Composition, thermal, and microstructural characteristics of mutton tallows in comparison with beef tallows
Abstract
The composition, thermal properties, and microstructure of some mutton tallows (MTs), such as sheep tallow (ST), goat tallow (GT), and sheep tail tallow (STT), were investigated and compared with those of beef tallow (BT). The results showed that the fatty acids (FAs) in the MTs were dominated by oleic, stearic, and palmitic acids, which were similar to those of BTs; also there were some natural trans and odd-carbon FAs occurred in these tallows. Comparison with STs, GTs, and BTs, STT had higher triunsaturated and diunsaturated-monosaturated triacylglycerols (TAGs) and lower monounsaturated-disaturated and trisaturated TAGs. Solid fat content (SFC) profile of STT was different those of STs, GTs, and BTs, whereas STs, GTs, and BTs had similar SFC profiles. In the range of 0–50°C, STT had a lower SFC compared with STs, GTs, and BTs at a given temperature. DSC results showed that MTs had melting and crystallization curves similar to BTs; and the GT, ST, and BT had higher melting and crystallization temperatures than STT. All MT and BTs crystals are β′ form; there also exists certain β form. The microscopic morphology of the STT was radial spherical aggregates, and those of other MT and BTs were consisted of radial inner core and low density outer halo area.
Practical Application: The results of SFC, microstructure, and morphology indicated mutton tallows containing the desired crystal form and crystal morphology could be used as shortening base fats. The research can provide a theoretical basis for expanding the application of mutton tallows in food industry.
1 INTRODUCTION
Tallow is considered a by-product of beef and mutton industry.[1] Edible tallows, including beef tallow (BT) and mutton tallow (MT), are produced by rendering the clean, sound, fatty tissues of bovine animals, sheep, and goat in good health at the time of slaughter and fit for human consumption.[2] BT and MT have a unique flavor and nutritional value that cannot be replaced by vegetable fats. They also contain natural conjugated linoleic acid (CLA) and odd-chain saturated fatty acid (OCSFA), which have important physiological functions.[3, 4] Tallow is used in the oleochemical industry as glycerol and the derivatives of fatty acids (FAs).[1, 2] BT is applied widely in the food industry as plastic fat, frying oil, and hot-pot oil.[1, 2, 5, 6]
However, compared to BT, MT, including sheep and goat tallow (GT), was less studied and rarely utilized in food industry. Currently, most research on MT has focused on the composition of FAs[7-12] and thermal property.[9, 10] MT was mainly composed of palmitic, stearic, and oleic acids.[9-11] MT was used to produce special fats by interesterification with other vegetable oils.[13, 14]
Sheep tail tallow (STT) was obtained from tail fat adipose tissues of sheep,[10, 15] and rich in flavor precursor substance and used in kavurma preparation[15] and traditional Chinese food such as soup and hot pot.[16] The major FAs in STT were also C16:0, C18:0, and C18:1c; however, saturated FAs (SFAs) in STT were lower than those in fats from kidney, back, and intestine tissues of sheep.[10] In order to expand the application of STT, it was fractionated into liquid fraction (olein) and solid one (stearin).[17, 18] The FA composition and thermal properties of the original STT and the fractionated fats,[17, 18] the microstructure of STT stearin[17] were studied.
Little is known about the microstructure and morphology of MT. The characteristics of fats, especially physical ones, effect the properties of the products containing these fats.[19] Microstructure and morphology of natural fats are crucial in the food industry since they are related to macroscopic properties of final products, including texture and sensory evaluation.[20-22]
In this study, the composition, thermal properties, microstructure, and morphology of different types of MTs were investigated and compared with those of BTs. The results could provide some theoretical reference for developing and expanding the application of MT in food.
2 MATERIALS AND METHODS
2.1 Materials
STT and sheep kidney tallow (SKT) adipose tissues were purchased from the local farmers’ market in Lanzhou (Lanzhou, China). Two kinds of GT (GT1 and GT2), which were derived from the vicinity of internal organs and subcutaneous fat-containing tissues, and BT adipose tissues were purchased from the local farmers’ market in Zhengzhou (Zhengzhou, China). Refined beef tallow (RBT) was kindly supplied by Yihai Kerry (Tai'an) Oil Industry Co. Ltd.
2.2 Preparation of samples
After removal of the blood clots, fascia, and other nonfatty debris from the fat adipose tissues, the fatty tissues were cleaned and cut into small pieces of 4–5 cm. The fats were extracted from the issues by wet rendering according to our previous report.[23] The fatty tissues (1 kg) with water (3 kg) were boiled in a stainless vessel (about 6 L) for 60 min. After removing fatty tissue residues, the liquid mixture was cooled to ambient temperature, the fat layer was separated and dried at 100°C under vacuum (about 50 mm of mercury absolute pressure) for 30 min. The STT, SKT, sheep tallow (ST), GTs (GT1 and GT2), and BT were obtained. After preparation of the MTs and BTs, these were kept at −18°C until further use. The acid value and peroxide value of them were analyzed. The acid values of STT, SKT, ST, GT1, GT2, BT, and RBT were 1.5, 1.5, 0.70, 0.57, 1.4, 0.03, and 9.1 mg KOH g−1, respectively. The peroxide values of STT, SKT, ST, GT1, GT2, BT, and RBT were 0.061, 0.068, 0.365, 0.045, 0.047, 0.005, and 0.137 g/100 g, respectively.
