Two-dimensional halide perovskite quantum-well emitters: A critical review
Funding information: Davidson School of Chemical Engineering of Purdue University; Office of Naval Research, Grant/Award Number: N00014-19-1-2296
Abstract
Two-dimensional (2D) halide perovskites can be regarded as natural organic-inorganic hybrid quantum wells, which exhibit very promising light-emitting applications due to their high photoluminescence quantum yield, narrow emission bandwidth, and large exciton binding energy. However, it remains a grand challenge to achieve reliable devices for both light-emitting diodes (LEDs) and lasers utilizing phase-pure 2D perovskites. Recently, exciting progresses have been made with respect to molecular design, optoelectronic property, and device fabrication for novel 2D perovskite hybrid quantum-wells. In this article, we critically review the key challenges of exciton losses, charge injections, and triplet issues associated with the light-emitting applications of such phase-pure 2D perovskites after examining their recent breakthroughs in LEDs and lasers. Lastly, we provide a new perspective on molecular engineering strategies to address the above-mentioned fundamental issues, which may open up a new avenue to the development of highly efficient quantum-well emitters for solid-state lighting and display.
1 INTRODUCTION
Metal halide perovskites have been established as a new class of solution processed semiconductor materials for various applications due to their outstanding structure and composition tunability along with their remarkable electrical and optical properties.1-7 Inspired by these promising properties, considerable efforts have been devoted to pursuing high performance optoelectronic devices, including solar cells, light-emitting diodes (LEDs), lasers, and photodetectors.8-19 However, the crystal lattice of three-dimensional (3D) perovskite can be easily damaged when exposed to ultraviolet light, high temperature, or moisture, accelerating the degradation of perovskite devices, and thus leading to poor long-term stabilities. Two-dimensional (2D) halide perovskites featuring an inorganic layer sandwiched between two organic spacer layers are emerging as one of the potential alternatives with considerably better intrinsic stability and moisture resistance compared to their 3D counterparts owing to their higher formation energy and the hydrophobic nature of the organic spacers, making them promising for practical applications.20-23
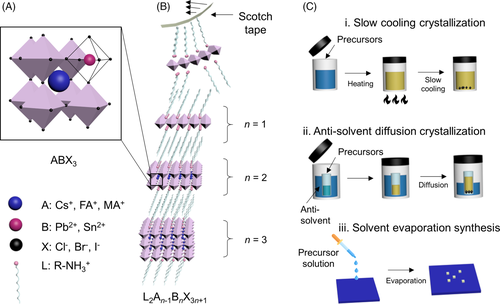
The 3D halide perovskite structure shown in Figure 1A is widely established with a general formula of ABX3, in which A is a small cation (e.g., methylammonium [MA+], formamidium [FA+], or cesium [Cs+]), B is a divalent metal cation (eg, Pb2+ or Sn2+), and X is a halide (eg, Cl−, Br−, or I−). 2D halide perovskites can be considered as slabs cut from their 3D parent structures.2 Generally, 2D perovskites arranged in the Ruddlesden-Popper phase (or RP phase) is the most commonly observed layered structure in halide-based perovskites.24 The RP phase takes the general chemical formula of L2An-1BnX3n + 1, in which L is a large organic cation (large-size or long-chain organic cations like phenylethylammonium, PEA+ or butylammonium, BA+). The variable n represents the number of inorganic [BX6]4− octahedral layers between the two layers of large organic cations. By changing the n variable, the structure's dimensionality can be modified, which is expected to affect the unique quantum well structures of 2D perovskites. When n is infinite, the resulting perovskites are thus approaching to the equivalent 3D counterparts. When n goes down to 1, a dimensional transition from quasi-2D to 2D is observed, and the structure eventually becomes an ideal 2D quantum well with only a single atomic layer of [BX6]4− isolated by L cations (Figure 1B). Stacking many repeating units together through Van der Waals forces will lead to the formation of 2D bulk crystals. Ideally, the exact value of n can be customized by carefully controlling the chemical stoichiometry, but obtaining phase-pure materials with higher n number tends to be very challenging due to phase separation.25 As a result, mixed-phase perovskites with different n numbers are usually obtained and their optical properties are dominated by the higher n phases (or 3D-like phases). Highly efficient LEDs and solar cells have been fabricated using this mixed-phase quasi-2D or 2D/3D mixed perovskites and many excellent reviews have been published recently.26-29
Here, from a different perspective, we mainly focus on 2D perovskites with n ≤ 3 because they behave more like true 2D quantum wells, are more stable, and are easily made phase pure. Importantly, previous works have demonstrated a significantly high solid-state photoluminescence quantum yield (PLQY ~80%), an extremely narrow emission linewidth (< 20 nm), as well as a large exciton binding energy (up to 470 meV) in 2D perovskite quantum-well configurations with type-I band-alignments, which are especially appealing for light-emitting applications.30-32 However, it has been very challenging to achieve reliable light-emitting devices in terms of both LEDs and lasers utilizing low-n phase 2D perovskite quantum wells, while the rationale behind this challenge remain obscure.
