Physical, Chemical, and Biological Characterization of Veiled Extra Virgin Olive Oil Turbidity for Degradation Risk Assessment
Abstract
Six different 300 kg batches of olive fruits are processed and the resulting six 20 kg batches of oil are collected at the end of the “decanter.” These batches of oil are subjected to four different water and solid particle separation treatments so as to obtain the following oil samples: veiled oil, filtered oil, “solid particle-only” oil, and “water-only” oil. The applied separation treatments show that water content has an important role in the degree of turbidity. High water content values (>0.2% w/w) are related to water activity values of >0.6 which are suitable for chemical and enzymatic reactions. The veiled oil samples are contaminated by microorganisms, but non-proportional behavior occurs between the microbial cell count and the water and solid particle contents.
Practical Applications: The results of this study recommend a multi-approach method to characterize turbidity, based on control markers such as the degree of turbidity, water content and water activity, solid particle content, microbial contamination, and phenolic compound content. In this way, each degree of turbidity can be associated with a different level of risk of veiled extra virgin olive oil degradation during shelf life.
1 Introduction
Veiled extra virgin olive oil (VEVOO) is always very attractive on the global market. For many consumers the quality level of VEVOO is higher than filtered extra virgin olive oil, since it is less processed, and therefore deemed healthier, and its sensorial characteristics are more greatly appreciated.1
VEVOO can also be considered a fine water-phase emulsion in a continuous non-polar phase. Water/olive oil micro-emulsion has been studied to understand the role of water in EVOO quality. Water is practically immiscible with oil, but micro-droplets, ranging from 1 to 5 µm, are likely to be found in the form of association colloids stabilized by endogenous emulsifiers such as phospholipids.2-4
The greater affinity of phenolic compounds for the water phase means that most of these compounds are dispersed in the water, that is, in the polar part of the water/oil emulsion. Indeed, in several studies VEVOO has shown a higher phenolic compound content than filtered oil, due to water remaining in the filter aids during filtration; therefore, it has been reported that water plays a stabilizing role in EVOO shelf life.5-10
Water is the most important substrate for the growth of microorganisms. Ciafardini and Zullo11 observed by microscopy that the micro-droplets of water and solid particles dispersed in VEVOO contain microorganisms, which are mainly yeasts.12
Fresh olive oil can contain yeast species which migrate into the oil from the fruits’ carposphere and other sources during the extraction process, but not all of these species can survive during processing and storage.2, 13, 14
The presence of yeasts affects the quality of EVOO, modifying its sensory properties and phenolic compound content.1, 15 Many sensory defects, such as fustiness, mouldiness, wine-vinegary taste, and muddy sediment, have been associated with the microorganisms’ endogenous enzyme activity.12, 14 The International Olive Council trade standards16 recommend an EVOO moisture content of ≤0.2 g per 100 g in order to avoid an off-flavor.
The opalescent appearance of VEVOO is also caused by the presence of solid particles (5–60 µm) deriving from the olive fruit and transferred into the oil during processing.17 The suspended solid particles are composed of polar phenols, carbohydrates, proteins, and phospholipids, and their presence has been related to the physicochemical stability of VEVOO.2, 18
The aim of this work was to make a synoptic characterization of VEVOO turbidity in order to indicate both lower and higher sensitivity to degradation, based on variation of the dispersion–suspension system through a driven change of the EVOO water and solid particle contents.
2 Experimental Section
2.1 Samples
-
¼ of the oil batches were untreated, forming the veiled oil samples for this study, that is, freshly extracted olive oil containing water and solid particles (i.e., samples VO#1 to VO#6).
-
¼ of the oil batches were filtered using a portable filter press (Colombo inox 12, Rover Pompe, Padua, Italy), which was equipped with five filter sheets (Rover 8, 3 µm cut-off, Rover Pompe, Padua, Italy). These formed the filtered oil samples for this study, that is, freshly extracted olive oil not containing water or solid particles (i.e., samples FO#1 to FO#6).
-
¼ of the oil samples were freeze-dried (Modulyo, Edwards, Milan, Italy), forming the “solid particle-only” samples for this study, that is, freshly extracted olive oil containing solid particles only and no water (i.e., samples SO#1 to SO#6).
-
¼ of the oil samples were filtered with glass wool using a filter aid to separate the solid particles, forming the “water-only” samples for this study, that is, freshly extracted olive oil containing water only and no solid particles (i.e., samples WO#1 to WO#6).
All of the oil samples obtained were bottled by hand in 0.25 L clear glass bottles with headspace of about 8% of the bottle's volume. They were stored at room temperature in dark conditions for maximum two days before the subsequent chemical, physical, and biological analyses.