2.3 Fatty acid composition
The fats were methylated according to the AOCS Official method Ce 2-66.[24] The composition of the FA methyl esters was determined using a gas chromatograph (Agilent 6890N, Agilent) equipped with a flame ionization detector (FID) and an SGE BPX-70 column (30 m × 250 µm × 0.25 µm) according to our previous research[23] (Li et al., 2023). The temperature of injector and detector were 250 and 300°C, respectively. The oven temperature was programmed from 170 to 210°C at the rate of 2°C min−1 and kept at 210°C for 20 min. The carrier gas was nitrogen with a flow rate of 1 mL min−1. The flow rates of hydrogen and air were 40 and 400 mL min−1, respectively. The FAs were characterized with reference to the retention times of various standard FA methyl esters performed at the same conditions. The content of each FA was estimated according to the area-normalization method.
The sn-2 FA compositions of these fats were analyzed according to our previous research.[23] The composition of the resulting FA methyl esters was determined by GC method as mentioned above.
2.4 Triacylglycerol composition
A high performance liquid chromatograph (Alliance E2695, Waters) equipped with an evaporative light scatter (ELSD2424, Waters) was used to analyze the composition of the triglycerides (TGs) according to the method reported by Li et al. with slight modification.[22] The sample (10 mg mL−1 trichloromethane solution) was eluted using a mixture of acetonitrile and dichloromethane (65/35, v/v) with a flow rate of 1 mL min−1. The TGs were characterized with reference to the retention times of the various standards and the equivalent carbon number, and the content of each TG was evaluated according to the area-normalization method.
2.5 Solid fat content analysis
The solid fat content (SFC) of the fat samples was determined by using a pulse-NMR (Minispec mq20, Bruker) according to AOCS Cd 16b-93.[25] About 5 g of melted fat samples were put into the NMR tube. The fat samples were heated at 80°C for 0.5 h, and then the oil samples were transferred to a water bath at 0°C for 1.5 h. The fat samples were placed at 5, 10, 15, 20, 25, 30, 35, 40, 45, 50, 55, and 60°C for 0.5 h before measuring.
2.6 Physical properties
The iodine values (IV) of fat samples were determined according to AOCS Cd 1d-92.[26] The slip melting points (SMP) of fat samples were determined according to AOCS official Method Cc 3-25-2009.[27]
2.7 Thermal behavior
The melting and crystallization properties of the fat samples were determined by a differential scanning calorimeter (NETZSCH DSC 204 F1) according to the method reported by Biswas et al. [28] with slight modification. The fat samples were weighed 8–10 mg and sealed in a special aluminum tray, heated from −50 to 80°C at a rate of 5°C min−1, kept for 10 min, and cooled from 80 to −50°C at a rate of 5°C min−1.
2.8 Polymorphism analysis
The polymorphism of fats was determined by an X-ray diffractometer (XRD) (Bruker D8 Advance, Bruker) with a Cu-Kα radiation (wavelength λ = 1.5406 Å, voltage 40 kV, and current 40 mA) as described by Li et al. [23] with slight modification. The sample was scanned with a 2θ range from 1° to 10° at a scanning speed of 1° min−1 with a step of 0.02°, and a 2θ range from 10° to 40° at a scanning rate of 4° min−1 with a step of 0.02°.
2.9 Crystal morphology
The fat samples were observed by using a polarized light microscope (Carl Zeiss Axioscope 5) according to the method used by Miskandar et al.[29] with slight modification. The fat sample was dipped into a glass sampling capillary and spotted on a slide, then slowly pushed away with a coverslip so that the fat sample was presented on the slide in a more uniform and suitable thickness for observation.
2.10 Statistical analysis
The data were analyzed by IBM SPSS Statistics for windows version 20.0. Duncan's multiple range tests were conducted to test significant difference at p < 0.05. The software Origin2021 (OriginLab Co.) was used to draw tables and graphs.