In this critical review, we summarize the up-to-date progress of 2D hybrid perovskite quantum wells (n ≤ 3) that have been employed as light-emitters. Their crystal structures, preparation methods, and optoelectronic properties are reviewed in detail. Then, we highlight the key challenges associated with the light-emitting applications of such 2D hybrid quantum wells after examining their recent breakthroughs in LEDs and lasers. Finally, a comprehensive outlook regarding molecular engineering strategies to address the above challenges is provided.
2 SYNTHESIS AND FABRICATION METHODS
Solution synthesis is a relatively less energy intensive process compared to other widely used methods for 2D semiconductors (graphene, transition-metal dichalcogenides, etc.) such as chemical vapor deposition, which requires either high temperature or vacuum conditions.33 Bulk 2D perovskite single crystals can be obtained from precursor solutions with conventional crystallization methods, such as slow cooling, anti-solvent diffusion, and solvent evaporation. Slow cooling method is the most widely utilized method, which uses an aqueous acidic solvent consisting hydrogen halide acid and hypophosphorous acid. As shown in Figure 1C(i), all the precursors are dissolved into the solvent upon heating and slowly cooled down until precursors self-assemble into a RP phase perovskite.34 Anti-solvent diffusion method is also widely employed for achieving high quality 2D perovskites, especially for n = 1 (Figure 1C[ii]). Temperature for this process is maintained constant while organic polar compounds such as N,N-dimethyl formamide (DMF) or gamma-butyrolactone (GBL) are used as a good solvent, non-polar compounds like chloroform and dichloromethane serve as the anti-solvents.35
To prepare atomically thin 2D layered perovskites with high quality, various approaches have been reported. Inspired by the method widely used in 2D semiconductors, mechanical exfoliation was initially investigated for isolating RP phase 2D perovskite bulk single crystals by overcoming the weak Van der Waals forces between the organic layers (Figure 1B). Niu et al36 and Yaffe et al37 reported the mechanical exfoliation of (BA)2PbI4 2D crystals, which helps produce ultra-thin 2D perovskite sheets that are as thin as 2.5 nm. Although exfoliated sheets are expected to be single crystals with high quality, they usually show random shapes and a wide distribution of thickness. Thus, mechanical exfoliation is deemed to be inadequate for producing uniform crystals with high throughput for device application. Solvent evaporation is another method of direct nanocrystal synthesis where large-area of atomically thin 2D perovskites is grown directly on a substrate (Figure 1C[iii]).21, 38 After a simple procedure of dissolving precursors into a ternary solvent system (a mixture of solvents and anti-solvents), the solution is drop-casted onto a substrate. Upon heating, solvent evaporation assisted nanocrystal growth is realized. The ternary system allows the control of solvent evaporation rate so that solute solubility could be constantly limited to inhibit the increase in concentration as solvent evaporates. However, there is still obscurity in controlling the thickness and density of the sheets as the local evaporation rate varies across the substrate.
In addition, vapor deposition of 2D perovskites has also been demonstrated. Islands of PbI2 thin flakes are first deposited onto a substrate followed by a gas phase introduction of butylammonium iodide (BAI), which leads to gas-solid phase intercalation and the formation of 2D perovskite phase. Spatial precision and thickness controllability of the thickness is a highlight for this method.39 Colloidal synthesis methods have been investigated to obtain large-scale of 2D perovskite sheets by employing hot injection method.31 With a wide range of crystal thickness, it is believed that temperature conditions can control the distribution of crystal size and varying crystal thickness can be separated with centrifugation. However, colloidal method has its own limitations, such as the tendency for large sheets to break and small sheets to aggregate in the suspension. Interface-confinement method was then developed to prepare thin sheets with large areas where a solution is injected between two quartz plates with limited space.40, 41 By evaporating the solvents or introducing anti-solvents, it is possible to get 2D perovskite sheets in between the two plates. Nevertheless, it is still difficult to precisely control the spatial location and the thickness of 2D sheets.