2.2 Analyses
The acidity (% oleic acid), peroxide value (meq O2 kg−1), and UV spectroscopic indices (K232, K270 and ∆K) were measured according to the official EU method.19 The extraction, identification, and determination of the phenolic compounds were performed in agreement with the official IOC method.20 The olive oil volatile organic compound content was determined according to the method described by the literature,21 using the HS-SPME-GC-MS technique.
The degree of turbidity was measured in nephelometric turbidity units (NTU) using a Hach Model 2100 turbidimeter (Hach, Loveland, CO).
Water content (% w/w) was analyzed with a Karl Fischer Kit for visual water determination without a titrator (37858 HYDRANAL – Moisture Test Kit, Honeywell Fluka, Bucharest, Romania). Water activity (Aw) was measured using a Rotronic Hygroskop DT hygrometer (Michell Italia Srl, Milan, Italy).
The solid particle content was measured using the method described by the literature.18 The solid particle content was calculated by weighing the difference, and quantified in % w/w.
The microorganisms were enumerated according to the method reported by the literature,22 with some modifications: an aliquot of each sample (i.e., ≈20 mL) was taken from each bottle in sterile conditions and filtered through a 0.45 µm sterile nitrocellulose membrane. Then, the membrane was transferred into a 50 mL sterile Falcon tube containing 20 mL of sterile physiological solution (NaCl 0.85%) and homogenized using an UltraTurrax (mod. T25 homogenizer, IKA Milan, Italy). Of each homogenized sample, 200 µL serial dilutions were plated onto a YPD agar medium. After 48–72 h of incubation at 28 °C, the colonies with different morphologies were counted and, for each kind, the cell morphology was observed through a light microscope.
CLSM experiments were performed with a Leica TCS SP2 laser scanning confocal microscope (Leica Microsystems GmbH, Wetzlar, Germany) equipped with a 100X oil immersion objective. A 488 nm laser line was used to acquire the fluorescent emission of Rhodamine 110 dissolved in the water droplets in the oil samples (green fluorescence measured between 498 and 520 nm). In particular, a fluorescent probe of about 1 mg was added to 5 g of the oil sample and stirred for more than 24 h at room temperature to distribute the fluorophore well in the oil matrix.
2.3 Data Processing
All analyses were carried out in triplicate for each oil sample. The analytical data were statistically processed according to a multifactor ANOVA using Statgraphics Centurion software (ver. XV, Statpoint Technologies, Warrenton, VA). Type III sums of squares were chosen and the contribution of each factor (i.e., oil, treatment, and replication) was measured after removing the effects of all of the other factors. The p-value test measured the statistical significance of each of the factors.
3 Results
The VO, FO, SO, and WO samples were extra virgin in conformity with the EC regulation.19
3.1 Degree of Turbidity, Water, and Solid Particle Contents
All the VO samples showed a high degree of turbidity, and a high water and solid particle content (Table 1) since they were collected at the end of the “decanter.”
| Veiled oil samples | Degree of turbidity [NTU] | Water content [%w/w] | Solid particle content [%w/w] | Water/solids ratio | Aw | Microbial cell count [UFC g−1] | Microbial cell count [log UFC g−1] | Total phenolic compound content [mgtyrosol kg−1] | Sum of C5 volatile compound content [mg kg−1] | Sum of C6 volatile compound content [mg kg−1] |
|---|---|---|---|---|---|---|---|---|---|---|
| VO#1 | 1677 ± 6a | 0.25 ± 0.01b | 0.25 ± 0.01b | 1.00 | 0.72 ± 0.01c | 2840 ± 903cd | 3.4 | 860 ± 100a | 8.7 ± 0.1d | 43.8 ± 0.4f |
| VO#2 | 1428 ± 13d | 0.37 ± 0.01a | 0.14 ± 0.07d | 2.64 | 0.73 ± 0.01c | 5220 ± 1202bc | 3.7 | 718 ± 54b | 10.41 ± 0.1c | 56.5 ± 0.5c |
| VO#3 | 845 ± 13e | 0.22 ± 0.01c | 0.16 ± 0.01cd | 1.38 | 0.62 ± 0.01d | 7540 ± 1029b | 3.9 | 602 ± 33c | 11.0 ± 0.1b | 59.8 ± 0.6b |
| VO#4 | 836 ± 10e | 0.21 ± 0.01cd | 0.33 ± 0.02a | 0.64 | 0.65 ± 0.01e | 1073 ± 875d | 2.9 | 671 ± 52bc | 13.8 ± 0.1a | 66.2 ± 0.6a |
| VO#5 | 1475 ± 12c | 0.16 ± 0.01e | 0.27 ± 0.08b | 0.59 | 0.78 ± 0.01b | 4913 ± 766bc | 3.7 | 660 ± 62bc | 7.7 ± 0.1e | 46.6 ± 0.2e |
| VO#6 | 1519 ± 8b | 0.20 ± 0.01d | 0.21 ± 0.04bc | 0.95 | 0.82 ± 0.01a | 17527 ± 2726a | 4.2 | 735 ± 30b | 7.2 ± 0.1f | 47.7 ± 0.4d |
Although the oil samples were extracted at the same oil mill, different degrees of turbidity were seen; the lowest degree was approx. 840 NTU for samples VO#3 and VO#4, and the highest approx. 1680 NTU for sample VO#1. The degree of turbidity was related to a statistically significant difference in the water and solid particle contents (Table 1), but not in a proportional way: most of the veiled oil samples did not show a high solid particle content (i.e., VO#1 vs VO#4) or a high water content (i.e., VO#5 vs VO#3). Moreover, the veiled oil samples with a similar degree of turbidity showed different water/solid content ratios (Table 1), such as for sample VO#3 (845 NTU and w/s 1.38) versus sample VO#4 (836 NTU and w/s 0.64).