3 RESULTS AND DISCUSSION
3.1 Fatty acid composition of tallows
FA composition is very crucial because it is related to the physiochemical properties of fat. The FA compositions of the tallows are shown in Table 1. Saturated and monounsaturated FAs (SFAs and MUFAs) are predominant in MTs. Oleic acid (O, 43.31%) was the main FA present in STT, followed by palmitic acid (P, 20.81%) and stearic acid (St, 10.70%). In other tallows, stearic (27%–36%) and oleic (24%–35%) acids were major FAs, followed by palmitic acid (19%–26%). GTs showed the highest content of SFAs than BTs and STs. In STT, unsaturated fatty acids (UFAs) were higher than SFAs; whereas the opposite results were observed in other tallows. Moreover, the content of oleic acid of STT was significantly higher than those of BT, ST, and GTs, and the content of stearic acid of STT was significantly lower than those of BT, ST, and GTs. The BT, ST, and GTs had small differences in the types and contents of the three major FAs. The major kinds of FA of MT and BTs are similar, with oleic acid, stearic acid, and palmitic acid dominating the total FA, which is consistent with those reported in the relevant literature.[15]
| RBT | BT | ST | SKT | GT1 | GT2 | STT | |
|---|---|---|---|---|---|---|---|
| Fatty acid composition (%) | |||||||
| C14:0 | 3.16 ± 0.00ab | 2.07 ± 0.02c | 2.3 ± 0.20c | 2.88 ± 0.11b | 2.14 ± 0.01c | 3.27 ± 0.01ab | 3.53 ± 0.43a |
| C15:0 | 0.64 ± 0.00abc | 0.65 ± 0.00abc | 0.78 ± 0.19a | 0.55 ± 0.06bc | 0.45 ± 0.00c | 0.72 ± 0.00ab | 0.81 ± 0.10a |
| C16:0 | 24.72 ± 0.01a | 19.9 ± 0.00c | 20.42 ± 0.29c | 22.84 ± 0.77b | 20.00 ± 0.11c | 25.61 ± 0.06a | 20.81 ± 0.67c |
| C16:1c | 0.37 ± 0.03c | 0.46 ± 0.05c | 1.00 ± 0.37b | 1.03 ± 0.05b | 0.37 ± 0.01c | 0.51 ± 0.00c | 2.62 ± 0.03a |
| C16:1t | 0.49 ± 0.00b | 0.66 ± 0.02b | 0.27 ± 0.13c | 0.33 ± 0.07c | 0.66 ± 0.01b | 0.52 ± 0.00b | 2.38 ± 0.10a |
| C16:2 | 2.03 ± 0.02a | 1.68 ± 0.11b | 0.87 ± 0.17d | 0.56 ± 0.08e | 1.17 ± 0.01c | 1.09 ± 0.00c | 1.00 ± 0.00 cd |
| C17:0 | 2.15 ± 0.01b | 1.98 ± 0.02c | 1.86 ± 0.17c | 2.57 ± 0.02a | 1.63 ± 0.10d | 2.40 ± 0.01a | 2.58 ± 0.01a |
| C17:1 | 0.71 ± 0.00b | 0.57 ± 0.01b | 0.71 ± 0.32b | 0.54 ± 0.09b | 0.18 ± 0.00c | 0.72 ± 0.00b | 1.69 ± 0.10a |
| C18:0 | 27.88 ± 0.05d | 32.76 ± 0.05b | 31.03 ± 2.08bc | 26.68 ± 0.45d | 35.34 ± 0.11a | 29.17 ± 0.05 cd | 10.70 ± 1.66e |
| C18:1c | 31.91 ± 0.01cd | 33.00 ± 0.05c | 34.69 ± 0.82b | 31.1 ± 0.25d | 24.28 ± 0.00f | 27.21 ± 0.01e | 43.31 ± 1.38a |
| C18:1t | 3.31 ± 0.01c | 3.49 ± 0.03c | 2.51 ± 0.52c | 6.02 ± 0.46b | 8.11 ± 0.45a | 5.32 ± 0.04b | 5.97 ± 1.10b |
| C18:2 | 1.76 ± 0.01d | 1.72 ± 0.05d | 2.01 ± 0.07d | 4.15 ± 0.19a | 4.33 ± 0.24a | 2.38 ± 0.21c | 3.32 ± 0.06b |
| C18:3 | 0.28 ± 0.01d | 0.45 ± 0.00b | 0.63 ± 0.17a | 0.25 ± 0.01d | 0.32 ± 0.01bc | 0.42 ± 0.01bc | 0.41 ± 0.01bc |
| C20:0 | 0.58 ± 0.08b | 0.62 ± 0.10b | 0.92 ± 0.14a | 0.51 ± 0.01b | 1.04 ± 0.05a | 0.67 ± 0.01b | 0.88 ± 0.03a |
| SFA | 59.13 ± 0.01b | 57.97 ± 0.10bc | 57.31 ± 1.42 cd | 56.03 ± 0.18d | 60.6 ± 0.18a | 61.83 ± 0.15a | 39.3 ± 0.43e |
| MUFA | 36.8 ± 0.02c | 38.17 ± 0.05b | 39.18 ± 1.15b | 39.01 ± 0.28b | 33.59 ± 0.42d | 34.29 ± 0.05d | 55.97 ± 0.50a |
| PUFA | 4.07 ± 0.01c | 3.86 ± 0.06cd | 3.52 ± 0.27d | 4.96 ± 0.09b | 5.81 ± 0.24a | 3.89 ± 0.20cd | 4.73 ± 0.08b |
| TFA | 3.80 ± 0.01cd | 4.14 ± 0.05c | 2.78 ± 0.39d | 6.34 ± 0.38b | 8.77 ± 0.44a | 5.84 ± 0.04b | 8.34 ± 1.00a |
| Physical properties | |||||||
| IV | 44.9 ± 0.15d | 42.9 ± 0.27e | 42.8 ± 0.07e | 47.8 ± 0.19c | 49.2 ± 0.04b | 43.9 ± 0.52de | 59.7 ± 0.11a |
| SMP (°C) | 45.9 ± 0.07c | 47.0 ± 0.21b | 45.5 ± 0.56c | 45.7 ± 0.14c | 49.1 ± 0.28a | 47.7 ± 0.14b | 33.8 ± 0.14d |
- Note: Means within a row with different superscripts (a–f) differ significantly (p < 0.05).
- Abbreviations: BT, beef tallow; GT, goat tallow; IV, iodine value; MUFA, monounsaturated FA; RBT, refined beef tallow; ST, sheep tallow; STT, sheep tail tallow; SKT, sheep kidney tallow; SMP, slip melting point; SFA, saturated FAs; TFA, trans fatty acid.
