Stability and Bioaccessibility of β-Carotene in Oil in Water Emulsions: Impact of Antioxidant Type and Emulsifier
Abstract
The objective of this study is to explore the influence of proteins type and antioxidants on the physicochemical properties, in vitro digestion, and bioaccessibility of β-carotene laden emulsions. The combination of rutin and whey protein isolate provides optimal bioaccessibility while also stabilizing the emulsion system and effectively preventing β-carotene degradation. This is due to the amphiphilic rutin adsorbed into the interface of the emulsion stabilized by whey protein, resulting in partially replacing and further improving the physical and oxidative stability of the β-carotene emulsion. Meanwhile, the rutin emulsions remain stable and carotene content is still higher (22.38–32.07%) than others even after digestion. Hence, these emulsions show great potential to be applied for delivering β-carotene based on oil-in-water emulsions. This study provides valuable information to build up stable excipient emulsions for functional foods and nutraceuticals.
Practical applications: β-carotene is being exploited for a range of biomedical applications. Concentrate on the blend of antioxidants and proteins displaying various properties in β-carotene O/W emulsions that can more likely convey β-carotene to the body and better keep up with natural movement.
1 Introduction
β-carotene is a carotenoid, which is a vitamin A precursor, that plays an important role in quenching singlet oxygen, inactivating free radicals, regulating lipid metabolism, and expression of oxidation-related enzyme receptors.[1, 2] β-Carotene is largely utilized in the food industry as a colorant and nutraceuticals.[3] Although with its unique health benefits comes, β-carotene is known for its highly sensitive to light, oxygen, and heat, due to its conjugated polyunsaturated hydrocarbon chain for oxidation, isomerization, and degradation.[4, 5] Also, the gastrointestinal bioaccessibility and bioavailability limit its application in the food industry.[6-8] That is why encapsulating β-carotene into various delivery systems is a hot topic in order to improve its solubility, chemical stability, and bioavailability upon ingestion.
Functional lipophilic ingredients such as vitamin A, D, and E, β-carotene, lycopene, lutein, curcumin, resveratrol, and coenzyme Q10 can be incorporated into oil droplets of an oil in water (O/W) emulsion.[9, 10] In emulsions, oil droplets are protected by membranes formed by emulsifier molecules, which act as a barrier to protect the droplets from highly acidic environments, light, heat, singlet oxygen, transition metals, and free radicals.[11, 12] The interface layer, in particular, can prevent flocculation and coalescence by scraping out the pro-oxidant in the water phase via a physical barrier (sometimes an electrostatic barrier).[13, 14] Therefore, the formation of an emulsifying interface layer is absolutely essential.[15]
The addition of antioxidants and protein-type emulsifiers can effectively at prevent the oxidative degradation of β-carotene.[16-18] Protein emulsifiers such as milk proteins of whey protein isolate (WPI) and casein (CN) and plant protein of soy protein isolate (SPI) are widely used in food emulsions such as beverages, soups, sausages, and coffee whiteners, due to their excellent nutritional and functional properties.[19, 20] The interfacial protein between oil and water assisted by the different types of antioxidants can better prevent β-carotene oxidation in O/W emulsion.[21] The oxidative degradation of β-carotene has been shown to be significantly reduced by both oil-soluble antioxidants (such as coenzyme Q10 and α-tocopherol) and water-soluble antioxidants (such as ethylenediaminetetraacetic acid (EDTA),[22-24] ascorbic acid, and gallic acid compounds). Nonetheless, the criteria are not precisely selecting the proper antioxidant in a complex emulsion system, yet relies upon many variables. Because antioxidants with various properties in the same emulsion have different antioxidant results.[25, 26] As previously reported, the amphiphilic antioxidants ascorbyl palmitate and propyl gallate are more effective than the highly hydrophobic α-tocopherol in O/W emulsions stabilized with Tween 65 or Tween 80.[27] In addition, some proteins containing disulfide bonds might interact with antioxidants (i.e., polyphenols) in food systems by H-bonds, electrostatic attraction, and hydrophobic association, which stabilize the emulsion by interaction of molecules.[28-30] The conjugates have improved antioxidative, emulsifying, and steric stabilizing properties.[31] Therefore, obtaining an ideal match of protein and antioxidant is essential for building up protecting structures and better antioxidantive capacity to ensure the bioaccessibility of β-carotene.[32]
This work is focusing on two aspects: I) investigating the effect of three proteinaceous emulsifiers (WPI, SPI, and sodium caseinate (SCN)) for β-carotene emulsion system by the combination of three antioxidants with different solubility (ascorbyl palmitate, as representative of lipophilic; rutin as an example of amphiphilic, ferulic acid as a typical hydrophilic); II) selecting optimal match of antioxidants and three protein emulsifiers for β-carotene emulsion system by a series of evaluation indicators containing stability, particle size, ζ-potential, particle size index, and in vitro simulated digestion.