Although a variety of synthesis methods have been developed and high quality 2D perovskite single and nano crystals have been obtained, device integration remains a major challenge for the 2D perovskite crystals. Currently, polycrystalline thin films prepared using a simple spin-coating method is the most widely used in LED devices.
3 OPTOELECTRONIC PROPERTIES
The “soft” lattice of 2D halide perovskites offers immense structural and compositional flexibility resulting in high degree of tunability in optical properties. Figure 2A,B show the wide tunable range of absorption and photoluminescence (PL) spectra in 2D RP phase halide perovskites (L2An-1BnX3n + 1) by changing the halide anion (X), metal cation (B), small organic cation (A), and inorganic layer thickness (n). The difference in absorption/PL spectra with different organic cations (A) is quite subtle compared to an obvious shift in absorption/PL spectra with change in halide anions, metal cations, and inorganic layer thicknesses.31 Because their valence band maximum (VBM) is predominantly governed by the halide p-orbital hybridized with metal s-orbital and the conduction band minimum (CBM) is primarily determined by metal p-orbitals, significant band structure tuning can be induced with different X-site anions, B-site cations and n numbers in 2D perovskites.43 Additionally, all these PL emissions exhibit narrow peaks, which can be ascribed to the unique quantum well structures of 2D halide perovskites. In general, the highest occupied molecular orbital (HOMO) of the organic layers is below the VB of the inorganic layers and the lowest unoccupied molecular orbital (LUMO) of the organic layers is similarly above the CB of the inorganic layers in 2D halide perovskites. This leads to a type-I quantum well configuration for the confinement of both electrons and holes, which is very beneficial to enhance radiative recombination for light-emitting applications. Other types of band alignments including inverted type-I or type-II can be also achieved by introducing large organic cations with tunable HOMO and LUMO levels into the perovskite crystal lattice.35, 44 Thus, large organic cations (L) also play a functional role in tuning the overall electronic and optical properties of 2D halide perovskites.
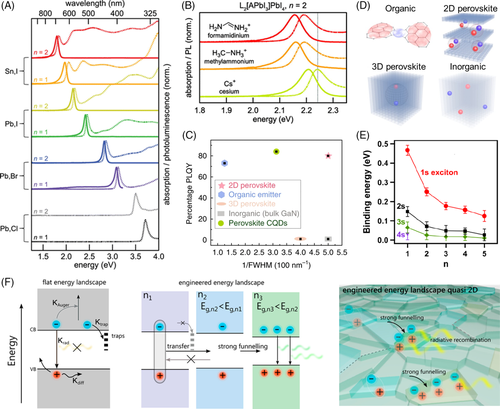
The quantum confinement effect in 2D halide perovskites can produce high PLQY and narrow PL emission linewidth. PLQY is a measure of the efficiency with which incident photons are absorbed by the material and converted to emitted photons. The full width at half maxima (FWHM) of the PL emission determines the color purity of the emitted photons. The interest in employing 2D perovskites for light-emitting devices can be largely ascribed to their promising high PLQY and narrow FWHM. Figure 2C provides a comparison of PLQY vs reciprocal FWHM of single crystalline 2D perovskites with organic, inorganic, 3D perovskites, and perovskite colloidal quantum dot emitters. The absence of defects and grain boundaries leads to a higher PLQY in single crystalline 2D perovskites. Moreover, the excitonic localization at the surface of 2D perovskites due to their ability to incorporate relatively large organic cations in the Van der Waals layered structure offers a unique advantage compared to their 3D counterparts. The high PLQY of ~79% and narrow FWHM of ~20 nm for 2D halide perovskite is achieved by increasing the crystal rigidity and reducing electron-phonon interactions utilizing bulky PEA+ cation rather than widely used BA+ cation.30
The photon emission in 2D halide perovskites is governed by the radiative recombination of excitons that are bound electron-hole pairs in the VB and CB. The bound excitons in 2D halide perovskites are schematically illustrated and compared with excitonic behaviors in organic, inorganic, and 3D perovskites in Figure 2D. The excitons in 2D perovskites are confined in the quantum wells and are consequently tightly bound by Coulombic interactions. The strong Coulombic interactions along with spatial and dielectric confinement effects lead to large exciton binding energies of several hundreds of meV, which can be further modulated by controlling the quantum well thickness of the 2D halide perovskites.45, 46 Figure 2E shows the variation in experimentally measured ground state (1s) and excited state (2s, 3s, and 4s) exciton binding energies with change in inorganic layer thickness (“n” number). The ground state binding energy of ~467 meV for n = 1 2D halide perovskite is much higher than that for MAPbI3 3D perovskite (~16 meV). The decrease in exciton binding energy with increase in “n” number can be attributed to reduced quantum confinement for quasi-2D and 3D perovskites compared to 2D perovskites.32, 47