Yilmaz and Karakaya reported GTs principally composed of palmitic (21%–24%), stearic (22%–39%), and oleic (21%–37%) acids.[9] It was reported that the Uruguayan MT was mainly composed of 20.4%–23.4% C16:0, 22.3%–30.5% C18:0, and 38.4%–41.9% C18:1c.[30] Goat body fat mainly composed of 25.8% palmitic, 29.2% stearic, and 28.9% oleic acids was reported by Upadhyay et al.[31] Moon et al. studied the FA composition of the fat in the goat meat,[32] the results showed that the fats in meat of loin, leg, and neck were mainly composed of oleic (28%–50%), palmitic (20%–30%), and stearic (14%–22%) acids; however, the major FAs of the fat in rib meat were palmitic (42%) and stearic (26%) acids. It was reported that BT primarily composed of 20.9%–28.9% palmitic, 7.0%–26.5% stearic, 30.4%–48.0% oleic acids; and MT principally consisted of 19.5%–21.3% palmitic, 17.6%–28.9% stearic, 33.2%–40.8% oleic acids.[33] Limmatvapirat reported the primary FAs in the BTs obtained by different rendering methods were 30%–45% oleic, 20%–30% palmitic, and 15%–30% stearic acids.[22]
There was a small amount of natural trans fatty acids (NTFAs) in the tallows (Table 1). The TFAs were 9t-16:1 and 9t-18:1. The NTFAs contents of RBT, BT, ST, SKT, GT1, GT2, and STT were 2.78%, 4.15%, 3.80%, 6.35%, 8.77%, 5.84%, and 8.35%, respectively. The result of a previous study showed that a certain amount of NTFAs also existed in beef kidney fat (BKF), beef intermuscular fat (BIF), and sheep tail fat (STF) with contents of 1.58%, 1.66%, and 3.10%, respectively.[15] It was reported NTFAs in beef and MTs were 1.3%–6.6% and 11%–14.6%, respectively.[33]
OCSFAs, such as pentadecanoic (C15:0) and margaric (C17:0) acids, also occurred in these tallows (Table 1). The contents of these FAs in the tallows ranged from 2.0% to 3.5%. The research indicated that OCSFAs could significantly inhibit non-small-cell lung cancer cell proliferation.[4] It was reported that BKF, BIF, and STF contained a certain amount of OCSFAs with a range of 2.0%–4.5%.[15] The OCSFAs in goat body fat was about 3.1%.[31] In the previous report, the OCSFAs in the MTs were normally lower than 2.5%.[13, 14, 34] OCSFAs are a class of healthful FAs which had important physiological functions. OCSFAs are protective against a wide range of human metabolic diseases compared to even chain SFAs.[3, 4] CLA is not detected in the tallows due to its lower content and the poor selectivity of the capillary column and low sensitivity of FID in our study. CLA is commonly detected by GC–MS.[35]
The FAs in the sn-1,3 and sn-2 positions have an important influence on the digestion and absorption of lipids in the human body and also determine the application value of edible oils.[36] For example, it has been shown that palmitic acid in the sn-2 position of the glycerol skeleton promotes mineral absorption in infants and young children, whereas palmitic acid at sn-1 and sn-3 is easily hydrolyzed into free palmitic acid by lipase in the intestinal tract and saponified with minerals such as calcium and magnesium, reducing the efficiency of fat absorption and utilization in the human body.[37] Therefore, it is important to detect the region specificity of FAs in TGs. The sn-2 FAs of MTs consist mainly of oleic (over 55%), followed by stearic and palmitic acids (Table 2). Natural trans and odd chain FAs were not detected in the sn-2 position of triacylglycerols (TAGs) in MT and BTs.
| FAs | Sn-2 fatty acid composition (%) | ||||||
|---|---|---|---|---|---|---|---|
| RBT | BT | ST | SKT | GT1 | GT2 | TaT | |
| C14:0 | 4.24 ± 0.18a | 5.06 ± 0.32b | 5.01 ± 0.23b | 4.15 ± 0.13a | 4.32 ± 0.03a | 5.24 ± 0.15a | 4.21 ± 0.26a |
| C16:0 | 12.73 ± 0.02b | 15.52 ± 0.05b | 15.62 ± 0.36b | 14.7 ± 0.81a | 15.72 ± 0.66b | 21.82 ± 0.95a | 7.56 ± 0.24c |
| C18:0 | 15.96 ± 0.04a | 12.45 ± 0.12d | 13.95 ± 0.83c | 14.17 ± 0.14b | 16.46 ± 0.06a | 17.93 ± 1.27a | 4.6 ± 0.34c |
| C18:1c | 62.31 ± 0.92b | 61.51 ± 0.03bc | 60.82 ± 1.06bc | 59.14 ± 0.25c | 60.18 ± 1.05bc | 49.53 ± 2.2d | 77.36 ± 0.69a |
| C18:2 | 4.76 ± 1.16bc | 5.46 ± 0.12bc | 4.61 ± 0.1c | 7.85 ± 0.54a | 3.32 ± 0.36c | 5.48 ± 0.14bc | 6.28 ± 0.37ab |
| MUFA | 62.31 ± 0.92b | 61.51 ± 0.03bc | 60.82 ± 1.06bc | 59.14 ± 0.25c | 60.18 ± 1.05bc | 49.53 ± 2.2d | 77.36 ± 0.69a |
| PUFA | 4.76 ± 1.16bc | 5.46 ± 0.12bc | 4.61 ± 0.1 cd | 7.85 ± 0.54a | 3.32 ± 0.36c | 5.48 ± 0.14bc | 6.28 ± 0.37ab |
| SFA | 32.93 ± 0.25b | 33.03 ± 0.14c | 34.57 ± 0.96bc | 33.01 ± 0.79b | 36.49 ± 0.68a | 44.99 ± 2.06a | 16.37 ± 0.33c |
- Note: Means within a row with different superscripts (a–f) differ significantly (p < 0.05).
- Abbreviations: BT, beef tallow; GT, goat tallow; MUFA, monounsaturated FA; RBT, refined beef tallow; ST, sheep tallow; SKT, sheep kidney tallow; SFA, saturated Fas.
