A preformulation study of marine alkaloid aaptamine: Physicochemical properties investigation and liposomal formulation development toward hepatocellular carcinoma treatment
Tianjiao Geng and Haitao Xue contributed equally to this work.
Abstract
Hepatocellular carcinoma (HCC) is a global health concern with high prevalence and mortality. A marine alkaloid, AP-427, has been reported to show potential for HCC treatment. However, its use is limited by low solubility and high toxicity. We aimed to investigate the preformulation parameters and develop AP-427 liposomes to improve its clinical suitability. A stability-indicating HPLC assay was established, and the physicochemical properties of AP-427 were analyzed. Afterward, AP-427 liposomes were prepared and characterized, and their cytotoxicity was evaluated. AP-427 had a low solubility at physiological pH, a LogD of 2.56 ± 0.03, and a basic pKa of 3.24 ± 0.12. An entrapment efficiency of 52.71 ± 3.2% was achieved after optimization. The resulting AP-427 liposomes were 147.2 ± 3.4 nm and stable up to three months when stored in a pellet form at 4°C. The crystallization of AP-427 in liposomes became less ordered, and AP-427 liposomes exhibited a controlled release fitted in Korsmeyer–Peppas model, indicating the release was driven by diffusion. Furthermore, AP-427 liposomes showed a 3.6 times reduced cytotoxicity against HepG2 cells compared with free AP-427, potentially enhancing its antitumor efficacy. In conclusion, the precise preformulation parameters advanced the AP-427 liposomal formulation development, which showed potential for HCC treatment.
Practical Applications: The aaptamine derivative AP-427 has shown cytotoxic effects against hepatocellular carcinoma. However, the low solubility and high toxicity limit its clinical application. The present study aims to prepare liposomal formulation to solve the current problems. Results obtained from this study shed light on challenges related to drug solubility and have paved the way for the development of an effective AP-427 liposomal formulation with promising application in hepatocellular carcinoma therapy.
1 INTRODUCTION
Hepatocellular carcinoma (HCC), the fifth most common malignancy and the second leading cause of cancer-related mortality worldwide, presents a formidable healthcare challenge.[1, 2] Despite various available treatments, including liver transplantation, resection, tumor ablation, chemotherapy, interventional radiology, biological therapy, the prognosis for HCC remains poor, evidenced by a mere 5-year survival rate of 18%.[3, 4] Systemic chemotherapy has become a common approach, which primarily involves broad-spectrum tyrosine kinase inhibitors (TKIs) such as Sorafenib and Lenvatinib. Nonetheless, these therapies are hindered by poor oral bioavailability and limited efficacy in improving survival rate. Furthermore, patients frequently develop substantial resistance to these chemotherapeutic agents.[5] This highlights the urgent need for novel therapeutic agents and delivery systems with enhanced efficacy and reduced side effects.
Aaptamine and its derivatives, an intriguing class of marine alkaloid featuring a 1H-benzo [de]−1,6-naphthyridine core structure from marine sponge Aaptos aaptos, have demonstrated substantial biological activities, including antioxidant, antiviral, antineoplastic and anticancer activities.[6, 7] In 2014, our research group identified AP-427, one of the aaptamine derivatives (structure shown in Figure 1A), and revealed its pronounced cytotoxic effects against HCC HepG2 cells.[8, 9] Furthermore, we found that AP-427 effectively inhibits the cyclin-dependent kinases 2 (CDK2) activation by modulating the expression of key proteins like p21 and SOX9, thereby influencing HepG2 cells proliferation.[10, 11] The CDK2 inhibitory activities of aaptamine has also been confirmed in a recent study by others,[11] indicating its great potential for anticancer therapy. Most importantly, to enhance the viability for clinical application, we have developed a scalable synthesis method for AP-427, addressing the previous limitations in sourcing issue.[6, 12] However, similar to other anticancer agents that possess nitrogen-containing heterocyclic skeleton such as Vinorelbine and Asulacrine, AP-427 encounters challenge due to its poor aqueous solubility and systemic toxicity. The low water solubility would result in the low bioavailability and also is linked to increased toxicity or heightened risk of phlebitis after intravenous administration.[13-15] Therefore, development of drug delivery systems capable of improving the drug solubility of AP-427 would greatly improve its efficacy and is crucial to mitigate these adverse effects, ensuring the effective and safe application of AP-427 in clinical settings.
Lipid-based nanoparticles, such as liposomes and solid lipid nanoparticles, are the most common class of nanomedicines approved by the United States Food and Drug Administration (FDA).[16, 17] Liposomes were first commercialized in the 1990s as doxorubicin-loaded liposomes (Doxil®) with great success in clinic for cancer treatment. Since then, ten liposome products have been clinically used in cancer therapy due to their capacity to enhance drug solubility and reduce toxicity associated with free anticancer drugs.[14, 18-20] Furthermore, liposomes have the ability to promote the accumulation of chemotherapeutics in tumors due to the enhanced permeability and retention (EPR) effect which has led to extensive research on both in vitro and in vivo drug delivery.[21] Additionally, due to the amphipathic (hydrophobic and hydrophilic) features of liposomes, a wide range of drugs have been loaded into liposomes, which can protect the encapsulated molecule against metabolic destruction.[22] Consequently, the development of liposomal formulations for AP-427 could effectively overcome the challenges associated with its poor solubility and systemic toxicity, thereby enhancing its therapeutic efficacy and suitability for clinical use.