2 Results and Discussion
2.1 ζ-Potential Analysis of Emulsion
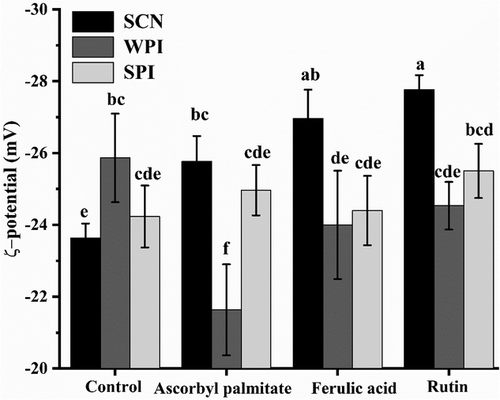
A strong negative charge was detected at a pH of 7, which prevented the accumulation of oil droplets and maintained the emulsion's stability. And the emulsion was relatively stable since a higher absolute value of the ζ-potential. The emulsions without antioxidants values were −56.36, −50.56, and −54.9 mV, as shown in Figure 1. Probably because ferulic acid and rutin have certain acidity and surface activity, and the ionized H+ will neutralize part of the hostile groups at the emulsion interface, which significantly reduced the absolute potential value of the WPI and SCN emulsion (p < 0.05).[33]
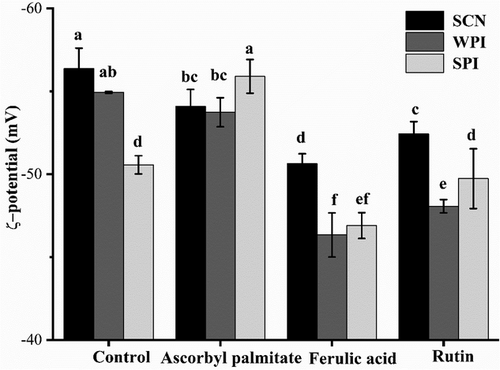
While, the addition of ascorbyl palmitate had non-significant effect on ζ-potential of milk-protein emulsions, except for SPI emulsion, which had slightly elevated (−55.9 mV). The increased absolute ζ-potential values of SPI emulsion with ascorbyl palmitate were possibly due to ionization of the ascorbic acid headgroup of ascorbyl palmitate (with a pKa = 4.2) at neutral pH conditions,[33] which provided a high energy barrier preventing particle aggregation and flocculation. These findings indicated that increased negative surface charge contributed significantly to decrease in particle size and ζ-potential, thereby improving emulsion physical stability.
2.2 Particle Size Analysis of Emulsion
As shown in Figure 2I, the droplets were relatively smaller (300–400 nm) across emulsions containing antioxidants. Our results showed adding ferulic acid and rutin could reduce the average droplet diameters by a significant amount compared to other emulsions. It's notable that the polyphenol compound like rutin and ferulic acid can form a thick and dense complex film with protein in the interface layer, which could increase the steric hindrance between droplets, inhibit the flocculation and coalescence, and improve the emulsion physical stability.[34] Similar results were obtained from ζ-potential results, despite ascorbyl palmitate is not a polyphenol compound, but it still improves the stability of the emulsion. Increasing the temperature (60 °C, Figure 2II) has been observed to adequate grow of the droplet sizes in different groups (300–500 nm), which indicated that the small droplets coalesced during storage and formed larger droplets.
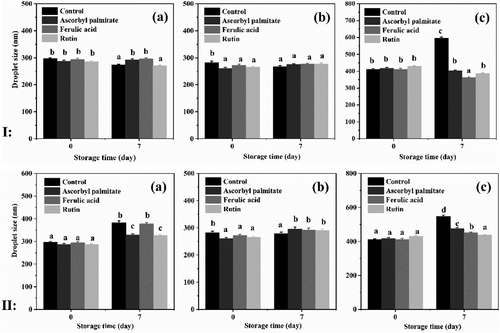
As well, the particle size of the WPI emulsion stored at different temperatures changed slowly. Indicating the emulsification stability of WPI, it showed higher levels than the other two protein emulsions. Different proteins with the same antioxidant may have different storage stability, for example, the particle sizes of SCN, WPI, and SPI emulsion containing ascorbyl palmitate after storing were 281.75, 269.8, and 639.1 nm, respectively. Furthermore, there was a distinctly different growth in the particle size of SPI emulsion, in agreement with the potential results.