3.2 Physical properties of tallows
IV was a measure of the unsaturation degree of a fat. The IVs of RBT, BT, ST, SKT, GT1, GT2, and STT were 44.9, 42.9, 42.8, 47.8, 49.2, 43.9, and 59.7 g I2/100 g, respectively (Table 1). The IVs of STT were higher than those of GT, ST, and BTs. The IVs of the fats from the subcutaneous, kidney, and intestinal adipose tissues of goat in the report of Yilmaz and Karakaya were 40.4, 34.4, 35.6, and 31.0 g I2/100 g, respectively.[9] The IVs of MTs from the subcutaneous, kidney, intestinal, and tail adipose tissues of sheep in a previous report were 36.9, 34.4, 35.6, and 42.6 g I2/100 g, respectively.[10]
SMPs of the tallows are presented in Table 1. Except for STT, other tallows had high melting points and poor oral melting properties. SMPs of BT, ST, and GTs ranged from 45 to 50°C, whereas STT showed lower SMP (33.8°C) than other tallows. GTs had higher SMPs than STs. The SMPs of the fats from the subcutaneous, kidney, and intestinal adipose tissues of goat in the report of Yilmaz and Karakaya were 41.6, 48.6, and 49.5°C, respectively.[9] The results of a previous research showed that the SMPs of MTs from the subcutaneous, kidney, intestinal, and tail adipose tissues of sheep were 37.6, 46.7, 46.8, and 37.4°C, respectively.[10] The SMPs of some MTs reported by Grompone were in the range of 43.0–47.1°C, and those of BTs were 45.0–48.0°C.[30] In the food industry, high melting point animal fats such as BT were usually optimized for their properties to make them suitable for use as base oils for margarine and shortening.[34, 38] The SMPs of STTs reported by Hou et al.[17] and Ünsal and Aktas[18] were 37.3 and 43.2°C, respectively, and higher than that of STT in our study, which may be due to the differences in the composition of samples.
3.3 Triglyceride composition of tallows
TGs, as the basic units that make up the fat system, have an important influence on the physicochemical properties of fats. The diversity of TGs is mainly caused by the arrangement of different kinds of FAs in the glycerol backbone, and this complexity influences the thermal properties, crystallization behavior, and microstructure of the fats.[1] The TAGs of several MT and BTs are shown in Table 3. The TAGs of RBT were mainly composed of StOSt (34.18%), StOO (14.54%), and POSt (12.93%), and the TAGs of BT were mainly composed of StOO (25.97%), POSt (18.66%), and StOSt (13.17%). The TAGs of ST are mainly composed of StOO (30.91%), POSt (25.37%), and POP/PPO (16.06%). The TAGs of SKT were mainly composed of POSt (23.18%), StOO (12.05%), and PPSt (9.58%). The TAGs of GT1 were mainly composed of StOO (24.49%), POSt (21.51%), and PStSt (10.62%), and GT2 was mainly composed of StOO (32.64%), POSt (14.99%), and POP/PPO (13.41%). List et al.[12] reported the triacylglycerol composition of MT calculated from the positional distribution of the FAs according to the 1,3-random-2-random distribution hypothesis, and MT was primarily composed of 6.7%–17.3% POO, 13.7%–16.9% StOO, 4.4%–10.0% POP, 18.3%–20.2% StOP, 9.8%–19.0% StOSt, 3.6%–5.8% PPSt, 3.7%–11.0% PStSt, and 1.2%–7.4% StStSt. Neff et al. analyzed the triacylglycerol composition of lard and ST using RP-HPLC coupled with APCI-MS, they reported that ST was principally composed of 2.3% OOO, 13.6% POO, 8.7% StOO, 6.5% POP, 17.4% POSt, 4.3% PPS, 13.5% StOSt, 7.0% PStSt, and 3.4% StStSt.[39] In a previous research, BT was mainly composed of 10.8% POO, 13.5% PLSt, 21.0% POSt, 11.8% PPS, 10.2% StOSt, and 8.2% PStSt; and MT was primarily consisted of 9.7% POO, 7.5% PLSt, 6.5% POP, 19.4% POSt, 10.6% PPS, 16.2% StOSt, 11.4% PStSt, and 5.2% StStSt.[40] The difference of the results between our study and the published reports may be related to the different adipose tissues, breeds, and growth conditions of sheep. The triunsaturated triacylglycerols (UUU) in STs and GTs were lower than 5%, diunsaturated-monosaturated triacylglycerols (UUS) in them were in the range of 17%–35%, monounsaturated-disaturated triacylglycerols (USS) ranged from 40% to 57%, trisaturated triacylglycerols (SSS) were 13%–26%.