In our endeavor to improve the solubility of AP-427 and advance its therapeutic potential, we have embarked on developing a liposomal formulation. Our initial step involved developing a rapid, reliable, and stability-indicating high-performance liquid chromatography (HPLC) assay, specifically designed for the accurate quantification of AP-427. Subsequently, we focused on the meticulous design and fabrication of liposomes encapsulating AP-427 (AP-427@liposomes). This process necessitated a comprehensive analysis of the physicochemical properties of AP-427, encompassing its solubility, pH-dependent chemical stability, ionization constant (pKa), and distribution coefficient (LogD). Furthermore, we thoroughly characterized the size distribution, zeta potential, morphology, crystallization, drug release profile, and physical stability of AP-427@liposomes. The culmination of this study was detailed comparative assessment of the cytotoxic effects of these AP-427@liposomes versus free AP-427 on HepG2 cells.
2 MATERIALS AND METHODS
2.1 Materials
The reagents and solvents for the synthesis of AP-427 were purchased from Bidepharm (Shanghai, China). The phospholipids, 1,2-dipalmitoyl-sn-glycero-3-phospocholine monohydrate (DPPC), and N-(carbonyl-methoxy-polyethylene-glycol-2000)−1,2-distearoyl-sn-glycero-3-phosphoethanolamine (DSPE-mPEG2000) were purchased from Avanti Polar Lipids (Alabama, USA). Cholesterol and phosphate-buffer saline (PBS, 1×, pH 7.4) were sourced from Sigma-Aldrich (Shanghai, China). Reagents for cell culture were purchased from Thermo Fisher Scientific (Shanghai, China). All other chemicals and solvents used in this study were of analytical grade.
2.2 Cell culture
The human HCC cell line HepG2 was obtained from American Type Culture Collection (ATCC, Manassas, VA). It was maintained in Minimum Essential Medium (MEM) supplemented with 10% fetal bovine serum (FBS), 100 U mL–1 penicillin, and 100 µg mL–1 streptomycin. Cell cultures were maintained in an environment with 95% humidity and 5% CO2 at 37°C.
2.3 Synthesis of AP-427
AP-427 was synthesized following the procedures outlined in our previous publications.[6, 12] The structural confirmation of AP-427 was achieved through 1H NMR, 13C NMR analyses, which were conducted using a 600 MHz Bruker Ascend 600 spectrometer (Bruker, Germany). High-resolution mass spectrometry (HRMS) utilizing electrospray ionization (ESI) was performed with a Waters Q-Tof premier™ mass spectrometer (Waters, USA) to further validate the structure of this compound.
2.4 Stability-indicating HPLC method development and validation
2.4.1 Instrumentation and chromatographic conditions
The Waters 1200 series HPLC system was configured with a 1525 Binary HPLC pump, a 2707 auto-sampler, and a 2998 photodiode array detector. The mobile phase employed in this analysis comprised a mixture of acetonitrile (ACN) and water in 1:1 (v/v) ratio. Prior to use, the mobile phase was filtered through 0.22 µm nylon filter and degassed in an ultrasonic bath. The auto-sampler was maintained at a temperature of 25°C, and 10 µL was injected into the HPLC system. All samples were pumped through a C18 column (S-5 µm, 250 × 4.6 mm) at a flow rate of 1 mL min–1, with detection performed at 254 nm using a UV detector. The output signal was continuously monitored and processed using the HPLC Chem Station Software.
2.4.2 Preparation of stock, standards, and quality control (QC) samples
Stock solutions of AP-427 were prepared by dissolving it in ACN to achieve a concentration of 1 mg mL–1. Analytical standards for AP-427 (2, 5, 10, 20, 30, 40, 60, and 80 µg mL–1) were then prepared by further diluting the stock solutions. This dilution was achieved using a mixture of ACN and water in a 1:1 (v/v) ratio. Quality control (QC) samples were also prepared following the same procedure, resulting in concentrations of 4, 20, and 50 µg mL–1. All prepared solutions promptly stored at −20°C until they were ready to be utilized in the analysis.
2.4.3 Specificity and forced degradation of AP-427
To assess the stability-indicating nature of the assay, AP-427 solutions were subjected to various stress conditions, including exposure to 10 mM HCl (pH 2.0), 10 mM NaOH (pH 12.0), and 3% H2O2 in a duration of 24 h, respectively.[14] In the experimental process, a 1 mg mL–1 stock solution of AP-427 in ACN was mixed with solutions of HCl or NaOH or H2O2 at a ratio of 1:19 (v/v). These mixtures were then placed in an oven set at 37°C. At different time intervals, samples were withdrawn and immediately analyzed by HPLC. It is important to note that these experiments were conducted in triplicate to ensure the reliability and consistency of the results.
2.4.4 Linearity
The linearity of the assay was validated across a concentration range of 2–80 µg mL–1 for AP-427. Standard regression methods were employed to generate the standard curves for analysis. It is worth noting that all standard solutions were shielded from light and subjected to immediate analysis to ensure data accuracy and consistency.
2.4.5 Precision and accuracy
The precision of the analytical method is the closeness between multiple injections of the same sample and is expressed in terms of standard deviation (SD) and relative standard deviation (RSD, %). The accuracy of the method, defined as the closeness of the agreement between the true value and the value found, is reported as the percentage recovery by assay with comparison to the known added amounts.[23] Intraday precision and accuracy were calculated by analysis of the samples at concentrations of 4, 20, and 50 µg mL–1 for three replicates. Interday precision and accuracy were calculated by analysis of three replicates of the same concentration over three consecutive days.