The oil samples were statistically compared in relation to the applied separation treatments in order to observe how the solid particles and water contents separately affected the degree of turbidity. A change in the turbidity, water and solid particle contents occurred with the treatments (Table 2).
| Oil samples from the different separation treatments | Degree of turbidity [NTU] | Water content [%w/w] | Solid particle content [%w/w] | Aw | Microbial cell count [UFC g−1] | Microbial cell count [log UFC g−1] |
|---|---|---|---|---|---|---|
| *** | *** | *** | *** | *** | *** | |
| VO#1–VO#6 | 1296 ± 364a | 0.24 ± 0.07a | 0.23 ± 0.07a | 0.72 ± 0.07a | 6519 ± 5825a | 3.7 |
| FO#1–FO#6 | 15 ± 4c | 0.05 ± 0.01c | 0.00 ± 0.00b | 0.39 ± 0.04c | 0 ± 0b | n.d. |
| SO#1–SO#6 | 181 ± 21b | 0.03 ± 0.01d | 0.24 ± 0.09a | 0.37 ± 0.05c | 18 ± 28b | 0.6 |
| WO#1–WO#6 | 59 ± 34c | 0.11 ± 0.01b | 0.00 ± 0.00b | 0.56 ± 0.12b | 276 ± 461b | 1.6 |
- n.d. not determined.
The VO samples showed the highest mean contents of water (0.24% w/w) and solid particles (0.23% w/w) and the highest mean turbidity value (1296 NTU). When both the water and soluble particle contents were separated from the oil, the degree of turbidity decreased: the FO samples showed an almost complete lack of water (0.05% w/w), no solid particle content and the lowest mean degree of turbidity (15 NTU). When only the water was separated from the oil (the SO samples), the degree of turbidity showed a decrease in the order of magnitude (181 NTU) compared to the VO samples, even though the solid particle content of the SO samples (0.24% w/w) was not statistically different from the VO samples (Table 2). When the solid particles were separated from the oil (the WO samples), the degree of turbidity decreased by almost two orders of magnitude (59 NTU) compared to the VO samples. This considerable decrease in turbidity could be related both to the complete removal of solid particles from the WO samples and to the combined decrease in water content (0.11% w/w) in the WO samples, highlighting the adsorption of some water on the solid particles.
3.2 Morphology of the Solid Particles and Water Distribution
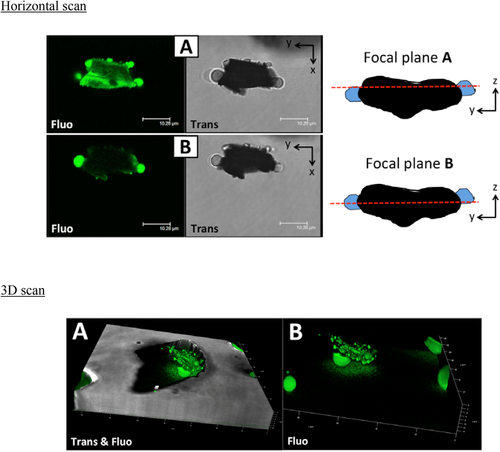
CLSM was applied to observe the morphology of the solid particles dispersed inside the organic matrix and the distribution of water inside the oil samples.