An increase in exciton concentrations could improve the PLQY of quasi-2D perovskites. This can be achieved by controlled synthesis of continuously graded quantum well structures. An efficient transfer of charge and energy from 2D to quasi-2D perovskites will outcompete the non-radiative decays and improve their photophysical properties for light-emitting applications. Figure 2F depicts a schematic representation of the radiative and nonradiative recombination pathways for decay of charge carriers in flat and engineered energy landscapes. By funneling the charge carriers from larger band gap n = 1 2D perovskites to smaller band gap quasi-2D perovskites, nonradiative decays are considerably minimized. Tailoring the synthesis and crystallization of graded 2D/quasi-2D perovskites, PLQY as high as ~60% has been obtained with a relatively low excitation intensity of ~1.8 mW/cm2.42
4 LIGHT-EMITTING APPLICATIONS
Halide perovskites are potential candidates for use in LEDs due to their tunable bandgap from visible to infrared regions as well as outstanding photoluminescent properties. The external quantum efficiencies (EQE) of LED devices using 3D or quasi-2D halide perovskites have been boosted from 0.1% to over 20% in just 6 years.48-51 For instance, a high EQE over 11% was achieved by employing a conjugated ligand, 1-naphthylmethylammonium (NMA+), which leads to the formation of multiple quantum wells with cascaded energy transfer in a mixed-phase quasi-2D halide perovskites film (n > 3).14 Nevertheless, the quasi-2D perovskite-based devices still suffer from significant ion migration and phase separation, which inevitably degrade the device stability and performance, especially under large electrical bias. An effective strategy to enhance the device performance is to incorporate phase-pure 2D perovskite-based quantum wells with low n number (n ≤ 3) into LEDs, which are intrinsically more stable. In addition, very large exciton binding energy together with fast radiative recombination rate and extremely high PLQY of the 2D perovskite quantum wells make them encouraging for LEDs.
Despite the great promises, there has been very little progress in achieving high-performance LED devices using such low n phase 2D perovskite quantum wells. 2D perovskite-based LED was firstly demonstrated in 1994 with (PEA)2PbI4, which exhibit green emission without EQE and brightness being reported.52 Since then, different 2D perovskites with different quantum well configurations, such as (PEA)2PbBr4, (PEA)2PbCl2Br2, (BA)2PbBr4, have been utilized to fabricate LED devices, which show the EQE as low as 0.31%.53-57 At the same time, lead-free Sn-based 2D perovskite quantum wells have also captured intense research interests for LEDs, but still suffer from limited EQE (<1%) and poor operational stability.58-63
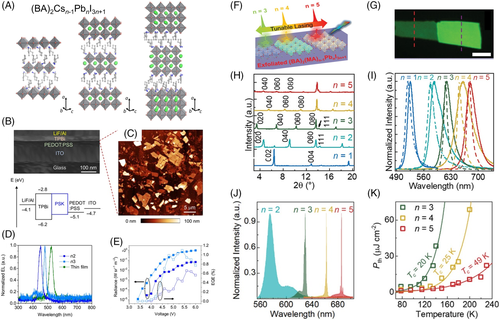
Recently, Yang et al64 synthesized a series of phase-pure 2D perovskite single-crystals, (BA)2Csn-1PbnBr3n + 1 (n = 1, 2, 3, denoted henceforth as n1, n2, n3, respectively). Figure 3A shows their single crystal structures, where the inorganic layers of n1, n2, and n3 are constructed by one, two and three layers of [PbBr6]4− octahedra, respectively. On the A-site of n2 and n3, Cs+ cations are enclosed to stabilize the octahedral layers by partially balancing the negative charges among the [PbBr6]4− octahedra. In comparison with mixed phases and complex compositions from spin-coated 2D perovskites thin films, such high-quality single crystals offer a great platform to investigate their intrinsic optoelectronic properties. Hereby, the authors fabricated single crystal LEDs with a device architecture of ITO/poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) (PEDOT:PSS)/RP phase 2D perovskite films/(2,2′,2″-(1,3,5,-benzinetriyl)-tris(1-phenyl-1H-benzimidazole)] (TPBi)/LiF/Al. Figure 3B shows the cross-section scanning electron microscopy (SEM) image of fabricated LED along with the energy diagram of constituent layers. Here, 2D perovskites single crystal thin films were prepared with a micromechanical exfoliation method to keep their single crystallinity, and the morphology of exfoliated nanosheets was further confirmed by atomic force microscopy image (Figure 3C). The blue electroluminescence (EL) emissions from single crystal-based LEDs are plotted in Figure 3D with voltage-dependent radiance and EQE shown in Figure 3E. The maximum EQEs for n2 and n3 devices are 0.7% and 1.1%, respectively. It is noteworthy to mention that the EL spectrum of n1-based LED is not conclusive because of a wavelength overlap with TPBi emission. Also, the radiance and EQE curves for n1-based LED devices are not available, which could be attributed to extremely weak emission and low efficiency of the n1-based single-crystal device.