2.4.6 Sensitivity
The sensitivity of the HPLC method was evaluated through the determination of the limit of detection (LOD) and limit of quantitation (LOQ).[24] These values were calculated based on the standard deviation (SD) of the response (σ) and the slope (S) of the calibration curves (n = 3) using the following equations:
2.5 Saturation solubility studies
The solubility of AP-427 was assessed across different pH levels, specifically at pH values of 4.0, 5.0, 6.4, 7.4, 9.0, and 10.0. Buffers were prepared by combining 0.2 M solutions of anhydrous disodium hydrogen phosphate (NaH2PO4) and sodium dihydrogen phosphate dihydrate (NaH2PO4) at appropriate ratios. The pH of these buffers was adjusted by the addition of 0.2 M NaOH or H3PO4. Excess AP-427 was mixed with 0.1 mL of each solvent at room temperature. Samples were collected at 24, 48, and 72 h to determine the time needed to reach the equilibrium. Afterward, the samples were centrifuged at 3000 rpm for 10 min, and the concentration of AP-427 in the supernatant was quantified using HPLC. These experiments were conducted in triplicate to ensure reliability and consistency of the results.
2.6 pH dependent degradation and kinetics
These experiments were conducted in triplicate to ensure the robustness of the results.
2.7 Distribution coefficient
2.8 Ionization constant (pKa)
The ionization constant (pKa) of AP-427 was determined using a spectrophotometric method.[26] In the experimental procedure, absorption maxima of the neutral and ionized species were determined by scanning the absorbance spectra of AP-427 in aqueous solutions with pH values of 4.0, 5.0, 6.4, 7.4, and 9.0, utilizing a Shimadzu UV-3600i Plus Spectrophotometer (Kyoto, Japan). A wavelength of 498 nm was selected for pKa determination. The AP-427 solution was titrated with either NaOH or H3PO4, and the absorbance at 498 nm was measured as a function of pH at room temperature. The shift in absorption was considered, and a sigmoidal curve was constructed, correlating the pH of the solutions with the absorption at 498 nm. Finally, the pKa was determined using Graphpad Prism 9.5.0, providing precise information about the ionization behavior of AP-427 under various pH conditions.
2.9 Preparation and characterization of AP-427 liposomes
2.9.1 Preparation of blank liposomes
The lipids of DPPC, cholesterol and DSPE-mPEG2000 were combined at molar ratios of 6:3:1, and dissolved in a mixture of chloroform: methanol (3:1, v/v) within a 25 mL round-bottom flask. The solvent was gently evaporated using an IKA®-RV8 rotary evaporator (Beijing, China) under reduced pressure, resulting in the formation of a thin liquid film on the interior of the flask. The film was maintained in a vacuum chamber until completely dry. Subsequently, the lipid film was hydrated by adding 1 mL of ammonium sulfate solution (250 mM) to create a multilamellar vesicle suspension. The suspension underwent 7 freeze-thaw cycles, which involved freezing it in liquid nitrogen and then immersing it in a water-bath at 45°C. Following this process, the liposomes were extruded through 200 nm and then 100 nm polycarbonate membranes to obtain vesicles with an approximately uniform diameter. To eliminate any residual free ammonium sulfate, the suspension was subjected to ultracentrifugation at 188 272 × g for 1 h using the equipment from Beckman (USA). This step was essential for further refinement of the liposomal formulation.
2.9.2 AP-427 loaded into liposomes
These calculations provided essential information about the encapsulation efficiency and drug loading of AP-427 within the liposomes, which are crucial for drug delivery systems.
2.9.3 Size distribution, zeta potential and morphology
The size, polydispersity index (PDI), and zeta potential of both blank liposomes and AP-427 loaded liposomes were measured by Dynamic Light Scattering (DLS) in triplicate. The measurements were conducted with a Zetasizer Nano ZS instrument (Malvern Panalytical Ltd., UK) at a detection angle of 173° and a laser wavelength of 633 nm. The temperature during the measurements was maintained at 25°C.
To visualize the morphology of the liposomes, cryogenic transmission electron microscopy (cryo-TEM) was employed. For this purpose, a sample containing 10 mg lipid mL–1 was applied to a copper grid and was then bolted to leave a thin film stretched over grid holes. The grid was subsequently dipped into liquid ethane and cooled to 90 K with liquid nitrogen. The samples were observed using a Talos F200C G2 electron microscope (Thermo Fisher Scientific, USA) operating at 120 kV, which allowed for a detailed examination of the liposome structure.
2.9.4 Differential scanning calorimetry (DSC)
DSC analysis was performed using an instrument PerkinElmer Inc. (Waltham, MA, USA) in the temperature ranging from 30°C to 200°C, with a heating rate of 10°C min–1. Each sample with 5 mg was inserted in stainless steel capsules that were hermetically sealed and support internal pressures of 24 bar.
2.9.5 In vitro release profile
This calculation allowed for the quantification of the drug released from the liposomes over time under the specified conditions.
The zero-, first order, Higuchi, and Korsmeyer–Peppas models were applied to ascertain the AP-427 release kinetic from liposomes. The best-fitting one was defined considering the highest regression values (R2) of the analyzed release data. Moreover, the n value, obtained from the slope of the equation referring to the Korsmeyer–Peppas model, was employed to define the release mechanism of AP-427 as well.