As an example, the results are shown for samples VO#2, SO#2, and WO#2 as follows. Figure 1 shows the horizontal and 3D scans of sample VO#2. The confocal images revealed the presence of green polar droplets dispersed in the oil phase and adsorbed on the surface of the solid particles. Thus, in the VO samples, water was present as isolated droplets and in smaller droplets adsorbed on the solid particles’ surface. Furthermore, the emission signal of the probe was also visible on the surface of the solid particle, perhaps owing to the adsorption of the fluorophore on the polar surface of the solid and/or to the presence of a water film on the solid surface.
The confocal scans acquired on sample SO#2 are shown as in Figure S1, Supporting Information. The oil samples did not contain water droplets, since no spherical green domains containing the hydrophilic probe were visible. Thus, the observed fluorescence could be attributed to the adsorption of Rhodamine 110 chloride on the polar surfaces, such as pulp or other polar residual solid particle components.
The confocal scans acquired on sample WO#2 are shown as in Figure S2, Supporting Information. Small water droplets were present together with some residual solid particles, as revealed by the presence of small rounded green areas.
3.3 Microbial Contamination
All the VO samples were contaminated by microorganisms (Table 1) with microbial counts in the range of 3–4 log UFC g−1. The microorganisms were mainly yeasts, according to the microscopic observations and following the data in the literature.12
A non-proportional relationship occurred between the microbial cell counts and the degree of turbidity, water, and solid particle contents (Table 1). The VO samples with a high degree of turbidity did not show a high microbial cell count (i.e., sample VO#1) and vice versa (i.e., sample VO#3). Similarly, the VO samples with a high water or solid particle content did not show a high microbial cell count (i.e., the VO#2 and VO#4 samples, respectively) and vice versa (the VO#6 and VO#2 samples, respectively).
All the VO samples showed water activity values of >0.6 with a range from 0.62 to 0.82 Aw (Table 1), which makes the VO oil samples a potentially suitable medium for microbial growth and activity.23
The comparison of the oil samples in relation to the applied separation treatments showed an effect on the microbial cell counts (Table 2). The microbial cell count was mainly affected by the decrease in water content. The FO samples contained no microorganisms; the SO and WO samples had a surviving microbial population (i.e., 18 and 276 UFC g−1, respectively), but the relevant microbial cell counts were not significantly different from the FO samples. Consequently, the water activity of the oil samples was affected by the separation treatments applied (Table 2). The water activity values of the FO, SO, and WO samples were <0.60 with a range from 0.37 to 0.43 Aw, namely, they were not suitable for enzymatic activities.23
3.4 Phenolic Compound and Volatile Organic Compound Content
The phenolic compound content of the oil samples was studied as total content, content of groups of phenolic compounds and content of single representative compounds in the EVOO.24 These compounds (i.e., biophenols) are responsible for the important nutraceutical properties of EVOO.
All the VO samples resulted in a high phenolic compound content which was not proportional to the degree of turbidity, water or solid particle contents (Table 1). Comparison of the oil samples in relation to the applied separation treatments showed an effect on the phenolic compound content (Table 3). The mean total phenolic compound content of the VO samples was higher than the FO samples (708 mgtyrosol kg−1 vs 559 mgtyrosol kg−1). Due to the affinity of phenolic compounds for the water phase, the removal of water through a filtration treatment led to an approx. 20% decrease in the total phenolic content. A decrease also occurred for the different groups of phenolic compounds and it was comparable to the total decrease in phenolic compound content. Instead, no statistically significant differences occurred for the 3,4-DHPEA-EDA and p-HPEA-EDA contents.
| Phenolic compound content [mgtyrosol kgoil−1] | VO#1 -VO#6 | FO#1 -FO#6 | WO#1 -WO#6 | SO#1 -SO#6 | p-value |
|---|---|---|---|---|---|
| Sum of oleuropein and its derivatives | 376 ± 35b | 296 ± 55c | 390 ± 23b | 448 ± 29a | *** |
| 3,4-DHPEA-EDA | 97 ± 17a | 88 ± 29a | 104 ± 19a | 115 ± 41a | n.s. |
| Hydroxytyrosol (3,4-DHPEA) | 5 ± 6ab | 3 ± 1c | 4 ± 2bc | 7 ± 6a | *** |
| Sum of ligstroside and its derivatives | 185 ± 70a | 155 ± 46b | 169 ± 46ab | 152 ± 66b | ** |
| p-HPEA-EDA | 98 ± 41a | 92 ± 37a | 86 ± 40a | 81 ± 45a | n.s. |
| Tyrosol (p-HPEA) | 3 ± 1a | 2 ± 1b | 3 ± 1a | 3 ± 1a | *** |
| Phenolic acids | 31 ± 8a | 17 ± 9c | 24 ± 8b | 28 ± 5ab | *** |
| Lignans | 77 ± 9a | 68 ± 10b | 74 ± 10ab | 74 ± 10ab | * |
| Flavonoids | 13 ± 3a | 11 ± 4b | 13 ± 6a | 15 ± 5a | ** |
| Total phenolic compounds | 708 ± 88ab | 559 ± 89c | 681 ± 55b | 737 ± 116a | *** |
- n.s. not statistically significant different.