Halide perovskites are also promising in the realm of nanolasers owing to their large optical gain coefficients (comparable to that of the industrial GaAs), broadly tunable bandgaps, and facile solution processability (easily self-assembled into high-quality optical resonators).66 As early as 1998, amplified spontaneous emission (ASE) was demonstrated with 2D perovskite films, (PEA)2PbI4, at a cryogenic condition.67 Later in 2007, room-temperature stimulated emission was observed in microcrystalline CsPbBr3 films, which opened up the possibility of exploring perovskite materials for lasing applications.68 By 2014, Xing et al and Zhu et al demonstrated ASE or lasing from 3D halide perovskite films or nanowires, respectively, at room temperature,15, 16 which thus expand the capabilities of perovskite lasers and unlock a wide range of prospective applications. After that, RP phase 2D perovskites have attracted intensive attention in laser applications because of their enhanced environmental stability and exciton confinement compared to their 3D counterparts. Essentially, ASE and lasing behaviors have been demonstrated with solution-processed RP phase quansi-2D perovskite thin films.69-73 However, multiple RP components with mixed n numbers would inevitably form different bandgaps that activate cascaded carrier transfer from low n components to high n components, in which emission can only originate from the highest n number or even 3D components embedded in the quasi-2D perovskite matrix.
Most recently, Zhang et al65 demonstrated a wavelength-tunable lasing from homologous RP phase 2D perovskites, (BA)2(MA)n-1PbnI3n + 1 (n = 3, 4, and 5) single crystalline thin flakes (Figure 3F), which was mechanically exfoliated from bulk crystals (Figure 3G as representative). The as-grown perovskites bulk crystals, (BA)2(MA)n-1PbnI3n + 1 (n = 1-5), shows intense X-ray diffraction patterns corresponding to the RP phase layered perovskites (Figure 3H), where the potential well thickness varies from 0.641 to 3.319 nm when n increases from 1 to 5, respectively. The PL spectra of those 2D perovskite bulk crystals exhibit a single peak (Figure 3I), indicating that the as-prepared 2D perovskites are homologous and contain a single compound. Accordingly, multicolor lasing is realized with wavelengths ranging from 630 nm to 687 nm by increasing n from 3 to 5 (Figure 3J), respectively. Furthermore, as temperature decreases, the lasing threshold decreases exponentially (Figure 3K), which was ascribed to the suppression of thermally activated nonradiative processes. It should be noticed the laser emission from n ≤ 2 is unattainable even at 78 K, which could be attributed to the stronger Auger recombination and exciton-phonon interaction in the lower-n 2D perovskites resulting from enhanced quantum confinement.