2.9.6 Stability of AP-427 loaded liposomes
The stability of the liposomes was assessed over a period of 4 months. During this time, the samples were stored at 4°C in a pellet form after ultracentrifugation and were shielded from exposure to light. Regular evaluations were conducted to monitor various parameters, including size, PDI, zeta potential, and drug content. This rigorous stability testing ensured that the liposomal formulation remained intact and maintained its key characteristics throughout the storage period, which is essential for assessing its suitability for pharmaceutical applications.
2.10 In vitro cytotoxicity study
The cytotoxicity of both AP-427 and its liposomal formulation was assessed using the CCK8 assay. Briefly, cells were seeded in 96-well plates at a density of 5000 cells per well. After 24 h of cell attachment, free drug and drug-loaded formulations prepared in 100 µL medium were added to each well (in triplicate or quadruplicate wells). Following 72 h of treatment, 10 µL of CCK-8 solution was added to each well, and the plate was incubated at 37°C for 3 h. The absorbance of the plate was then read at 450 nm using a microplate reader (Varioskan™ LUX, Thermo Fisher Scientific, Shanghai, China). The drug concentrations that resulted in 50% inhibition of viable cell density compared to the untreated controls (IC50) were calculated using Graphpad Prism 9.5.0 based on data from three independent experiments. The results were expressed as the percentage variation of control cell viability, with control cells considered as 100%. This assay allowed for the determination of the cytotoxic effects of AP-427 and its liposomal formulation on the tested cells, providing valuable information about their potential as anticancer agents.
2.11 Statistical analysis
All experiments were conducted in triplicate, and the data are presented as mean ± standard deviation (SD). Statistical analysis involved least square linear regression and one-way Analysis of Variance (ANOVA) with Tukey's multiple comparisons for data comparison. Graphpad (Version 9.5.0) was used for statistical analysis, and the significance level (p value) was set at 0.05.
3 RESULTS AND DISCUSSION
3.1 Synthesis of AP-427
AP-427 was synthesized, and its structure was identical to the reported natural compound isolated from sponge Aaptos aaptos.[9]
1H NMR (500 MHz, CDCl3) δ 8.74 (d, J = 4.4 Hz, 1H), 8.37 (s, 1H), 7.46 (d, J = 4.4 Hz, 1H), 7.09–7.04 (m, 1H), 6.60 (s, 1H), 3.97 (s, 3H), 3.37 (t, J = 6.5 Hz, 2H), 2.13 (hept, J = 6.7 Hz, 1H), 1.12 (s, 3H), 1.10 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 175.85, 157.90, 150.44, 144.35, 136.32, 136.04, 134.25, 129.69, 121.61, 117.89, 106.15, 55.57, 50.38, 28.51, 20.34. HRMS (ESI): calcd for C16H18N3O2 [M+H]+, 284.1399; found 284.1408.
3.2 Development and validation of stability-indicating HPLC method
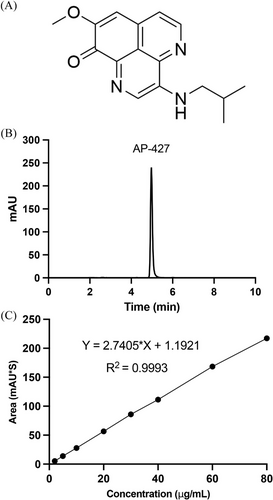
The HPLC analysis using a mobile phase composed of ACN: water (1:1, v/v) resulted in a distinct AP-427 peak with a retention time (tR) of 4.9 min, as illustrated in Figure 1B. The assay demonstrated linearity across a concentration range of 2 to 80 µg mL–1 (R2 = 0.9993, n = 3), as shown in Figure 1C.
A summary of the precision and accuracy of AP-427 HPLC analysis is presented in Table 1. Both interday and intraday precision and accuracy are satisfied, with the RSD ranging from 0.64% to 4.26% and the accuracy values between 92.0% and 104.5%. The LOD and LOQ for AP-427 were determined to be 0.197 and 0.602 µg mL–1, respectively. All results of the method validation undertaken complied with the criteria presented in ICH Q2 (R1) guidelines,[28] and underscore the precision and sensitivity of the HPLC assay for quantifying AP-427.
| Theoretical concentration (µg mL–1) | Interday (n = 3) | Intraday (n = 5) | ||||
|---|---|---|---|---|---|---|
| Concentration found (µg mL–1) | Accuracy (%) | RSD (%) | Concentration found (µg mL–1) | Accuracy (%) | RSD (%) | |
| 4 | 4.97 ± 0.07 | 92.0 ± 3.2 | 3.49 | 3.97 ± 0.15 | 99.5 ± 3.7 | 1.05 |
| 20 | 20.80 ± 1.51 | 98.7 ± 1.4 | 4.26 | 20.08 ± 0.13 | 100.4 ± 0.6 | 0.64 |
| 50 | 50.96 ± 0.26 | 101.9 ± 0.5 | 0.51 | 52.25 ± 0.87 | 104.5 ± 1.7 | 1.67 |
3.3 Forced degradation studies
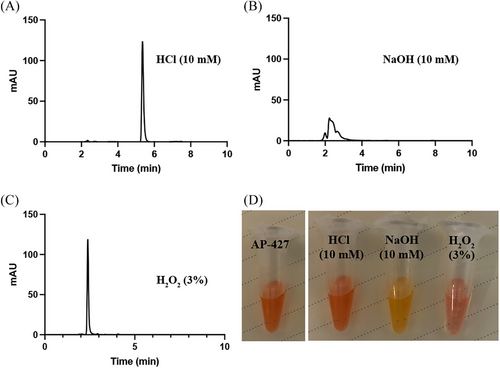
The chromatograms displaying AP-427 exposed to various conditions are presented in Figures 2A–C. Additionally, the color of drug solution changed under different conditions, as depicted in Figure 2D. Comparing all the stress conditions, AP-427 was resistant under acidic condition (10 mM HCl) for at least 24 h at 37°C, with the retention time remained same to that of AP-427 in the mobile phase, as shown in Figure 1B. However, when subjected to 10 mM NaOH, extensive degradation of AP-427 occurred, possibly due to the damage of the benzo[de][1,6]naphthyridin-9-one moiety of AP-427 in this alkaline condition. Compared to other conditions, AP-427 exhibited a great sensitivity in the presence of 3% H2O2, resulting in a peak with a retention time of 2.4 min, indicating that the majority of AP-427 was oxidized. Overall, these results suggest that the chemical stability of AP-427 was pH dependent, with resistance to acidic conditions but susceptibility to alkaline and oxidative environments. This seems to correlate with the solubility outcome. The stability of AP-427 found in our study is in line with the forced degradation behavior of bortezomib[29] and ixazomib,[30] which were prone to decomposition in H2O2 and alkaline solution. The forced degradation study data show the chemical behavior of the AP-427 which in turn helps in the development of formulation and package.