| TAG | Triacylglycerol composition (%) | ||||||
|---|---|---|---|---|---|---|---|
| RBT | BT | ST | SKT | GT1 | GT2 | STT | |
| OLL | – | – | – | – | – | – | 1.06 ± 0.03a |
| PLL | – | – | – | – | – | – | 1.15 ± 0.04a |
| LOO | – | – | – | – | – | – | 3.27 ± 0.1a |
| StLL | 0.90 ± 0.01b | – | 0.69 ± 0.27b | – | 1.79 ± 0.26a | 1.51 ± 0.27a | 0.90 ± 0.01b |
| PLO | 0.94 ± 0.14d | 0.57 ± 0.08d | 1.17 ± 0.36d | 5.57 ± 0.14b | 1.09 ± 0.1d | 2.91 ± 0.31c | 7.14 ± 0.34a |
| PLP | 1.55 ± 0.07c | 0.50 ± 0.01d | 1.79 ± 0.40c | 6.02 ± 0.16a | 1.96 ± 0.18c | 2.73 ± 0.20b | 1.82 ± 0.36c |
| OOO | 1.18 ± 0.09e | 1.71 ± 0.12d | 1.62 ± 0.42de | 4.10 ± 0.01b | 2.01 ± 0.02d | 2.78 ± 0.27c | 11.24 ± 0.14a |
| POO | 1.47 ± 0.13d | – | 1.70 ± 0.15d | 8.66 ± 0.12a | 6.07 ± 0.06b | 2.05 ± 0.35d | 4.21 ± 0.80c |
| StOO | 14.54 ± 0.21d | 25.97 ± 0.56c | 30.91 ± 4.78bc | 12.05 ± 0.32d | 24.49 ± 0.35c | 32.64 ± 0.05b | 56.57 ± 2.07a |
| POP | 8.05 ± 0.1bc | 6.17 ± 0.12d | 16.06 ± 3.05a | 9.00 ± 0.14bc | 10.52 ± 0.76b | 13.41 ± 0.32a | 9.13 ± 0.63bc |
| PPP | 3.37 ± 0.05b | 1.23 ± 0.28cd | 4.44 ± 0.79b | 7.86 ± 0.03a | 3.01 ± 0.24bc | 2.63 ± 1.98bc | – |
| POSt | 12.93 ± 0.14c | 18.66 ± 0.51bc | 25.37 ± 0.05a | 23.18 ± 0.56ab | 21.51 ± 1.87ab | 14.99 ± 1.62c | 3.86 ± 0.15d |
| PPSt | 11.8 ± 0.11a | 7.5 ± 1.38ab | 5.5 ± 0.55ab | 9.58 ± 0.23ab | 5.16 ± 1.07ab | 12.4 ± 1.14a | 0.26 ± 0.00b |
| StOSt | 34.18 ± 0.28a | 13.17 ± 0.39b | 6.95 ± 0.29c | 5.79 ± 0.15c | 6.91 ± 1.93c | 5.91 ± 0.56c | 0.25 ± 0.00d |
| PStSt | 4.78 ± 0.2b | 10.32 ± 0.43a | 3.13 ± 0.13b | 4.58 ± 0.03b | 10.62 ± 1.43a | 5.13 ± 0.72b | – |
| StOA | – | 2.11 ± 0.53a | – | – | – | – | – |
| StStSt | 4.32 ± 0.04b | 12.08 ± 2.56a | 0.67 ± 0.17b | 3.62 ± 0.07b | 4.86 ± 3.87b | 0.92 ± 0.16b | – |
| UUU | 2.78 ± 0.27 | 1.71 ± 0.12 | 1.62 ± 0.42 | 2.01 ± 0.02 | 4.10 ± 0.01 | 1.18 ± 0.09 | 15.56 ± 0.27 |
| UUS | 39.11 ± 1.69 | 26.55 ± 0.71 | 34.47 ± 3.94 | 33.44 ± 0.37 | 26.28 ± 0.36 | 17.84 ± 0.11 | 69.07 ± 1.16 |
| USS | 37.05 ± 0.29 | 40.61 ± 0.50 | 50.17 ± 2.99 | 40.9 ± 4.38 | 43.98 ± 0.74 | 56.71 ± 0.29 | 15.07 ± 0.84 |
| SSS | 21.07 ± 0.72 | 31.13 ± 1.33 | 13.74 ± 1.37 | 23.65 ± 3.99 | 25.64 ± 0.37 | 24.27 ± 0.32 | 0.26 ± 0.00 |
- Note: Means within a row with different superscripts (a–e) differ significantly (p < 0.05).
- Abbreviations: A, arachidic; BT, beef tallow; GT, goat tallow; L, linoleic; O, oleic; P, palmitic; RBT, refined beef tallow; S, saturated; St, stearic; ST, sheep tallow; SKT, sheep kidney tallow; UUU, triunsaturated triacylglycerols; U, unsaturated.
STT was mainly composed of UUS (69.07%). Compared with other MT and BTs, its content of SSS was the lowest, accounting for only 0.26% of the total TAG content, which corresponds to its lowest melting point.
3.4 Solid fat content of tallows
SFC is one of the important indicators for evaluating the functional properties of food-specific fat products.[41, 42] The SFCs of the tallows under different temperature are presented in Figure 1. When the temperature was lower than 50°C, the SFC of STT was more highly poor than those of the other tallows at each given temperature. This was presumably related to its higher content of UFA than the remaining tallows (Table 1). In the previous research, SFCs of STF at 0, 5, 10, 15, 20, 25, 30, and 35°C were 22.5%, 21.6%, 15.2%, 10.3%, 6.8%, 5.1%, 3.0%, and 0.8%, respectively,[17] which was different from the SFCs of STT of our study. This difference might be due to the cultivar types and growing condition of sheep. When the temperature was lower than 15°C, there was small fluctuation of the SFCs of the MT and BTs with the temperature increasing. This is probably because of the complex compositions of TAG of the MT and BTs. Some TAGs components with higher SMPs recrystallized following the unstable crystal structures melting, which probably led to the appearance of the fluctuations. The SFC of STT was slowly decreasing when the temperature was higher than 10°C, whereas the other tallows were sharply melting when the temperature was over 15°C. The SFCs of the STs and the BTs were no significant difference from 15 to 50°C. The SFCs of the GTs were slightly higher than the sheep and BTs from 0 to 50°C. At 55°C, the SFCs of all the tallow samples decreased to 0, indicating that the tallows were completely melted at this temperature.
