Excellent Properties of Dimer Fatty Acid Esters as Biolubricant Produced by Catalyst- and Solvent-Free Esterification
Abstract
Currently most technologies available to produce esters require acid or base catalysts for esterification or transesterification reactions. Production of dimerate esters (DE) exhibiting potential as a biolubricant for low temperature applications using catalyst- and solvent-free approaches is presented in this article. Hydrogenated C36 dimer acid and alcohol are reacted under the following conditions: dimer acid/alcohol (1:4.5 molar ratio), 150–200 °C, 24 h, 3Å molecular sieve (15% w/w). The performances of four DE species—dibutyl, dihexyl, di-(2-ethylhexyl), and dioctyl dimerate—as lubricant base stocks are evaluated by kinematic viscosity, viscosity index, cloud and pour point (cold flow properties) as well as oxidative stability, and compared with commercial synthetic lubricant base stock and DE, Radialube 7121. High viscosity indexes ranging between 129 and 138 are observed for the synthesized DEs, which are comparable with two commercial base stock, polyalpha olefin (PAO), and polyolester (POE). Significantly low pour point, less than −42 °C, is observed for di-(2-ethylhexyl) dimerate attributed to the branching of the side chain. The DEs are categorized as ISO VG 68 based on their viscosity according to ISO 3448 classification and show potential as biolubricant with high viscosity index and excellent cold flow properties.
Practical Applications: DFAE obtained have high potential to be used as lubricant base stock for equipment and machinery operating at extremely low temperature.
1 Introduction
In response to raising concerns toward environmental sustainability, depleting petroleum reserves, and stringent government regulations, development of biolubricant for industrial application has become of great interest. Highly sustainable technologies and practices are required to cater the ever-increasing global population while moving toward a society with bio-based feedstock, processes, and products for a subsistence economy.1 Biolubricant, referring to lubricant derived from bio-based materials such as vegetable oils, may have to undergo some modification to overcome limitation in its original form. However, some are found to be comparable or having even superior performance to the conventional mineral lubricants, on top of their renewability and biodegradability.2 In addition to bio-based raw material, lubricants made from synthetic esters and petroleum oils that satisfy established biodegradability and toxicity criteria can also be regarded as biolubricant.3 Recently, Zainal et al. (2018) provided an extensive review on biolubricant research detailing on the chemistry, production and technologies developed.4
Vegetable oil-based dimer acid is an important chemical intermediate with unique polyfunctional structure.5 It is a high molecular weight dibasic acid produced by polymerization of unsaturated fatty acid such as oleic and linoleic acid obtained from vegetable oil catalyzed by clay catalyst. Yang et al. (2013) reported dimerization of oleic acid in the presence of Montmorillonite (9 wt%) at reaction temperature of 260 °C for 8 hours (h) affording 72% yield.6 Using similar catalyst, Yasa et al. (2017) reported polymerization of 10-undecenoic acid in addition of deionized water (2.5 wt%) affording mixture of iso-undecenoic acid (47–51%), dimer acid (30–32%), and higher polymeric acids (17–23%).7 Further investigation indicated that chemical modification on montmorillonite which led to the change in interlayer spacing and surface acidity has significantly affected the product yield.8
Dimer acid, which appears as a clear, viscous liquid, is a useful polymer building block as it can impart unique properties in polymer network such as elasticity, flexibility, hydrophobicity, and intrinsically low glass transition temperature (Tg).9, 10 Furthermore, bio-based and non-toxicity properties of dimer acid allow its usage as eco-friendly materials.
Two reactive functional groups in dimer acid enable transformation of this material into other useful functionalities for various applications such as resins, surfactants, adhesives,11 inks, coating,12 and other applications.13 Dimer acid-based polyurethane (PU) synthesized from waste soybean oil was shown to have improved mechanical strength compared to PU from commercially available polyol.14 This is in accordance to a study by Park et al. (2018) who demonstrated improved mechanical strength of synthesized dimer acid-based polyamide from waste vegetable oil.15 Reulier et al. (2016) showed enhanced storage modulus of blends of dimer acid-based polyurethane and polyamide incorporated with talc as biocomposite thermoplastics.16
Lee et al. (2015) showed that diester derivatives of dimer acid exhibited better thermal properties compared to petroleum-based dioctyl phthalate (DOP) as plasticizer.17 The ester derivatives or dimer fatty acid esters (DFAEs) generally exhibit good thermal and oxidative stability which make them an excellent lubricant.18
While industry-wide efforts are underway to replace conventional petroleum-based chemical feedstock with renewable resources, there is also a compelling need in the design of sustainable synthesis and processes emphasizing on waste minimization, atom economy, and less hazardous and energy efficient synthesis. Indeed, the synthesis of DFAE could be even more attractive when carried out via environmentally benign processes. Solvent-free approach ensured an environmentally benign experimental procedure is in practice. Due to volatile and toxic nature of organic solvents, which are normally used in huge amount for large-scale reactions, their use poses serious threat to environment. Correspondingly, the cost of solvent waste treatment or removal for industrial scale would be added up into the total production cost.