2.3 Interfacial Adsorption Behavior
Antioxidants will be distributed in different locations of the emulsion (oil, aqueous, and interfacial layers) due to their different polarities, and measuring the antioxidant and protein content of the interfacial layer will help to analyze the interaction between antioxidants and proteins more intuitively.
Figure 3A,B illustrates the protein and antioxidant interfacial concentrations in oil-in-water emulsions. As shown in Figure 3A, emulsions generated with ascorbyl palmitate had a greater protein load of around 7 mg mL−1, which was comparable to the control group without antioxidant, showing that ascorbyl palmitate significantly increased the quantity of protein adsorbed. Furthermore, the effects of ferulic acid and rutin on protein interface adsorption are similar.
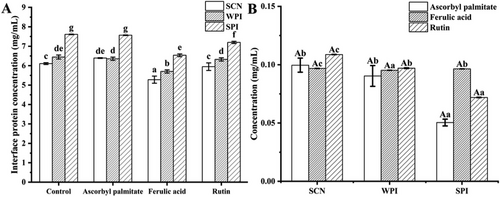
The interfacial rutin concentration for the three protein emulsions was definitely the greatest among the three antioxidant emulsions, as shown in Figure 3B. In other words, the emulsion with the highest interfacial rutin content may be more antioxidant. This finding was congruent with the findings of Cui et al.,[35] who demonstrated that rutin particles adsorb directly at the oil–water interface and interact with protein molecules. The results showed that ferulic acid and rutin were more widely distributed at the interface than ascorbyl palmitate, demonstrating that the presence of phenols can promote protein adsorption at the interface and alter emulsion properties such as emulsification and storage durability.
The stability and emulsified qualities of emulsions are intimately tied to the interactions between droplets and components, which are dependent on the structure and composition of the adsorbed protein layers at the surface of fat globules. The combination of SCN and WPI with rutin emulsions have the higher antioxidant content correspondingly consistent results were obtained in storage stability. Different to the SCN and WPI emulsions, SPI emulsions characterized with a higher interfacial protein content and a lower interfacial antioxidant content.
2.4 Changes of SRI During Emulsion Storage
The collisions between droplets became more frequent and aggregate formation accelerated as the temperature rose, resulting in the unstable emulsions. The SRI is an important parameter to characterize the physical stability. We maintained the same homogeneous pressure used in the emulsion preparation process, as previously reviewed.[36] No obvious distinction in fresh emulsion between the SCN and WPI groups (basically around 0.20) were observed in Figure 4. As the SRI did not change significantly after storage, reflecting that the emulsion was relatively stable at 30 °C. Higher SRI levels of SPI were observed in Figure 4 compared with the blank emulsion (0.267). The SRI associated with different antioxidants raised from 0.22 to 0.24, because the antioxidants could improve the SPI emulsion physical stability to certain extent. In addition, it could be found that the elevated growth in SPI emulsions compared to the WPI emulsions and SCN emulsions.
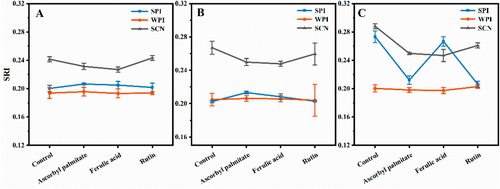
Along with temperature increased (60 °C), the minimum SRI was found in the WPI emulsions, particularly in ferulic acid. It is possible that the formation of protein-polyphenol conjugates (WPI-ferulic acid), as well as the thicker and denser interfacial layers at droplet surfaces, enhanced capacity to stabilize β-carotene emulsions. This phenomenon is easier to occur at a higher temperature, because the mixture can be formed through Maillard reaction based on the Amadori rearrangement.[37]
2.5 Degradation of β-Carotene During Storage
Considering the sensitivity of β-carotene to temperature, changes of β-carotene in different groups were determined,[33] as shown in Figure 5. Whether at 30 or 60 °C, the degradation of β-carotene in SCN emulsion and SPI emulsion occurred and significantly increased on the fourth day. On the contrary, the WPI emulsion is relatively stable and the β-carotene content still has higher values even at 60 °C (60.83% in emulsion with ascorbyl palmitate, 49.42% in the emulsion adding ferulic acid, and 86.05% in emulsion with rutin).