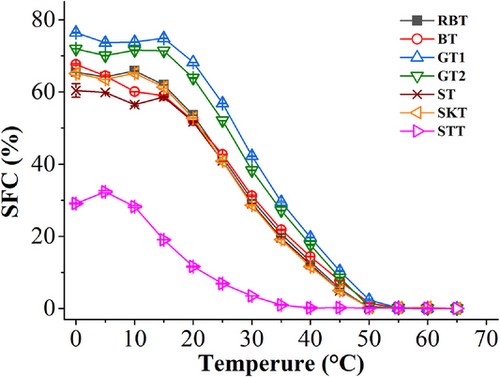
The SFC profile was a good reflection of the plasticity range of shortenings: 16%–30% at 21.1°C, 26°C 13%–27%, 33°C 7%–21%, and 40°C 2%–11%,[43, 44] the SFC of shortening was generally controlled at 10%–37.5% when the temperature was at 40–10°C. Among the tallows, STT had the widest plasticity range and was relatively most compatible with the requirements of SFCs at different temperature, whereas the other tallows alone did not meet the requirements and were usually modified in the food industry to product target shortening.
3.5 Thermal characteristics of tallows
The differential scanning calorimetry can be used to determine the properties of melting and crystallization of nature fats during heating and cooling process. The onset, peak, and offset temperatures were also described during the phase change occurring.[45] The heating and cooling profiles of the tallows are shown in Figure 2. Some subpeaks appeared in the heating and cooling processes of the MT and BTs, which could not be considered peaks because they did not return to the baseline.
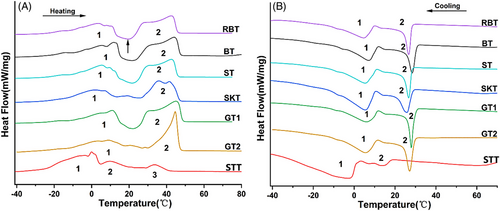
In the heating process (Figure 2A), BTs, ST, SKT, and GTs had two broad endothermic peaks at −20–20°C (peak 1) and 20–55°C (peak 2), respectively. A shoulder peak was observed in peak 2, which might had a relation with the earlier melting of some unstable crystal structures and the recrystallization of the remaining TAGs fractions to form more stable crystal structures. These new crystal forms may melt at higher temperatures, leading to the formation of shoulder peaks.[46] For RBT, BT, ST, and GT1, there was an exothermal peak appeared between peak 1 and peak 2 in the thermogram (Figure 2A). These exothermal peaks might be due to the polymorphic transformation during the heating process.[47] The BTs, ST, SKT, and GT1 presented wide melting peaks, suggesting the simultaneous melting of two or more TAGs in them, leading to an overlap of two consecutive thermal transitions. Compared to GT1, the peak 2 of GT2 was narrower and sharper, which indicated that GT2 had more TAGs with similar melting points between 20 and 55°C. The offset temperatures (Toffs) of peak 2 for RBT, BT, ST, SKT, GT1, and GT2 were 45.6, 47.3, 45.8, 47.0, 48.4, and 46.8°C, respectively, which were largely close to their SMP (Table 1), respectively. The transition from peak 1 to peak 2 of RBT, BT, ST, and GT1 showed a downward convex peak below the reference line, which likely associated with a crystallographic transition of the lower-melting fraction during the melting process.[43] STT showed two broad endothermic peaks and a weak one, namely, peak 1, peak 2, and peak 3, with summits at 0, 9.7, and 33.7°C, respectively. The Toff of peak 3 was 41.2°C. In the heating process, STT showed lower Ton and Toff than other tallows, which might be due to the difference of composition in STT. There was higher UFA (Table 1) and UUU/UUS (Table 3) in STT.
In the cooling process (Figure 2B), it showed two evident exothermic peaks of all the tallows samples. The peaks (peak 1), located in the higher temperature region, between 35 and 10°C in the thermograms, represented the crystallization of the fraction rich in saturated TAGs. The peaks (peak 2), ranging from 10 to −10°C, represented the crystallization of the phase rich in unsaturated TAGs. BT, ST, and GTs had two exothermic peaks 1 and 2 between −10–10 and 10–40°C, respectively. Compared with peak 1, peak 2 was narrower and more intense, which indicated that the high melting point components crystallized rapidly in this temperature interval. The TPs of peak 1 for RBT, BT, ST, SKT, GT1, GT2, and STT were 10.1, 7.1, 5.4, 5.4, 6.0, 17.2, and −3.0°C, respectively, and the Tps of peak 2 were 26.7, 28.4, 26.7, 25.8, 27.9, 27.1, and 13.8°C, respectively. STT presented one weak and broad peak (peak 1, at 20–5°C), and one large and broad peak (peak 2, at 5 to −35°C). STT had lower Ton of crystallization than other tallows. The peak 2 of STT was more significantly weak than other MT and BTs. This was presumably connected to the lower TAGs with melting points at 10–40°C in STT.
3.6 Crystal microstructure of tallows
The fats were scanned separately by XRD to obtain information on the molecular organization of crystals.[48] Small and wide angle diffractograms of the MT and BTs are shown in Figure 3.
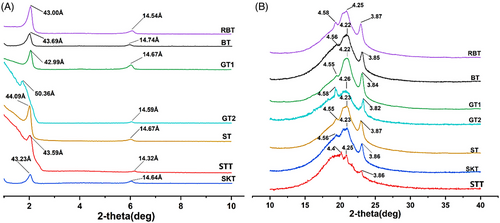
The information in the small angle region could reflect the interlayer arrangement of fat crystals. The diffraction peaks at about 41 and 14 Å indicated that TAGs in crystals were stacked in double chain length (2L) form, and the appearance of peaks at 61 and 31 Å showed that TAGs were stacked in triple chain length (3L) form.[49] It was known that the crystal near 39–44 and 14 Å were also an indication of the 2L-packing.[50] As can be seen from Figure 3A, GT2 showed two diffraction peaks at 50.4 and 14.6 Å. The diffraction peaks of other samples were close to 43.5 and 14.5 Å. Which indicated that the stacking form of the TAGs of these fats were 2L.