Various green technologies for esterification have been investigated including self-esterification,19 heterogeneous catalysis, and enzymatic reactions,20, 21 and there are several previous studies highlighting solvent-free esterification for biolubricant production.22, 23 However, to the best of our knowledge, most of DFAEs are produced by esterification of dimer acid and alcohol catalyzed by organic acid such as p-toluenesulfonic acid,17, 24 or dimerization of methyl ester of fatty acids.25 In order to investigate the performance of DFAE as a biolubricant, in the present study, we extended our previous study26 to produce DFAE by catalyst- and solvent-free esterification which was then compared to tin-catalyzed esterification. Synthesized DFAEs were then characterized, and the performance as lubricant base stocks were evaluated and compared to commercial DFAE, Radialube 7121.
2 Experimental Section
2.1 Materials
Hydrogenated C36 dimer acid (98% of dimer and 1% monomer, and 1% trimer acid), 3 Å molecular sieve (powder form) and tin(II) oxalate (98%) were obtained from Sigma-Aldrich. 1-Butanol and 1-octanol were purchased from Fischer Scientific (UK). 1-hexanol and 2-ethylhexanol were obtained from Aeros Organic and Merck, respectively. All the reagents were used as supplied, without any purification and stored according to the manufacturer's recommendations. Commercial sample of di-(2-ethylhexyl) dimerate, Radialube 7121 (R7121) was generously supplied by Oleon.
2.2 Methods
2.2.1 Sample Preparation
Catalyst- and Solvent-Free Esterification of Dimer Acid
The DFAEs—dihexyl, di-(2ethylhexyl) and dioctyl dimerate—were obtained using reaction conditions as previously reported.25 In general, a mixture of dimer acid (114.0 g, 0.2 mol), alcohol (0.9 mol), and molecular sieve (17.2 g, 15 wt%) were transferred into a single neck 500 mL round bottom flask. The flask was then attached to a Dean–Stark apparatus and subjected to heating at 200 °C for 24 h after which the reaction was ceased. The reaction was then cooled down to ambient temperature and molecular sieve was removed by filtration. Excess of alcohol was removed by vacuum distillation to yield the reaction product.
However, dibutyl dimerate was prepared with slight modification of above procedure, that is, heating at a lower temperature, 150 °C. The product was isolated as yellowish oil (124.3 g, 92%) upon removal of excess of alcohol by vacuum distillation. 1H NMR (600 MHz, CDCl3) δ 4.04 (t, J = 7.0 Hz, 4H), 2.27 (t, J = 7.2 Hz, 4H), 1.60 (br. s, 8H), 1.44—1.03 (m, 64H), 0.87 (t, J = 6.5 Hz, 12H); 13C NMR (151 MHz, CDCl3) δ 174.0, 64.1, 34.5, 30.8, 29.8. 29.7, 29.3, 25.1, 22.8, 19.2, 14.6, 14.2, 13.9, 13.8; FT-IR (neat)/cm−1 2922, 2853, 1736, 1460, 1377, 1170, and 1119.
Tin(II) Oxalate-Catalyzed Esterification of Dimer Acid
Tin(II) oxalate (1 wt%) was added into three-neck round bottom flask containing dimer acid (114.0 g, 0.2 mol) and 1-octanol (142.2 mL, 0.9 mol). The flask was then attached to a Dean–Stark apparatus and the mixture was subjected to heating at 200 °C for 24 h after which the reaction was ceased. The reaction was cooled down to ambient temperature and the mixture was separated by centrifugation. Excess of alcohol was removed via vacuum distillation.
