalysts, emphasizing the need for standardized protocols and rigorous data interpretation methodologies. Only through comprehensive and systematic comparisons can researchers glean meaningful insights into catalyst performance and guide the development of next-generation materials.
Furthermore, developing new techniques for gaining valuable information from experiments is crucial. In particular, in-situ and real-time analysis play a significant role in capturing the dynamic behavior inherent in the OER. By embracing these advanced techniques, researchers can gain deeper insights into reaction mechanisms and catalyst performance, ultimately driving innovation in the field. Finally, it is essential to consider how these techniques can be applied in real-world systems. For example, integrating them into membrane electrode assemblies for water electrolyzers is critical, as there remains a significant gap between fundamental developments in half-cells and their practical application in full cells.111, 112 Bridging this divide will be instrumental in translating laboratory advancements into tangible solutions for sustainable energy production.
In conclusion, advancing the field of OER catalysis requires a commitment to precision in measurement techniques and activity comparison methodologies. By embracing these principles and fostering collaboration across disciplines, researchers can drive innovation and pave the way for sustainable energy technologies.
AUTHOR CONTRIBUTIONS
Hongmin An was responsible for investigation, writing, and editing, review of the manuscript. Woncheul Park and Heejong Shin contributed to review and editing. Dong Young Chung supervised project.
ACKNOWLEDGMENTS
This work was supported by the Korea Institute of Energy Technology Evaluation and Planning (KETEP) and the Ministry of Trade, Industry & Energy (MOTIE) of the Republic of Korea (No. RS-2024-00404073) and also supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2021R1A5A1028138).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Biographies

Hongmin An received his B.Sc. (2023) in the Department of Chemical and Biomolecular Engineering from Korea Advanced Institute of Science and Technology (KAIST), Republic of Korea. He is currently a Ph.D. student in the research group of Prof. Dong Young Chung in the Department of Chemical and Biomolecular Engineering at KAIST. His research interest focuses on the electrocatalytic engineering of water electrolysis and in-situ analysis of the electrochemical reaction.

Wonchul Park has been attending undergraduate courses in the Department of Chemical and Biomolecular Engineering at Korea Advanced Institute of Science and Technology (KAIST), Republic of Korea. Currently, he is devoting his efforts to the Undergraduate Research Participation Program within the research group of Prof. Dong Young Chung in the Department of Chemical and Biomolecular Engineering at KAIST. His area of research interest takes root in analyzing and designing catalysts regarding water electrolysis.

Heejong Shin received his Ph.D. in Chemical and Biological Engineering from Seoul National University in 2020, under the supervision of Prof. Yung-Eun Sung. He is currently a postdoctoral researcher in Prof. Edward H. Sargent's research group in the Department of Chemistry at Northwestern University. His research interests include developing electrochemical technologies and materials for energy and environmental applications, such as energy conversion, catalysis, green synthesis, and energy devices.

Dong Young Chung has been serving as an assistant professor at the Korea Advanced Institute of Science and Technology (KAIST) since 2022. He obtained his PhD from Seoul National University and subsequently held a postdoctoral position at Argonne National Laboratory. His research centers around fundamental electrochemistry for energy conversion and storage, and he employs in-situ analysis techniques to uncover reaction mechanisms, taking a surface science approach to bridge the gap between fundamental scientific understanding and real-world applications.