5 CHALLENGES AND PERSPECTIVES
5.1 Key challenges
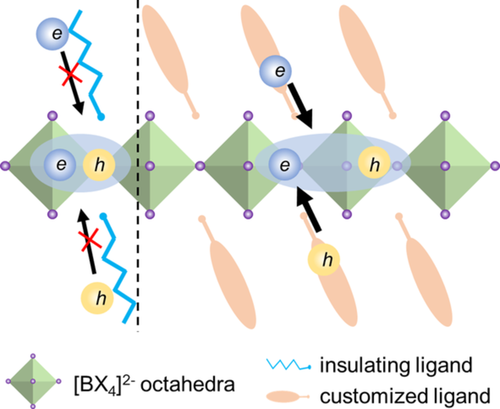
The 2D halide perovskites have demonstrated high PLQY, extremely narrow FWHM as well as large exciton binding energy. Despite these remarkable optoelectronic properties, it remains a great challenge to achieve faithful light-emitting devices in terms of both efficiency and stability using phase-pure 2D perovskite quantum wells (n ≤ 3). Based on previous literature reports, we hypothesize that several key issues listed below may play a critical role in restricting the overall device performances of 2D perovskite quantum wells.32, 74, 75 First, there are several unique exciton losses paths in the strongly confined 2D perovskites, such as Auger recombination, exciton-exciton annihilation, and electron-phonon interaction, etc., which impedes their device performances. Specifically, Auger recombination is a three-body process76 and it will only become significant in non-equilibrium conditions at high carrier density.65, 77 Exciton-exciton annihilation is another kind of many-body interactions, where recombined excitons non-radiatively transfer its energy to other excitons.74 Electron-phonon interaction is a scattering effect in polar semiconductors such as perovskites,78 which could result in fast non-radiative decay, thus worsening their optoelectronic properties.30 Second, the commonly used organic cationic ligands L, such as PEA+ or BA+, are not conductive enough for charge transport (Figure 4A[i]) and set a high energy barrier for charge carrier injection into the quantum well structures. Third, such organic cations like PEA+ or BA+ are relatively short and flexible, rendering them ineffective in inhibiting ion migration and stabilizing the perovskite matrix. Fourth, the dark triplet states in perovskites will bring many triplet-related losses, resulting in poor device performance. Finally, better thin film deposition and defect passivation strategies are still to be developed. To address these fundamental issues, we propose an organic cation design strategy to modify and improve the 2D perovskite quantum well's optoelectronic properties for light-emitting applications.
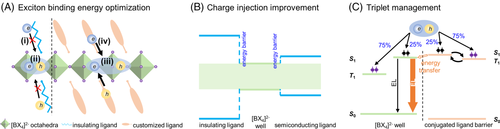
5.2 Exciton binding energy optimization
A huge exciton binding energy (Figure 4A[ii]) resulting from strong quantum and dielectric confinement in 2D perovskites will not only give rise to enhanced radiative recombination, but also cause serious exciton-exciton annihilation or Auger recombination. To overcome these exciton losses, it is possible to design new polar cationic ligands with weak dielectric confinement to optimize the exciton binding energy (Figure 4A[iii]) in 2D perovskites, which could suppress the exciton-exciton annihilation and Auger recombination, thereby maintaining radiative recombination at a relatively high rate.75 Recently, Yuan et al79 demonstrated the very efficient PeLEDs with a peak EQE of 20.36% and a record luminance of 82 480 cd m−2 by suppressing the Auger recombination. A polar molecule, p-fluorophenethylammonium (p-FPEA+), is employed to generate quasi-2D perovskites, where the recombination kinetics disclose the Auger recombination rate decreases to one-order-of magnitude lower compared to its PEA+ analogues, which was attributed to reduced exciton binding energy (Eb) from p-FPEA+. Basically, Auger recombination rate is proportional to the third power of Eb in strongly confined materials. Reducing Eb can be expected to suppress the Auger recombination rates in quasi-2D perovskites. One effective way to decrease Eb is to simply increase the n values, but increasing n values usually leads to inefficient energy transfer in quasi-2D perovskites. Another approach is to weaken the dielectric confinement by the manipulation of organic cations. Generally, organic cations with small dielectric constants are less polar, which leads to decreased dielectric screening of electron-hole Coulomb interaction. In principle, increasing the dielectric constant of organic cations can reduce the dielectric-confinement and then result in decreased Eb. Here, the presence of an electron-withdrawing F atom would polarize the electronic state of p-FPEA+ to generate a strong molecule dipole moment, which facilitates the charge separation, leading to increased dielectric constants. In this sense, novel aromatic cationic ligands with strong electron-withdrawing group may be employed to construct the 2D halide perovskites with optimized Eb by manipulating the dielectric confinement effects.