2.2.2 Characterization of DFAEs
The synthesized DFAEs were characterized by 1H NMR, 13C NMR, and FT-IR. 1H and 13C NMR spectra were recorded in Fourier Transform mode on a 600 MHz JNM-ECZR spectrometer (Jeol). The chemical shift for both 1H and 13C NMR spectra were recorded in ppm. All spectra were processed using Delta NMR processing and control v5.0.5.1 software. Deuterated chloroform (CDCl3) was used as solvent and reference (7.26 ppm). Multiplicities in the NMR are described as: s = singlet, d = doublet, t = triplet, td, triplet of doublets, q = quartet, sept. = septet, m = multiplet, br. = broad, coupling constants, J values are reported in Hertz (Hz). Infrared spectra were recorded as neat samples using a Perkin–Elmer Spectrum 100 FT-IR Spectrometer. FT-IR spectra were collected using 64 scans in the spectral range of 4000 to 600 cm−1. All IR data were manipulated using Spectrum v6.3.5 software.
2.3 Chemical Analysis
2.3.1 Acid Value
The acid value is defined as the number of milligrams of potassium hydroxide (KOH) required to neutralize the fatty acids in 1 g of sample and was determined using MPOB Test Method c2.7: 2004. This method is applicable to all fatty acids and polymerized fatty acids.27
The measurement was made in duplicate and presented as mean value.
2.4 Physicochemical Analysis
2.4.1 Density
Density was measured using digital density meter DMA 5000 M (Anton Paar). The sample (2 mL) was filled into the measuring cell slowly using 2 mL syringe until a drop emerged from the sample outlet adapter. Any presence of bubble in the measuring cell should be avoided and it can be detected using U-View. The measurement was made in duplicate for each sample at 25 °C and presented as mean value.
2.4.2 Kinematic Viscosity
Kinematic viscosity (KV) was calculated using the official ASTM method D 445–12. The measurement was carried out using PMT Tamson NVB Classic (Normalab, France) with viscometer bath set at 40 and 100 °C. The testing was made in duplicate for each sample and average values were recorded. Selection of different types of viscometry capillary was made according to the official ASTM method D 446. Ubbelohde capillary viscometers and the constant temperature bath were purchased from Cannon Instruments, State College, PA.
2.4.3 Viscosity Index
2.4.4 Cloud Point
Cloud point (CP) was measured using PP Classic purchased from Normalab (Belgium) to an accuracy of ± 1 °C according to the official ASTM method D 2500–09. The sample (50 mL) was placed in a test jar and observed in 1 °C decrement. The cloud point is defined as the temperature at which haze or cloud is first observed at the bottom of the test jar.
2.4.5 Pour Point
Pour point (PP) was determined using the official ASTM method D 97–12 to an accuracy of ± 3 °C The measurement was carried out using PP Classic purchased from Normalab (Belgium). The pour points were determined by placing a test jar containing 50 mL of sample into a cylinder submerged in ethanol (95%) as the cooling medium. The test jar was held in a horizontal position for 5 s in every 3 °C decrement and stopped when the sample was no longer flowing within 5 s in horizontal position. The temperature at which the sample was no longer flowing would be added 3 °C and recorded as the pour point. The pour point is defined as the lowest temperature at which the sample can still flow, or the movement of sample can be observed.
2.4.6 Oxidative Stability
The oxidative stability was determined according to the official ASTM method D 6186–98 (Reapproved)Ɛ1 using pressure differential scanning calorimeter (PDSC) model DSC Q20 purchased from TA Instruments, coupled with a high-pressure cell (Q20P) under the isothermal conditions in pure oxygen atmosphere. The equipment was calibrated using high purity indium as a standard. A small amount of samples (3.0 ± 0.2 mg) were weighed into an uncovered aluminium pan and placed onto the pressure DSC cell platform for sample pan. New empty pan with the same configuration was placed onto the pressure DSC cell platform for reference pan. Temperature of the cell was increased from ambient temperature to 80 °C at a rate of 100 °C min−1 and was allowed to equilibrate for 2 min. The cell was then slowly pressurized by pure oxygen to 3.5 ± 0.2 MPa and the purge gas flow rate was adjusted at 100 ± 10 mL min−1. Obtained diagram was analyzed using TA Universal Analysis 2000 software. The oxidation induction time (OIT) was measured from the time the oxygen valve was opened, measured as the intersection of the extrapolated baseline and a line tangent to the oxidation exotherm constructed at its maximum rate. All OIT data presented in this study are mean values of duplicate samples.