3.4 pH solubility
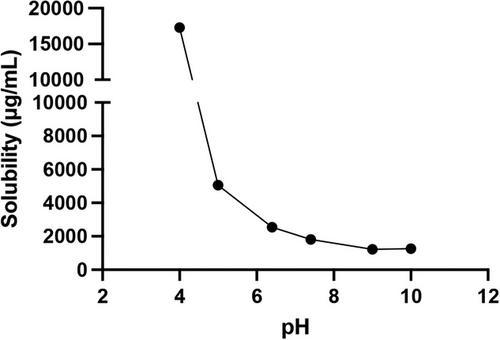
AP-427 achieved equilibration after 24 h, with no further dissolution observed up to 72 h. Figure 3 illustrates the solubility of AP-427 after 24 h of equilibration at room temperature as a function of pH. Notably, AP-427 exhibited lower solubility in basic environment, while an increase in saturation solubility was observed in solutions with pH < 7.4. This increased solubility at acidic pH levels can be contributed to the present of basic chemical groups in the chemical structure of AP-427.[31] Specifically, the solubility of AP-427 at pH 7.4 was determined to be 1.82 ± 0.14 mg mL–1. The low solubility of AP-427 at physiological pH is problematic, as it has been introduced as one of the most important reasons for the low bioavailability,[32] and may also lead to drug precipitation after intravenous injection.[33] Consequently, the clinical application of AP-427 would not be feasible without the development of a suitable formulation.
Various formulation strategies have been devised to address the solubility challenges of poorly water-soluble drugs, ultimately enhancing their bioavailability. These strategies include the use of salt forms, amorphous forms, lipid-based formulations, and nanocarrier-mediated formulations.[34] Among these approaches, liposomes have been attracted particular interests due to their biodegradability and versatility. So far, extensive research have been conducted on liposomes as an approach to improve the solubility and bioavailability of low water-soluble anticancer drugs, including paclitaxel,[35] curcumin,[36] and resveratrol.[37] Furthermore, liposomes have also demonstrated the potential to prevent post-injection drug precipitation by encapsulating the lipid soluble drug within their bilayer structures.[38] Therefore, liposomes may offer a promising solution to the solubility challenges associated with AP-427, ultimately enhancing its therapeutic efficacy and suitability for clinical applications in cancer therapy.
3.5 pH-degradation and kinetics
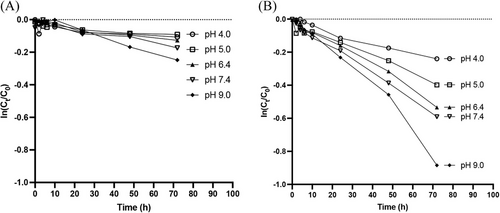
The degradation profiles of AP-427 at different pH levels and temperatures (25°C and 45°C) is depicted in Figure 4. These profiles reveal that the semilogarithmic plot of AP-427 fraction remaining versus time exhibited a linear trend, indicating pseudo-first order degradation kinetics.[39] The corresponding half-lives of AP-427 under various conditions are summarized in Table 2. It is noteworthy that AP-427 degradation was relatively slow in all tested conditions, with noticeably faster degradation occurring at higher temperature (45°C) compared to lower temperature (25°C). This outcome aligns with the expected behavior, as elevated temperatures can accelerate the decomposition of molecules.[40] Additionally, AP-427 exhibited faster degradation rate as the condition became basic in both 25°C and 45°C. This observation is consistent with the earlier findings present in results shown in Figure 2, further highlighting the pH-dependent stability of AP-427. Our results are valuable in estimating the shelf-life, handling, and storage conditions for AP-427 solutions in vitro and in vivo experimental studies. Importantly, they also help in the development of the pharmaceutical formulation.