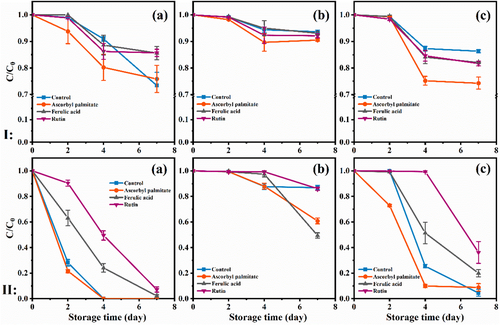
From the perspective of antioxidants, the influence of antioxidants on lipid oxidation will significantly affect the stability of β-carotene.[38] Li et al.[15] found that ascorbyl palmitate acted as a free radical inhibitor for automatic oxidation at low concentrations. The presence of antioxidants impedes the reaction between β-carotene and singlet oxygen or other free radicals.[38, 39] The ability to capture free radicals of antioxidants often depends on the physical distribution in the emulsion. When the polarity of the antioxidant is reduced or its surface activity is strong, it is more easily distributed in the emulsion interface where oxidation often occurs in the oil, thus promoting the antioxidant inhibition of emulsion oxidation. In other words, the polarity and surface activity of the antioxidants lead to the equally effect on the β-carotene stability.
Further analysis showed that the SRI value had no significant relationship with the loss of β-carotene degradation. Indeed, the smaller size emulsion is meaning the larger surface area and the oxidative degradation rate of β-carotene will be accelerated.[2, 5] In particular, the emulsion in this study presented a different trend. The degradation rate of β-carotene of SPI emulsion at 60 °C increased with the emulsion particle size. Although the blank group was larger, the final degradation rate of β-carotene showed an increasing trend under the combined influence of multiple factors such as free radicals and pro-oxidation factors in the emulsion. Moreover, the emulsions with rutin and other two antioxidants showed smaller emulsion particle sizes, the final degradation rate of β-carotene decreased gradually because the antioxidant was able to capture free radicals and offset the adverse effect of the smaller particle size.
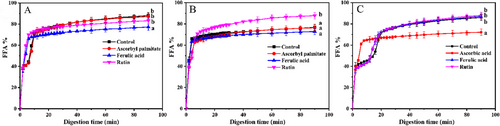
2.6 FFA Release Rate of β-Carotene Emulsions During Digestion
The digestion in the gastrointestinal tract is a complex process, and the release behavior plays an essential role in the absorption, distribution, and bioavailability of bioactive ingredients.[40] As shown in Figure 6, the release of FFA increased rapidly in the first 10 min, indicating that oil was rapidly hydrolyzed by pancreatic lipase and bile salt, resulting in the generation of large amounts of diglycerides, monoglycerides, and free fatty acids. Ferulic acid and ascorbyl palmitate can delay the digestion speed of SCN emulsion and SPI emulsions. The possible reason is that ferulic acid and ascorbyl palmitate will interact with part of pancreatic lipase, reducing the available lipase content, and thus reducing the digestion speed. Due to the strong surface activity of rutin, the competitive adsorption with the WPI emulsion interface makes some of the proteins replaced, increasing the thickness of the oil film, which is more conducive to pancreatic lipase and lipid contact reactions,[41] and promotes the occurrence of the digestive process.