3 Results and Discussion
3.1 Catalyst- and Solvent-Free Production of DE
In our preliminary study, we carried out solvent-free esterification of dimer acid and 1-octanol (mole ratio 1:2) catalyzed by tin(II) oxalate (1 wt%) by heating at 200 °C for 7 h. We employed tin(II) salt as the Lewis acid catalyst as it is a stable solid, highly tolerant to water, easily handled, less corrosive, inexpensive, and accessible commercial Lewis acid catalysts, and have shown high activity on esterification reactions.28 Due to these properties, the use of organotin as a potential catalyst to produce esters for biodiesel,29-32 lubricant,33 and alkyd resin34, 35 has become a new interest. In addition, this tin-based catalyst is found to be efficient in solvent-free reaction conditions.28, 36
Under similar reaction conditions, we carried out catalyst-free esterification and the result is presented in Figure 1. The reaction progress was monitored by reduction of acid value. To our surprise, after 7 h, slightly higher acid value for tin(II) oxalate-catalyzed reaction was obtained compared to catalyst-free reaction, 21.9 and 17.3 mg KOH g−1 of sample, respectively. This lower reactivity is presumably due to high molecular weight dimer acid used in this reaction. This is in accordance with report by Khrouf et al. (1998) on low reactivity of tin(II) oxalate in high molecular weight polycondensation reaction.37
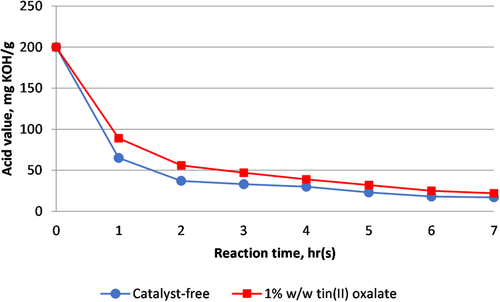
Thus, in our study, we adopted catalyst- and solvent-free approach which are more efficient to obtain DE with lower acid value compared to tin-catalyzed reaction. Using the optimized solvent- and catalyst-free reaction conditions we previously established,26 four species of DE were synthesized as shown in Figure 2. Compared to conventional acid-catalyzed process, this approach offers several advantages: 1) eliminates several post-synthesis steps, that is, neutralization, washing and drying processes, which are required to remove the acidity when homogeneous acid catalyst is employed;7, 17, 24 2) excess of alcohol used could be retrieved and reused; and 3) without solvent, the production is safer, greener, and of lower cost.
Occurrence of water in the reaction mixture from humidity accompanying the raw materials coupled with water formed in situ as by-product led to low formation of ester due to competitive formation of carboxylic acid. Since the reaction is controlled by equilibrium, it would require certain conditions to push the reaction in favor of ester formation.38 On top of reacting at temperature above water boiling point to continuously remove water, the addition of molecular sieve absorbed trace of moisture in the reaction mixture leading to favorable ester formation. Improved reaction rate and yield were reported with addition of molecular sieve as water scavenger in acid-catalyzed39 and enzymatic esterification.40 Acid value of the DE obtained were in the range of 0.06–5.62 mg KOH g−1 of sample (Table 1).
| DFAE | Molecular | Acid value [mg KOH per g] | Appearance | |
|---|---|---|---|---|
| weight [g mol−1] | formula | |||
| Dibutyl | 677.14 | C44H84O4 | 5.62 | Light yellowish oil |
| Dihexyl | 733.24 | C48H92O4 | 0.06 | Light yellowish oil |
| Di-(2-ethylhexyl) | 789.35 | C52H100O4 | 1.65 | Light yellowish oil |
| Dioctyl | 789.35 | C52H100O4 | 0.54 | Light yellowish oil |
Crude DFAE containing desired product and excess of alcohol were purified by vacuum distillation and characterized by 1H, 13C NMR and FT-IR spectroscopy. In the 1H NMR, formation of esters was confirmed by a triplet peak at δ 4.05–3.94 ppm corresponds to the methylene group protons of ester (─COOCH2─). The 13C NMR for carbonyl (─C═O) group of ester peak appeared at 174.0 ppm slightly upfield compared to carbonyl in the dimer acid at 180.5 ppm, at 64.1 ppm for O─CH2 of ester, 34.5 ppm for methylene carbon adjacent to carbonyl group (─CH2─C═O) (Figure 3). These key peaks can be a good indication for monitoring the formation of DFAE using NMR spectroscopy. It is a powerful tool with simple and quick sample preparation.