| Temperature (°C) | pH 4.0 | pH 5.0 | pH 6.4 | pH 7.4 | pH 9.0 |
|---|---|---|---|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
3.6 Distribution coefficient of AP-427
Figure 5A presents the LogD predictions for AP-427 using the predication software, which indicated that the highest LogD value of AP-427 was 2.17, occurring at a pH value around 6.0. The LogD value remained relatively stable as the pH increased, signifying its highest lipophilicity at pH > 6.0. It is crucial to predict the LogD value early and accurately by computer-aided drug design technology before conducting in-depth pharmacodynamic experiments.[41] Furthermore, by predicting Log D, it is easily to compare with the experimental results in the next step.[42]
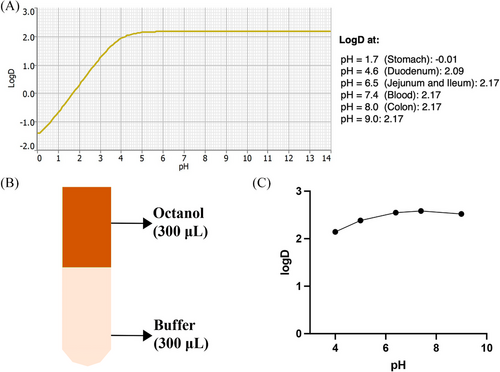
Experimental results in the octanol-buffer system revealed that equilibrium distribution was reached within 24 h for all tested pH values (Figure 5B). The LogD of AP-427 at pH > 7.4 averaged around 2.56 ± 0.03, with a slightly lower value of 2.15 ± 0.05 observed at pH 4.0 (Figure 5C). The relatively larger LogD in higher pH is possibly because the AP-427 would be expected to remain mostly unprotonated and negatively ionized in basic pH environments. This is in agreement with the lower solubility of AP-427 in basic condition, as indicated in Figure 3.
LogD value has been traditionally identified as a good predictor for drug delivery.[43] While there were slight differences between the predicated and experimental results, both sets of data indicated that AP-427 exhibited lipophilic characteristics at pH 7.4. This parameter is important for AP-427 loading into liposomes as the loading depends on the distribution process, and a high LogD suggests a rapid partition of the drug to the liposomal phospholipid bilayer.[14] Furthermore, the lipophilic nature of AP-427 at pH 7.4 underscores the potential for phlebitis following intravenous injection, highlighting the importance of formulation development to mitigate this issue.
3.7 Ionization constant (pKa)
Figure 6A illustrates the ionization behavior of AP-427, while Figure 6B displays the UV absorbance spectra of 0.1 mg mL–1 AP-427 aqueous solutions across different pH levels. A wavelength of 498 nm was chosen for analysis, as it exhibited a slightly greater difference in absorbencies among the solutions.[44] Utilizing the data, a sigmoidal curve was constructed to estimate the pKa value of AP-427, which was determined to be 3.24 ± 0.12 (Figure 6C). This pKa value is indicative of the basic nature of the acridine nitrogen group in AP-427. It correlates with the observed increase in solubility and decrease in LogD at lower pH values, as demonstrated in Figures 3 and 5, respectively.
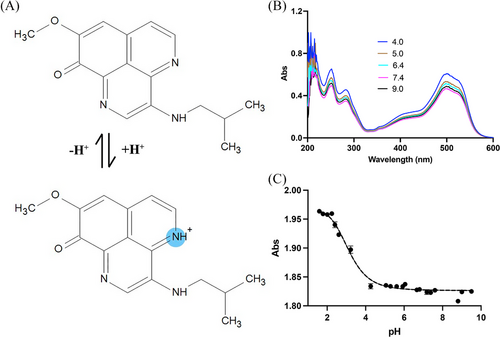
Measuring the pKa of AP-427 is important as it provides insight into the ionization state of the compound, which is crucial for understanding drug absorption in various parts of the gastrointestinal tract, including stomach, jejunum, ileum, and colon. Additionally, it aids in interpreting pH-solubility profiles.[45] Furthermore, this data sheds light on the potential cause of phlebitis following intravenous infusions. In the bloodstream, the drug solution can neutralize, causing the local drug concentration to exceed its solubility limit, which may lead to postinjection precipitation, and phlebitis can occur when the lipophilic drug deposits on the vein wall.[14]
3.8 Characterization of AP-427 liposomes
3.8.1 AP-427 loaded into liposomes
Liposomes were prepared using the thin-film hydration method, as depicted in Figure 7A. Since AP-427 has a weak basic pKa of 3.2, active loading with ammonium sulfate was employed to create pH gradient for AP-427 loading.[46] Ammonium sulfate has proven effectively in establishing a transmembrane pH gradient for the active loading of basic drugs.[47, 48] Compared to passive loading, active loading generally offers higher drug loading efficiency.[47] The mechanism of AP-427 loading into liposomes is outlined in Figure 7B. Ammonium sulfate creates a gradient that drives the influx of the unionized AP-427 species through the liposome membrane. Once inside, these molecules become protonated, inhibiting their diffusion out of the liposome membrane. After removing the excess extraliposomal ammonium sulfate, the higher concentration of ammonium ions inside the liposome core promotes the efflux of the neutral ammonia molecules.[48, 49] Additionally, given that the intraliposomal pH is 5.6 with 250 mM ammonium sulfate, the majority of AP-427 (a weak base, pKa = 3.2) becomes ionized after transport into the liposomes core.[48] As a result, the ionized form of AP-427 becomes trapped within the liposome core, facilitating efficient drug loading in this setting.