The short spacing information in the wide angle region could characterize the crystal polymorphism. The strong diffraction peak around 4.15 Å corresponded to α form; meanwhile, the peaks appearing at 4.2 and 3.80 Å represented the β′ form; the strong diffraction peak at 4.60 Å was the β form.[51] As can be seen from Figure 3B, the BT, ST, and GTs all had two weak peaks at 4.2, 4.6 Å, and a strong peak at 3.85 Å, indicating that these tallows were mainly β′ form but also had some β form (Table 4).
| Samples | Spacing (Å) | Polymorphic form (s) |
|---|---|---|
| RBT | 4.58, 4.25, 3.87 | β < < β′ |
| BT | 4.56, 4.22, 3.85 | β < < β′ |
| ST | 4.55, 4.23, 3.87 | β < < β′ |
| SKT | 4.56, 4.23, 3.86 | β < < β′ |
| GT1 | 4.55, 4.22, 3.84 | β < < β′ |
| GT2 | 4.58, 4.26, 3.82 | β < β′ |
| STT | 4.40, 4.25, 3.86 | β < < β′ |
- Abbreviations: BT, beef tallow; GT, goat tallow; RBT, refined beef tallow; ST, sheep tallow; SKT, sheep kidney tallow.
The STT had diffraction peaks at 4.4, 4.25, and 3.86 Å, which was similar to the result reported by Hou et al.,[17] indicating that the crystalline type of the crystals was β′ form. The β′ crystal polymorphism could form a dense network and was the crystal polymorphism required for shortening and margarine. Therefore, the MT and BTs in this study could meet the crystal form requirements of shortening or margarine and could be used as the base oil for shortening or margarine.
3.7 Morphology of tallows
In general, good quality shortening has strict requirements for crystal form, crystal size, and inter-crystal density.[52] If the size of the crystal aggregates was too large, it would result in a product with a severe sandy feel and a relatively rough texture. On the contrary, if the crystal size was too small, the hardness of the product may be relatively high.[53] The microscopic morphology of the MT and BTs was observed by using a polarized light microscopy, and the results are shown in Figure 4. From the polarized pictures, it was clear that the crystal morphology of these fats was slightly different. At a magnification of 100×, the aggregation of crystals to form large crystal clusters was observed. At a magnification of 200×, STs and GTs had similar morphology, were presented as globular crystal cluster, there was a clear nucleus, the nucleus was surrounded by a large number of irregular flocculent crystals. The similar microstructural morphology of palm oil was observed by Marangoni and Rousseau.[54] The micromorphologies of BTs were similar to the performance of irregular clusters of granular crystals. Notably, compared to unrefined BT, the globular crystal size of RBT was smaller and the crystals were more tightly packed, indicating that RBT had a more desirable crystal structure and was suitable for use as a base fat for shortening or margarine.
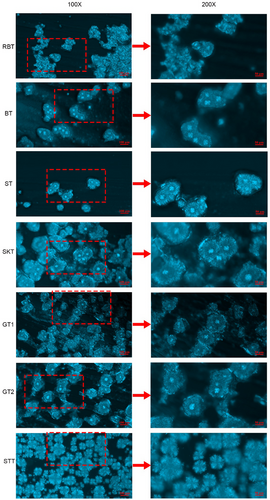
The 100× polarized picture showed that the crystals of STT were mainly irregularly shaped, spherical aggregates of varying sizes. From the 200× polarized image, it was observed that the microstructure of STT was composed of many tiny needle-like crystals aggregated to form snowflakes of varying sizes. Compared with the crystal morphology of GTs, STs, and BTs, no obvious flocculated areas were observed around the crystal aggregates of STT. The significant difference between the microscopic morphology of STT and the other MT and BTs may be explained by their significant differences in composition.[55]
4 CONCLUSION
The total FA and sn-2 FA composition of MT and BTs were dominated by oleic, stearic, and palmitic acids; SFAs were higher than unsaturated FAs in BTs, GTs, and STs, whereas SFA content was lower than unsaturated FA in STT. Some natural trans and odd chain FAs were presented in MT and BTs; however, they were not detected in sn-2 position of TAGs. The IV of STT was higher than those of BTs, STs, and GTs; however, the SMP of STT was significantly lower than those BTs, STs, and GTs. With the exception of STT, MT, and BTs had high SFC (more than 30%) at room temperature. STs, GTs, and BTs had higher USS/SSS and lower UUU/UUS than STT. The melting and crystallization profiles of BTs, GTs, and STs were similar and different significantly from those of STT. In terms of microstructure, the stacking mode of TAGs in all MT and BTs was double chain length structure (2L), and all of them contained fine β′ form crystal, but a certain amount of granular textured β form was also present. The aggregates of the fat crystals of STs and GTs were similar and presented as globular cluster, there was a clear nucleus, the nucleus was surrounded by a large number of irregular flocculent crystals; the micromorphologies of BTs were similar to the performance of irregular clusters of granular crystals; the microstructure of STT was composed of many tiny needle-like crystals aggregated to form snowflakes of varying sizes. Overall, the MT was suitable for use as a base stock for animal fat-based plastic fats (such as shortening or margarine) in addition to lard and BT, and the ideal crystalline form of STT as well as its lower SFC made it suitable for use as a liquid oil stock in plastic fats. This work provides a theoretical support for expanding the application of MTs in food industry.
ACKNOWLEDGMENTS
This work was sponsored by the National Natural Science Foundation of China (No. 31772003).
CONFLICT OF INTEREST STATEMENT
All authors declare that there are no conflicts of interest in this research.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