2.7 Changes of ζ-Potential in the β-Carotene Emulsion During Digestion
Compared with the potential value of fresh emulsion, the negative potential value of emulsion decreased obviously after digestion, and the potential of all emulsions ranged from −22 to −27 mV in Figure 7. Antioxidants and proteins have a limited effect on the emulsion system potential. Due to the large amount of negatively charged surface-active substances such as lipids and bile salts in the digested β-carotene emulsion, these substances are easily replaced by proteins and adsorb on the surface. The amount of negative charge of lipids and bile salts is lower than interface proteins, so the total interface charge of the emulsion decreases when proteins are replaced out of the interface.[42]
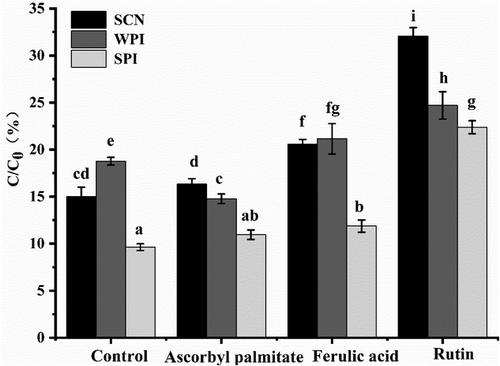
2.8 Degradation of β-Carotene in the Digested Emulsion
During simulated digestion, the original interfacial membrane in the O/W emulsion is destroyed, and the proteins used to assist in stabilizing emulsions are hydrolyzed into small peptides, which have much lower emulsification capacity than the protein itself.[43] The bare surface of the oil droplets is more prone to resist collision and coalescence, and the β-carotene in emulsion is more prone to oxidative degradation.[44] Figure 8 showed the β-carotene retention after digestion. For β-carotene emulsion embedded with SCN, the final retention rates of blank and emulsion with antioxidants of ascorbyl palmitate, ferulic acid, and rutin emulsion were 15.00%, 16.33%, 20.57%, and 32.07%, respectively, indicating that oxidation occurs in the gastrointestinal digestion period and antioxidants can significantly inhibit the degradation of β-carotene in SCN emulsion (p < 0.05). It is probably because rutin with strong surface activity can easily adsorb to the interface and form a natural barrier to block free radicals. The final retention rates of SPI emulsions were 9.63%, 10.95%, 11.87%, and 22.38% and that of WPI were 18.77%, 14.76%, 21.15%, and 24.70%, respectively. It was found that ascorbyl palmitate and ferulic acid had relatively low antioxidation capacity compared with rutin, which was similar to the previous results of the storage oxidation experiment. (Figure 5)
3 Conclusion
This study showed an ideal combination of antioxidant and protein can be utilized to improve the chemical and physical stability of the emulsions. Further, the combination can improve the solubility of β-carotene and its bioaccessibility. The order of the three antioxidants improving SPI emulsion's physical stability was: rutin > ferulic acid > ascorbyl palmitate > blank. However, the effect of the three antioxidants for SCN and WPI emulsions was not obvious. When storage the WPI emulsions at 60 °C, ferulic acid and ascorbyl palmitate had a limited effect on inhibiting degradation of β-carotene. For the emulsions stabilized with SCN and SPI, the antioxidants could significantly inhibit the degradation and the order was rutin > ferulic acid > ascorbyl palmitate > blank. After digestion, the emulsion's absolute ζ-potential dropped visibly. Three kinds of antioxidants can inhibit the decomposition of β-carotene in different degrees during the digestion of protein emulsion, among which rutin is the best followed by ferulic acid. As expected, milk protein-excipient emulsions are good candidates for improving physical stability and the bioaccessibility of lipophilic active compounds such as β-carotene if choose the proper protein and the excipient. Therefore, the combination of rutin with an amphiphilic property and strong antioxidative stability and WPI was proven as a good partner to deliver β-carotene.
4 Experimental Section
Chemicals
The β-carotene was purchased from Yuanye Biotechnology Co., Ltd. (Shanghai, China). Pine nut oil was obtained from the local market. Whey protein isolate (WPI), soybean protein isolate (SPI), sodium caseinate (SCN) with a protein content of 91–92% used in this study were supplied by Glanbia Nutritionals Ltd. (Ireland). All the other chemicals were of analytical grade.
Preparation of β-Carotene Emulsion
The emulsions preparing method was measured by the previous method.[45] First, the endogenous antioxidants in pine nut oil were removed according to the reported multilayer chromatographic method by the packed column with silica gel, Celite 545, activated carbon, and granulated sugar.[23] Second, the β-carotene (0.25% w/w) was completely dissolved by stirring and heating the oil phase (pine nut oil) at 140 °C for 30 s. For the water phase, WPI (1% w/v) was dispersed in the phosphate buffer (10 mm, pH 7.0), stirred magnetically for 5 h and hydrated overnight. Then sodium azide (0.02%, w/v) was added to the dispersion as an antimicrobial agent. Ascorbyl palmitate, ferulic acid, and rutin were dissolved in the aqueous phase to a final concentration of 0.15 mg mL−1, and the WPI dispersion without any antioxidant was used as the control group. Cui et al.[35] reported a method for preparing rutin dispersion that was modified slightly. Rutin was added to a screw-capped 50 mL Erlenmeyer flask filled with methanol solution and agitated for 1 h. Following that, the methanol in the mixture was evaporated using a spray of nitrogen, and the rutin was dispersed with water and sonicated for 5 min. Accordingly, we continued to magnetically stir for 3 h to completely dissolve and form an aqueous phase (90%, v/v). Mixing the oil phase and the water phase at 12 000 rpm for 2 min using a high-shear mixer (model IKA-ULTRA-TURRAX T25 basic, IKA Works, Inc., Wilmington, NC). Next, the final emulsion was prepared through the high-pressure homogenizer (model GYB 30–6S, Donghua High Pressure Homogeniger Factory, Shanghai, China) three times (90 s) at the homogenization pressures of 35 MPa, pH adjusted to 7.0.