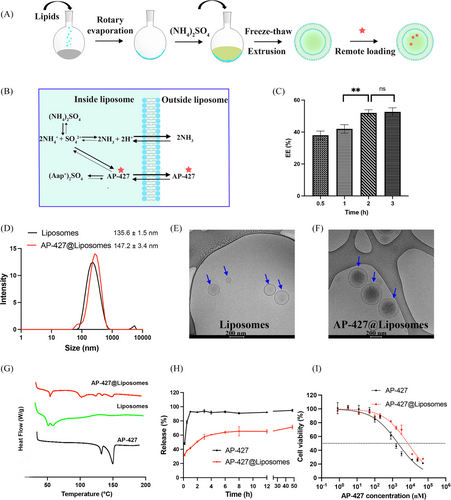
Through this method, a maximal EE of 52.71 ± 3.2% was achieved after a 2 h incubation period at 37°C, with no further benefit observed when extending the incubation time to 3 h (Figure 7C, **p < 0.01). Consequently, a 2 h incubation at 37°C was selected for loading AP-427 into liposomes for the downstream applications. Accordingly, the DL of AP-427 was 4.76 ± 0.01% at this condition. This drug equilibrium in the liposome and drug solution was similar compared with our previous study which loaded doxorubicin into liposomes.[50]
3.8.2 Size, PDI, zeta potential, and morphology
Figure 7D illustrates that the liposomes exhibited a uniform distribution with a size of 135.6 ± 1.5 nm and a zeta potential of −27.6 ± 1.2 mV. After loading AP-427, the AP-427 liposomes displayed a slightly increased size, measuring 147.2 ± 3.4 nm (p > 0.05). The zeta potential was slightly naturized to −20.4 ± 1.3 mV (*p < 0.05), indicating that the addition of AP-427 resulted in slightly positive charges, possibly due to the interaction of AP-427 and liposomes. The change of zeta potential in positive direction can be useful for improving penetration function of particles in tumor cell.[51] The morphology of the liposomes remained intact (Figures 7E and F). However, the drug-loaded liposomes appeared darker and denser in comparison to those without AP-427, indicating successful AP-427 loading into the liposomes. This outcome aligns with the previous observations.[52]
3.8.3 Thermal analysis
DSC is a thermal analysis widely used for the characterization of drug-loaded nanoparticles in terms of crystallinity degree, drug-lipid interactions, and polymorphism.[53] DSC thermograms of the pure AP-427, liposomes and AP-427@liposomes are shown in Figure 7G. AP-427 showed sharp endothermic melting peaks at 135.29°C and 153.88°C, respectively. Empty liposomes displayed an endothermic transition at 44.58°C. Compared to the pure AP-427 and empty liposomes, AP-427@liposomes showed a melting enthalpy decline of AP-427, suggesting that AP-427 in liposomes was in a less ordered crystal state. The crystal state change may be due to the AP-427/lipid matrix interactions.[54] The less ordered crystal state of AP-427 in liposomes could result in a higher solubility and a greater bioavailability.[55]
3.8.4 Release profile
Figure 7H illustrates the release profiles of AP-427 and its liposomal formulation in physiological pH. In our system, we observed an initial burst release of 60.5 ± 4.2% from AP-427@liposomes within the first 4 h, followed by a sustained release, reaching up to 71.1 ± 2.4% with no complete release of AP-427 during the 48 h period observation. The fast initial release suggests that some of the AP-427 compound was entrapped in the lipid bilayers, and the subsequent sustained release indicates that the liposomes effectively trapped AP-427 in their core.[56] The rate of release decreased over time as it requires more time for the drug inside to transport from the inner aqueous phase to the lipid bilayers.[57] In contrast, free AP-427 was completely released after 1 h, further confirming the advantage of the developed AP-427 liposomal formulation. The controlled release of AP-427 from liposomes is a desirable feature for cancer therapy, as premature drug leakage from the nanocarriers in the circulation can lead to inadequate drug delivery to the cancer cells.[58] Additionally, achieving spatial and temporal control of AP-427 release can enhance its performance, and reduce toxicity by allowing the lower administered doses.[59] The better-sustained release property in pH 7.4 indicated a good long-term absorption in the neutral medium, such as in the small intestine, and the potential application in oral drugs.[37]
The in vitro drug release profile can fit into different models, including zero-order, first-order, Higuchi, and Korsmeyer–Peppas kinetic models.[60] The corresponding kinetic parameters obtained from the models are reported in Table 3, and the highest R2 allowed to define the kinetic which better describes the AP-427 release from the liposomes. Based on the analysis, the release of AP-427@liposomes was fitted best into the Korsmeyer–Peppas model (R2 = 0.8977). The Korsmeyer–Peppas model is considered a comprehensive equation for polymer-based drug-release systems, as well as the kinetics of the medium and the structural/geometric properties of the carrier. The n value, Fickian constant, is an important parameter of the Korsmeyer–Peppas model, which indicates the release mechanism of the active substance and is used to determine the role of diffusion in a drug release system.[61] In this study, the n value of AP-427@liposomes was 0.1357 as shown in Table 3, which demonstrated that AP-427 would release from liposomes mainly driven by diffusion followed Fick's law (n ≤ 0.5).[62] The result agrees with previously published data involving drug-loaded liposomes where the best fit into the Korsmeyer–Peppas model, with n value of 0.32, was found.[63]
|
Zero order Qt = Q0 – k0t |
First order Qt = Q0(1 – exp−k1t) |
Higuchi Qt = kHt1/2 |
Korsmeyer–Peppas Mt/M∞ = ktn |
|||||
|---|---|---|---|---|---|---|---|---|
| R2 | K0 (h−1) | R2 | K1 (h−1) | R2 | KH (h−1/2) | R2 | K (h−n) | n |
| 0.3808 | 0.6072 | 0.6212 | 1.7611 | 0.6652 | 5.6266 | 0.8977 | 45.1599 | 0.1357 |
3.8.5 Stability
Table 4 presents the stability data. The liposomes were stored at 4°C in a pellet form after ultracentrifugation as used by others.[52, 64] Over the course of 4 months storage, there were no significant differences observed in the particle size, PDI, zeta potential of AP-427@liposomes. The physical stability could be attributed to the negative zeta potential of liposomes, which resulted in electrostatic repulsion between particles.[64] However, after four months, drug leakage began to occur (*p < 0.05), possibly because the entrapped AP-427 in the lipid bilayer diffused out of the liposomes. Similar stability results were observed in a previous study when loading the anticancer drug SN25860 into liposomes, stored as pellets at 4°C.[46] Lyophilization is commonly applied as an important way to improve liposomal drug stability, and facilitate transportation, storage and improve the shelf-life.[65] It is of great interest to investigate the storage of liposomes by lyophilization in the future.