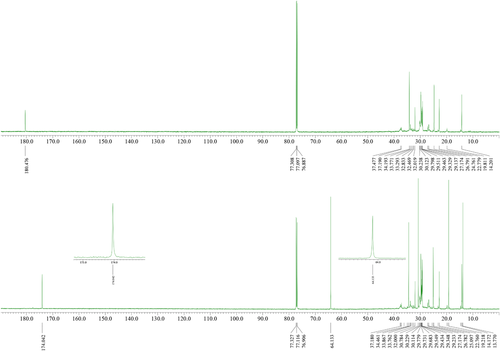
The FT-IR spectra of DFAE showed a strong peak at 1736–1737 cm−1 which can be assigned to stretching vibration of carbonyl (─C═O) group. The carbon–oxygen (C─O) stretching peak appeared at 1169–1172 cm−1. The ester carbon–hydrogen (C─H) stretching and bending vibrations were observed at 2853–2923 and 1461–1463 cm−1.25
3.2 Physicochemical and Lubricant Properties of DFAEs
All of the synthesized DFAEs were characterized for their physicochemical and lubricant properties such as density, kinematic viscosity, pour point, cloud point, and oxidation induction time (OIT) using standard test methods and the results are shown in Table 2. The properties of synthesized DFAEs are compared to commercial DFAE, R7121 that has similar molecular structure as the branched DFAE, di-(2-ethylhexyl) dimerate. This is to evaluate the properties of DFAE synthesized using our procedure and DFAE produced commercially
| Properties | Dibutyl | Dihexyl | Di-(2-ethylhexyl) | Dioctyl | R7121 |
|---|---|---|---|---|---|
| Density [g cm−3] at 25 °C | 0.9097 | 0.9071 | 0.9198 | 0.9023 | — |
| Kinematic viscosity [cSt] at 40 °C | 65.53 | 62.37 | 80.50 | 77.53 | 92.70 |
| 100 °C | 10.81 | 10.56 | 11.90 | 12.30 | 13.60 |
| Viscosity index (VI) | 136 | 138 | 129 | 135 | 131 |
| Cloud point [°C] | −27 | −18 | n/da | −16 | n/da |
| Pour point [°C] | −33 | −27 | −42b | −21 | −33 |
| Oxidation induction time (OIT) [min] | 4.87 | 3.36 | 3.33 | 3.13 | 4.21 |
- a First formation of cloud or haze is hardly observed;
- b pour point could not be determined due to the limitation of instrument.
3.2.1 Kinematic Viscosity and Viscosity Index
The kinematic viscosities of prepared esters ranged between 62.37 and 80.50 cSt at 40 °C and between 10.56 and 12.30 cSt at 100 °C as shown in Table 2. Kinematic viscosity values of the DFAEs increased with increasing molecular weight except for dibutyl which has higher viscosity than dihexyl. It was observed that the branched-chain ester, di-(2-ethylhexyl) dimerate showed the highest viscosity. The viscosity of ester lubricants is highly dependent on molecular weight and amount of branching. As the structure becomes more branched, the molecule becomes bulkier (steric geometric hindrance) which makes it more difficult to bend around and flow over itself41-43 compared to its linear counterpart. Based on ISO Viscosity Grade (ISO VG)–ISO 3448 classification, these esters can be categorized as ISO VG 68 lubricant base stocks.
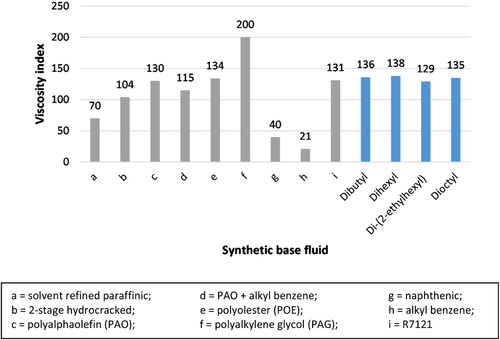
The viscosity index of DFAE synthesized is in the range of 129–138. In contrast with kinematic viscosity, di-(2-ethylhexyl) dimerate has the lowest VI due to the restricted rotational freedom around the ester linkage and decreased ratio of length to cross-section.18 The synthesized DFAEs were then compared to other synthetic base stock.44 As can be seen in Figure 4, viscosity index of DFAE is comparable to commercial polyalphaolefin (PAO) and polyolester (POE) which are commonly used in refrigeration compressor, and automotive, and industrial lubricants.