Finally, the β-carotene emulsion was acquired with the volume fraction of the oil phase of 10%, a mass fraction of β-carotene of 0.025%, and a mass fraction of WPI of 0.9% was obtained. The other two groups were made in the same way, using SCN and SPI dispersion.
Particle Characterization
Each emulsion's droplet particle size and ζ-potential were determined by dynamic laser light scattering by NICOMP 380 ZLS analyzer (Particle Sizing System, Santa Barbara, CA, USA) at 25 °C. Before each measurement, the emulsion was diluted 100 times with distilled water.
Determination of Particle Size Index
Determination of β-Carotene Retention
Interface Protein and Antioxidant Concentrate
The interface protein content of excipient emulsion droplets was determined according to the previous reports.[35, 47] The emulsion was taken 1 mL in a centrifuge tube and centrifuged at room temperature for 50 min at 15 000 × g. After centrifugation, the emulsion was separated into oil–water phase and the aqueous phase. And the amount of unadsorbed protein remaining in the serum phase was removed the bottom with a syringe and put it over the 0.45 µm membrane and determined by Bradford method using a Protein Assay Kit (Beyotime Biotechnology, Shanghai, China).
Second, absolute ethyl alcohol (2:1, v/v) was added to another portion of the serum phase and sonicated for 5 min to sufficiently extract the unadsorbed rutin.[48] The amount of rutin in the serum phase was determined using the method given by Cui and Gan.[35, 39] The absorption spectra of samples were investigated using UV-spectrophotometry (Shimadzu UV 2600) at 258 nm. Similar to the process of rutin, the ascorbyl palmitate contents were measured by the previous method with some modified.[25, 49] The unadsorbed ascorbyl palmitate from the supernatant was quantified using reverse-phase high-performance liquid chromatography (RP-HPLC). The inclusion content of ascorbyl palmitate was calculated by subtracting the unadsorbed ascorbyl palmitate from the total added ascorbyl palmitate. RP-HPLC analysis was carried out using the Waters 2695–2998 HPLC and a Shield RP-C18 column (250 × 4.6 mm i.d., 5 µm particle size) was utilized for HPLC and analytes were eluted at a flow rate of 1.0 mL min−1. Solvent A was water acidified with phosphoric acid (1% v/v) and solvent B was methanol/acetonitrile 1:1 (v/v). Gradient conditions were as follows: 0–1 min, 40% B; 1–5 min, 40–60% B; 5–12 min, 60–95% B and 12–20 min, 95% B. The injection volume was 10 µL and the detection was by UV absorbance at 243 nm. Pure palmitic acid and ascorbyl palmitate served as standard. And the ferulic acid was measured by Tian et al. by UV 2600 at 320 nm.[50, 51]
Simulate In Vitro Digestion
To simulate the β-carotene bioaccessibility of ingested samples, the previous digestion model was carried out using with some modifications.[43, 52-54]
At the stage of simulated gastric digestion, 10 mL of the emulsion was mixed with 30 mL of simulated gastric fluid (SGF) containing 0.0032 g mL−1 pepsin to mimic gastric digestion. The mixture was finally adjusting the pH to 1.2 and incubated with a stirring speed of 200 r min−1 at 37 °C for 90 min.
Data Processing
All measurements were assayed in triplicate. Data were processed by Origin 8.0 and SPSS 16.0 software (SPSS Inc., Chicago, IL, USA), and the difference between the mean values of samples was compared by Duncan method (p < 0.05).
Acknowledgements
This research was supported by the National Natural Science Foundation of China (Grant No. 31901614).
Conflict of Interest
The authors declare no conflict of interest.
Author Contributions
J.L.: Formal analysis; Investigation; Writing—review and editing. X.C.: Formal analysis; Investigation; Writing—original draft. C.L.: Formal analysis; Investigation. H.X.: Writing—review and editing. G.M.: Writing—review and editing. X.Z.: Funding acquisition; Methodology; Resources; Supervision; Writing—review and editing.
Open Research
Data Availability Statement
The data that support the findings of this study are available upon reasonable request.