| Time (month) | Size (nm) | PDI | Zeta potential (mV) | Drug loading (%) |
|---|---|---|---|---|
| 0 | 147.2 ± 3.4 | 0.15 ± 0.1 | −20.4 ± 1.3 | 4.76 ± 0.01 |
| 1 | 145.9 ± 1.9 | 0.12 ± 0.1 | −21.5 ± 2.1 | 4.77 ± 0.01 |
| 2 | 148.1 ± 2.7 | 0.21 ± 0.2 | −21.6 ± 1.9 | 4.67 ± 0.02 |
| 3 | 145.9 ± 4.1 | 0.18 ± 0.1 | −19.8 ± 2.2 | 4.62 ± 0.02 |
| 4 | 147.3 ± 2.1 | 0.16 ± 0.2 | −20.1 ± 1.4 | 4.21 ± 0.01* |
- Statistical analysis: *p < 0.05.
3.9 In vitro cytotoxicity
An in vitro cytotoxicity study was conducted to evaluate the impact of AP-427 and its liposomal formulation on HepG2 cell viability (Figure 7I). The free AP-427 inhibited HepG2 cells growth in a dose-dependent manner, and the IC50 value was determined to be 2.03 ± 0.15 µM. In contrast, the AP-427@liposomes exhibited a 3.6-fold increase in IC50 (7.33 ± 0.90 µM) compared to free AP-427. This reduced cytotoxicity can be attributed to several factors. Firstly, the controlled release of AP-427 from liposomes (Figure 7H) led to lower exposure of the active compound to HepG2 cells. Additionally, the gradual endocytosis of liposomes, potentially hindered by PEGylation due to the DSPE-mPEG2000 used in the liposomes, resulted in a slower accumulation of AP-427 within the cells compared to the rapid diffusion of free AP-427 in the cell culture model.[58] The reduced AP-427@liposomes cytotoxicity against HepG2 cells, combined with the controlled release profile, hold significant promise for altering the pharmacokinetics of AP-427. By ensuring a sustained release of the drug, this formulation has the potential to enhance its antitumor activity.[66] These findings align with our previous study where doxorubicin loaded liposomes have demonstrated similar behavior and reduced their cytotoxic activity to glioblastoma U87 cells compared to free doxorubicin.[50]
4 CONCLUSIONS
A stability-indicating HPLC assay for AP-427 was developed. AP-427 has a solubility of 1.82 ± 0.14 mg mL–1 at pH 7.4, and a LogD and pKa of 2.56 ± 0.03 and 3.24 ± 0.12, respectively, suggesting its low bioavailability and high toxicity. The AP-427 liposomal formulation was developed with a maximal EE of 52.71 ± 3.2% and a favorable size and morphology. These liposomes had a less ordered crystal state and a long-term storage stability at 4°C in a pellet form. Additionally, AP-427@liposomes showed a controlled release profile under physiological condition followed by the Korsmeyer–Peppas model, and a 3.6 times reduced cytotoxicity against HepG2 cells, offering the potential to alter the pharmacokinetics of AP-427 and improve its therapeutic outcomes.
In conclusion, our comprehensive preformulation studies have shed light on challenges related to drug solubility and have paved the way for the development of an effective AP-427 liposomal formulation. This formulation has the potential to increase the solubility, reduce the toxicity, and mitigate drug precipitation issues during infusion, thereby improving therapeutic efficacy. Future investigations into the in vivo effects of AP-427 liposomes are warranted to validate their clinical utility and potential impact on cancer treatment.
AUTHOR CONTRIBUTIONS
Tianjiao Geng: Funding acquisition; methodology; investigation; formal analysis; writing—original daft. Haitao Xue: Investigation; formal analysis; writing—review and editing. Can Kong: Methodology; formal analysis; writing—review and editing. Luping He: Methodology; writing—review and editing. Shuping Wang; Ling Ding; and Xiaoyan Zou: Writing—review and editing. Zhichun Gu; Hongze Liao; and Houwen Lin: Conceptualization; investigation; resources; supervision; funding acquisition; formal analysis; writing—review and editing. All authors have read and agreed to the published version of the manuscript.
ACKNOWLEDGMENTS
The authors wish to thank National Key Research and Development Program of China (2022YFC2804100); National Natural Science Foundation of China (22137006); Shanghai Pujiang Program (23PJ1407500); Innovative Research Team of High-level Local Universities in Shanghai (SHSMUZDCX20212702). Tianjiao Geng also wishes to thank her grandfather Fuqian Geng who was diagnosed with HCC and passed away on October 13, 2023. This promotes her to investigate the efficient drug delivery system for HCC therapy.
CONFLICT OF INTEREST STATEMENT
The authors report there are no competing interests to declare.
Open Research
DATA AVAILABILITY STATEMENT
Research data are not shared.