3.2.2 Cold Flow Properties
The cold flow properties which we evaluated by cloud and pour point are defined as the crystallization and flow behavior of lubricant in low temperature environment. Lower cloud and pour point indicating better performance of lubricant at low temperature. For linear-chain esters, dibutyl dimerate has the lowest cloud point of −27 °C followed by dihexyl and dioctyl dimerate, −18 and −16 °C, respectively. Meanwhile, cloud point of branched-chain ester, di-(2-ethylhexyl) dimer fatty acid could not be determined as first formation of haze or cloud was hardly observed. This is also observed in the commercial DE R7121.
Similarly, the pour point increased with longer linear-chain with dibutyl dimerate exhibiting lowest pour point (−33 °C) followed by dihexyl (−27 °C) and dioctyl (−21 °C) dimerate. Di-(2-ethylhexyl) dimerate exhibited much lower pour point of less than −42 °C and superior than commercial R7121.
The cold flow properties of these esters are affected by molecular weight (carbon chain length) and the structure (branched- or linear-chain). In linear-chain DFAE, the longer the carbon on the ester side, the worse the cold flow properties and this is accordance with several other findings.45, 46 This is mainly attributed by greater van der Waals interactions for longer chain molecules.47, 48 The presence of branching on the carbon chain of esters introduced steric hindrance which inhibit crystallization by disrupting the ability of this molecule to pack regularly in crystal form. In other words, the increase in branching in a molecule requires higher thermodynamic forces for crystallization.24, 42, 49-52
3.2.3 Oxidative Stability
Oxidation stability of the synthesized DFAE was determined using pressure differential scanning calorimeter (PDSC) subjected to oxygen at 3.5 MPa. PDSC provides oxidation induction time (OIT) indicating the oxidation stability of the samples. In the synthesized DFAE, OIT was found to be in the range of 3.13–4.87 min (Table 2). The highest OIT was observed at 4.87 min for dibutyl dimerate which has the shortest side chain followed by dihexyl dimerate and dioctyl dimerate, at 3.36 and 3.13 min, respectively. The OIT of these esters slightly decreased with longer chain length from C4 to C8 as longer chains are more susceptible to oxidative cleavage compared to shorter chains.53
In addition of carbon chain length, branching of molecules has been shown to affect oxidative stability. Dihexyl dimerate has higher OIT than its branched-chain counterpart, di-(2-ethylhexyl) dimerate. This is an expected result due to relative stability of linear-chain molecules toward oxidation. In general, rules of susceptibility toward oxidation of C─H bond is in the order of ─CH > ─CH2 > ─CH3.43 These results are in agreement with studies by other studies on synthetic esters.24, 53
4 Conclusions
Green and efficient approach of producing dimer fatty acid esters (DFAE) with low acid value (0.06–5.62 mg KOH g−1 of sample) has been developed in the absence of catalyst and solvent. Without use of acid catalyst in this esterification, we eliminated the acid neutralization and washing step while solventless approach introduced an environmentally friendly synthesis design and significantly reduced manufacturing cost which would be a great interest for industry. The DFAEs were synthesized by reacting hydrogenated C36 dimer acid with different straight and branched alcohols followed by studies of their physicochemical and lubricant properties. The esters have excellent cold flow properties with cloud point between −16 and −27 °C and pour point between −21 and −42 °C. In comparison to commercial base stock, viscosity index of the synthesized esters is comparable to commercial synthetic base stock PAO and polyolester POE and can be classified as ISO VG 68. The cold flow properties of di-(2-ethylhexyl) dimerate are superior compared to commercial DFAE, R7121. These promising results of synthesized DFAE using catalyst- and solvent-free approach indicating their potential as lubricant base stock for industrial lubricating oil especially for low temperature applications.
Abbreviations
-
- DFAE
-
- dimer fatty acid esters
-
- DOP
-
- dioctyl phthalate
-
- ISO VG
-
- ISO viscosity grade
-
- PAO
-
- polyalpha olefin
-
- POE
-
- polyolester
-
- PU
-
- polyurethane
Acknowledgements
This work was supported by Malaysian Palm Oil Board (MPOB). The authors would like to thank Director-General of MPOB for the permission to publish this article. The authors are also grateful for technical assistance provided by staff of Specialty Chemical (SC) Group for the synthesis and analysis work; Ahmad Lutfi Md Yusof and Selasiah Abdullah, lubricity properties evaluation by Industrial Chemical (IC) Group; Mohd. Zambri Yusof and Sapiah Hashim. The authors would like to express their gratitude to Analytical Testing Services Lab (ATS) of QEA unit, and MPOB for carrying out density measurement for all the synthesized dimer fatty acid esters.
Conflict of Interest
The authors declare no conflict of interest.