The role of water was confirmed by the phenolic compound content of the SO samples. Since the applied freeze-drying treatment was able to remove the water only, without solutes, no statistically significant difference occurred between the phenolic compound content of the SO and VO samples. A decreasing trend in the phenolic compound content was also evidenced in the WO samples, which had a lower moisture content than the VO samples (Table 2).
The volatile organic compound content of the oil samples was studied as groups of compounds with five and six carbon atoms, which are usually associated with the LOX pathway and, consequently, with the “fruity” and “green” positive sensory attributes in EVOO.25
Data from all the VO samples (Table 1) showed that C6 compounds from linolenic acid were the most abundant compounds, in agreement with the literature,25, 26 demonstrating that the LOX pathway had a preferential action on the linolenic acid.
The C5 and C6 volatile compounds in the VO, FO, and WO samples were not different from each other (Table S1, Supporting Information). Instead, a decrease in the volatile compounds occurred in the SO samples; a stripping of the volatile compounds was observed due to operating under vacuum conditions during the freeze-drying treatment.
4 Discussion
The separation treatments applied in this study showed that water content had an important role in the degree of turbidity (Table 2). Our confocal images of the oil samples (Figure 1 and Figures S1 and S2, Supporting Information) showed water distribution in the form of i) droplets dispersed in the oil phase, and ii) droplets and a water film, which were adsorbed on the surface of the solid particles. This was the reason why the water content also decreased in the WO samples, which were treated by separating the solid particles with glass wool.
The presence of water in VEVOO is a well-known cause of enzymatic and non-enzymatic degradation phenomena on triglycerides, phenolic and volatile compounds during shelf life.27 In particular, water activity (Aw), that is, water not bound to molecules, is the key factor in the above phenomena. In this study, high values of water content (>0.2% w/w) were only related to Aw values of >0.6, which are suitable for chemical reactions (Tables 1 and 2).
Microbial activity is highly dependent on Aw.23 Spoilage microorganisms, mainly yeasts, are closely associated with the microbiota of the olive fruit carposphere and phyllosphere. During olive processing, microorganisms can migrate into the oil through both solid particles of olive fruit and micro-drops of vegetation water.12, 13 Therefore, water and solid particles could be indirect sources of spoilage microorganisms.
In agreement with the above phenomena, the VO samples were contaminated by microorganisms, whereas the FO samples presented no microorganisms (Tables 1 and 2). However, the behavior between microbial cell counts and water and solid particle contents was not proportional; for example, some VO samples had a difference of 1 log-cycle microbial cell count even though they had a similar moisture content. Indeed, the literature data show that microbial contamination could also be related to the sanitary conditions of the olive fruits and the hygiene conditions of the olive oil mill.2, 14
In this study, moisture content was also shown to have an effect on oil biophenols, whereas no moisture effect occurred on the volatile organic compound content in the oil, which is associated with positive sensory attributes. In particular, a decrease in the water content in the oil samples caused a decrease in both the total phenolic compound content and most of the single phenolic compounds (Table 3), thus highlighting the phenomenon of affinity of phenolic compounds for the water phase.10, 28, 29
5 Conclusions
According to the results of this study, the term “turbidity” should instead be used in the plural, since VEVOO can be characterized by a wide spectrum of “turbidities,” first of all in combination with the water and solid particle contents.
Each degree of turbidity could be associated with a different level of risk of a drop in VEVOO quality during shelf life; for example, this study showed that VEVOO at high risk of degradation has a degree of turbidity which is characterized by high water activity, high microbial contamination and a low phenolic compound content. Therefore, the planning and control of oil turbidity should start from i) adjustment of the water content with suitable application of the normal separation treatments after oil extraction by “decanter”; ii) good manufacturing practices to minimize microbial contamination during the olive oil processing chain.
Acknowledgements
This study was supported by the COMPETiTiVE project (AGER project – AGroalimentare E Ricerca, CdG 29 June 2016).
Conflict of Interest
The authors declare no conflict of interest.