5.3 Charge injection improvement
The charge transport in 2D perovskites can be improved by replacing alkyl chain-based insulating ligands with conjugated cation-based semiconducting ligand (Figure 4A[iv]). Also, the conjugated cations usually have smaller HOMO-LUMO gap, which is favorable to decrease the energy barrier height in 2D hybrid quantum-well configurations for better charge injections (Figure 4B).35 In addition, such semiconducting cations are typically bulky and rigid, further improving the intrinsic stability of the 2D perovskites, inhibiting potential ion migration under device operation80 as well as suppressing the electron-phonon interaction.38 Although several of such energy-level tunable ligands have been synthesized and reported,35, 81-84 their applications in high-performance devices remain exclusive. In 2018, Stupp et al81 reported a series of n = 1 2D perovskites with different aromatic moiety of naphthalene, pyrene, and perylene, which exhibits enhanced out-of-plan conductivity owing to better energy level matching between the inorganic layers and organic spacers. Such enhanced conductivity together with visible absorption of these materials enables a power conversion efficiency of 1.38%. For the optoelectronic applications like solar cells, the parallel orientation of n = 1 layered perovskite requires transport of charge carriers perpendicular to the inorganic layers and through insulating organic layers. Here, employing naphthalene, pyrene, or perylene-contained cations to produce 2D perovskites exhibits substantially higher conductivity than those containing aliphatic moieties, thus improving the device performances. Meanwhile, the trend in conductivity (perylene > pyrene > naphthalene) indicates the better alignment of energy levels between the HOMO, LUMO of perylene cations and the VB, CB of inorganic lattice. Moreover, the introduction of hydrophobic bulky aromatic cations demonstrates enhanced stability while maintaining higher conductivity comparing to those aliphatic cations. This work suggests that the design of rigid and conjugated cations with tunable electronic characters is favorable to enhance charge transport and stability in these materials. Very recently, our group also reported tin-based n = 1 hybrid quantum wells employing molecularly tailored organic semiconducting barrier layers. Introducing a bulky small bandgap organic barrier in the HQW, charge transport is enhanced and ion migration is greatly suppressed, which enables efficient and stable lead-free perovskite LEDs.85 We envision that this organic cation design strategy could be a new promising direction for the development of highly stable and efficient perovskite LEDs and lasers in the future.
5.4 Triplet management
Triplet issue is detrimental to the device performance of 2D perovskite, especially for LEDs. Based on the spin statistics, electrically injected charge carriers (electrons and holes) will likely generate singlet (S1) and triplet (T1) excitons with a 1:3 ratio in such organic-inorganic hybrid semiconductors. In most cases, triplet excitons are non-emissive in 2D perovskites, which can cause significant exciton losses via triplet absorption, triplet-triplet annihilation, triplet-singlet annihilation, etc.73, 86 This also severely limits the internal quantum efficiencies of electroluminescent devices. Accordingly, we could employ customized organic cations as a triplet manager to tackle this issue. In 2019, Adachi et al86 reported highly efficient perovskite LEDs based on a triplet management strategy. Notably, the management of singlet and triplet excitons is critical to the design of highly-efficient organic light-emitting diodes, while the triplet issues have not been considered for perovskite LEDs and the understanding of the triplet nature of excitons in perovskites remains fundamental.87 In this work, the authors demonstrate that triplet excitons are key to efficient emission in quasi-2D perovskite devices and the quenching of triplets by the organic cation is a major exciton loss path. Interestingly, the authors found that employing an organic cation with a high triplet energy level (PEA+ with a triplet states at 3.30 eV) can help harvest the triplets for emission in a quasi-2D perovskite. On the other hand, the use of an organic cation with a low triplet energy (NMA+ with a triplet state at 2.60 eV) results in poor performance since the triplets are transferred away from the radiative recombination centers, leading to non-radiative recombination. This strategy has been further extended to room temperature continuous-wave lasers with suppressed triplet-related loss by utilizing organic cations as triplet managers.73 These two works provide a deep understanding on the role of triplets in perovskite light-emitting devices, which are expected to pave the way for the pursuit of future current-injection perovskite lasers. To this end, a possible new direction is to incorporate thermally activated delayed fluorescence molecules featuring a reverse intersystem crossing from triplets to singlets as a triplet manager in 2D perovskites (Figure 4C).88, 89 Although triplets have been considerably addressed and discussed in a few recent works,73, 86, 87 more intensive studies are required to provide a deeper insight into the triplets in perovskites from both the fundamental and device aspects.
5.5 Defect passivation
In addition to the above-mentioned fundamental materials-related challenges and possible mitigation strategies, thin film morphology optimization,90, 91 surface and bulk defect passivation,51, 92, 93 device interface engineering94 are also critically important for further boosting the device performance, which have been widely studied in many recent works and reviews. Especially, defects have been identified as a major efficiency-limiting factor for perovskite LEDs. They are generally believed to be associated with intrinsic point defects, such as vacancies, interstitials and anti-site occupations. For the defect passivation, a variety of organic molecules with different anchoring groups have been reported as molecular passivators to passivate perovskite defects. For instance, surface halide vacancies can be suppressed by softer Lewis bases passivators, such as alkylphosphonates and sulfonates.95 Diamine-based agents are expected to coordinate with unsaturated Pb sites by forming weaker hydrogen bonding with organic cations.51 In addition, P=O and As=O groups have also been demonstrated to bind with unsaturated Pb sites in reduced-dimensional perovskites with enhanced PLQY.96 Very recently, our group systematically investigated the passivation effect of different conjugated anchoring groups, including primary ammonium, formamidinium and imidazolium, where the formation low-dimensional perovskite on the surface of 3D perovskite nanograins are suggested to be responsible for defects passivation, thus leading to enhanced device performances.93 On this basis, a deep understanding of how the anchoring groups of molecular passivator interact with the perovskite defects would be very significant for the rational design of passivation agents to further improve the perovskite-based device performances.
All in all, for highly efficient light-emitting devices, the overall optoelectronic properties of 2D perovskites need be improved regarding optimal exciton binding energy, enhanced charge injection, as well as triplet management and defect passivation. In this case, we can introduce the polar groups into those conjugated semiconducting ligands to produce 2D perovskites with better band-alignment and matched triplet states level after a clear understanding on the effect of large organic cations on determining the optoelectronic properties of 2D perovskites. We believe that the cationic ligand design will play a very important role in optimizing the optoelectronic properties of 2D perovskites for high-performance light-emitting devices regarding LEDs and lasers, where there is a huge space for materials chemists to contribute.
6 CONCLUSION
2D hybrid perovskite materials have become an important class of semiconducting materials for light-emitting applications, in which the large organic cations play a significant role in determining their overall optoelectronic properties. The interplay between the organic barrier layers and inorganic well layers is worthy of further investigation to better control and manipulate excitons at the organic-inorganic interface. Obviously, there is a wide space for molecular engineering to further tune the optical and electronic properties of 2D perovskites with customized organic cations, which provide great potential for high-performance devices. Using these strategies, we anticipate that this newly emerging class of materials, devices and opportunities will attract broad interest of chemists, materials scientists, physicists, and engineers to contribute to this field in a way that will eventually deliver these materials into commercial products.
ACKNOWLEDGMENTS
This work is supported by Office of Naval Research (Grant No. N00014-19-1-2296, Program Manager: Dr. Joe Parker and Dr. Paul Armistead) and Davidson School of Chemical Engineering of Purdue University.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Biographies

Kang Wang is currently a postdoctoral research associate in the Davidson School of Chemical Engineering at Purdue University. He obtained his PhD degree from Institute of Chemistry, Chinese Academy of Sciences in 2019 before joining Prof. Letian Dou's group. His current research focus on the synthesis and characterization of hybrid semiconducting materials and their applications in light-emitting devices.

Jee Yung Park is currently a PhD student in Davidson School of Chemical Engineering at Purdue University. He obtained his bachelor's degree in chemical and biological engineering from Korea University in 2019 before joining Purdue University. His research interests include the synthesis and characterization of two-dimensional halide perovskite materials and their applications in optoelectronics.

Akriti is currently pursuing her PhD degree from Davidson School of Chemical Engineering at Purdue University. She obtained her BE degree in Chemical Engineering from BITS Pilani—Goa, India in 2013. She then worked as a Process Engineer in Shell before joining Purdue University in 2017. Her research interests include two-dimensional halide perovskites and heterostructures, and their application in optoelectronics.

Letian Dou is currently the Charles Davidson assistant professor of Chemical Engineering at Purdue University. He obtained BS in Chemistry from Peking University in 2009 and PhD in Materials Science and Engineering from UCLA in 2014. From 2014 to 2017, he was a postdoctoral fellow at the Department of Chemistry, UC-Berkeley and Materials Science Division, Lawrence Berkeley National Laboratory. His research interest includes the synthesis of organic-inorganic hybrid nanomaterials, fundamental understanding of their structure-property relationships, as well as applications in high performance optoelectronic devices. He is a recipient of Office of Naval Research Young Investigator Award, Highly Cited Researcher in Cross-Fields, MIT Technology Review Innovators Under 35-China Award, and Materials Research Society Graduate Student Award.